Receptor Binding Domain-Specific B Cell Memory Responses Among Individuals Vaccinated Against SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participant Details
2.3. Inclusion and Exclusion Criteria
2.4. Blood Collection and Sample Processing
2.5. Indirect Anti-RBD IgG ELISA
2.6. Expression and Purification of the RBD Protein of SARS-CoV-2 (Wuhan Strain-Hu-1)
2.7. Labelling of RBD Protein with the Alexa Fluor-488
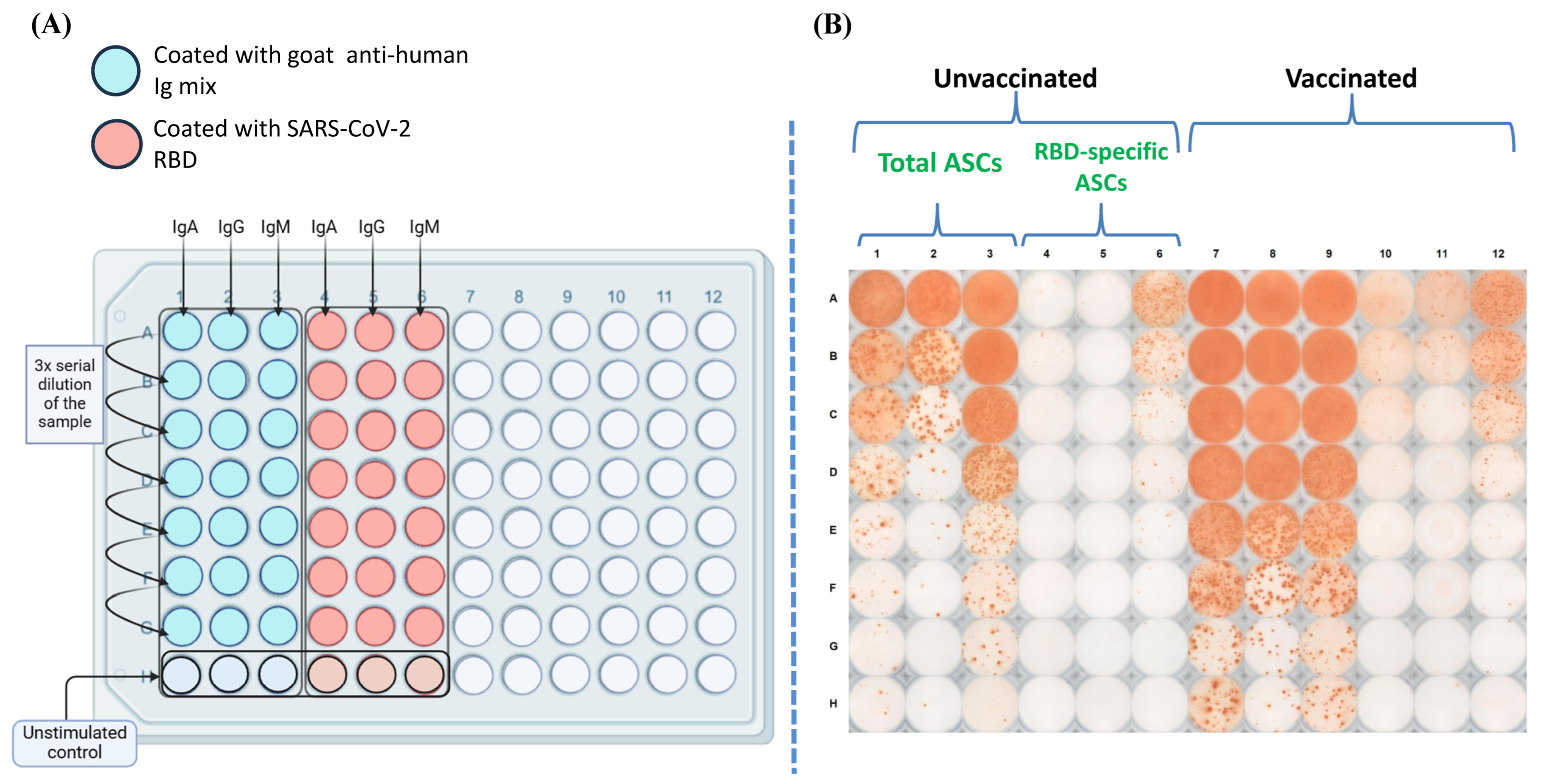
2.8. Estimation of Antibody-Secreting Cells
2.9. Estimation of Memory B Cells in the Peripheral Circulation
2.10. Statistics
3. Results
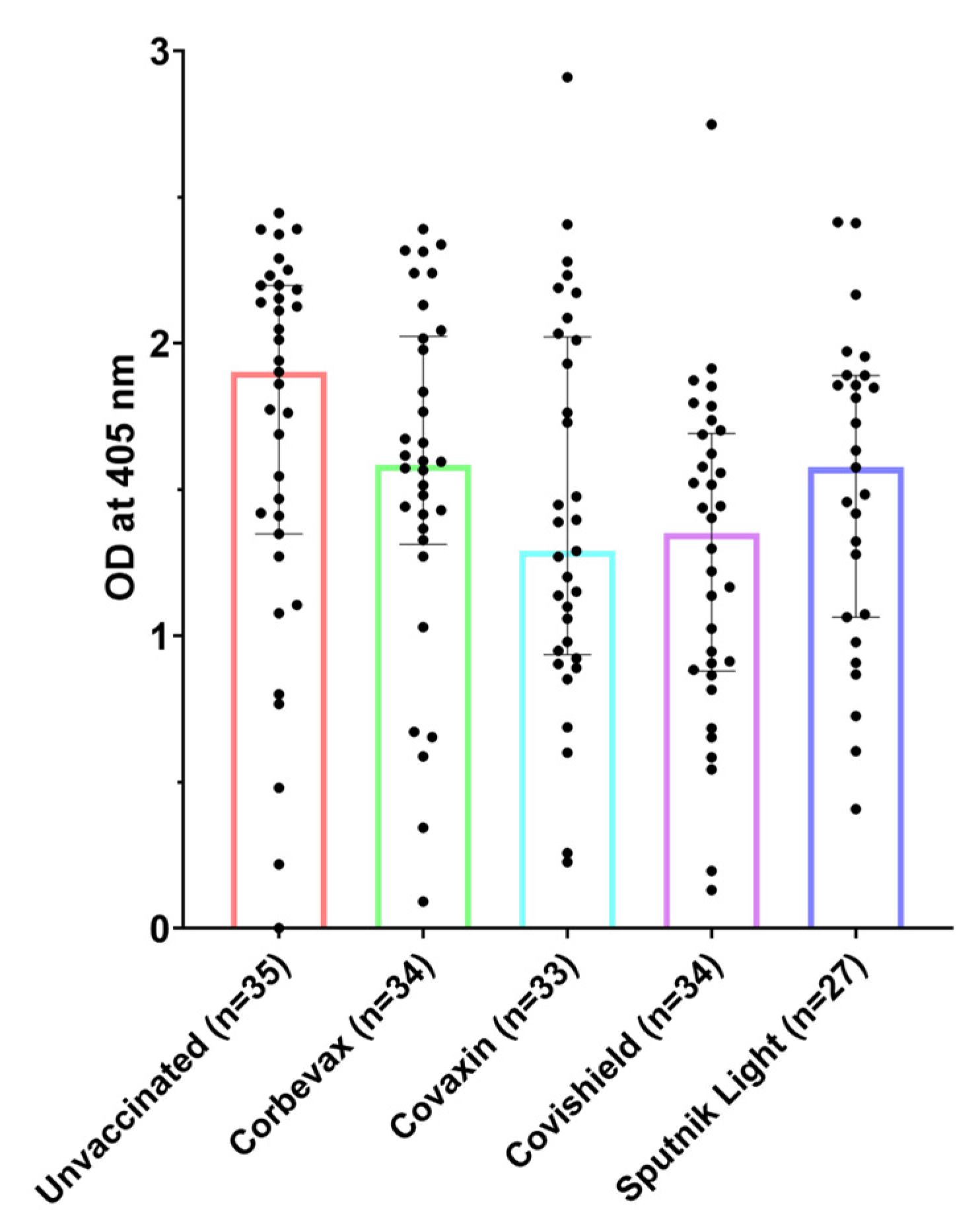
3.1. All Participants, Including Unvaccinated Individuals, Showed Marked Levels of Anti-RBD IgG Antibodies
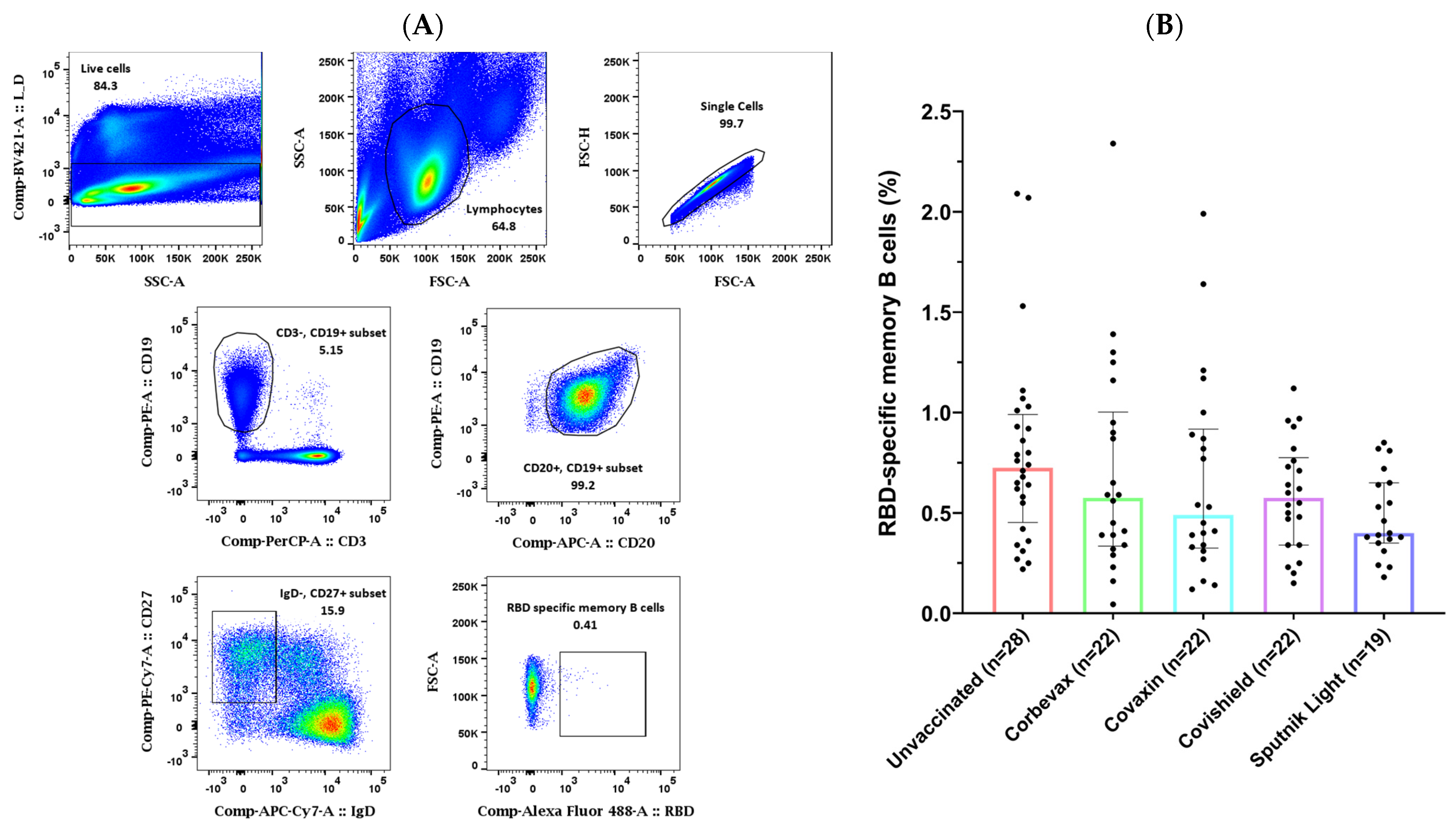
3.2. Study Participants Exhibited RBD-Specific Memory B Cells in the Peripheral Circulation
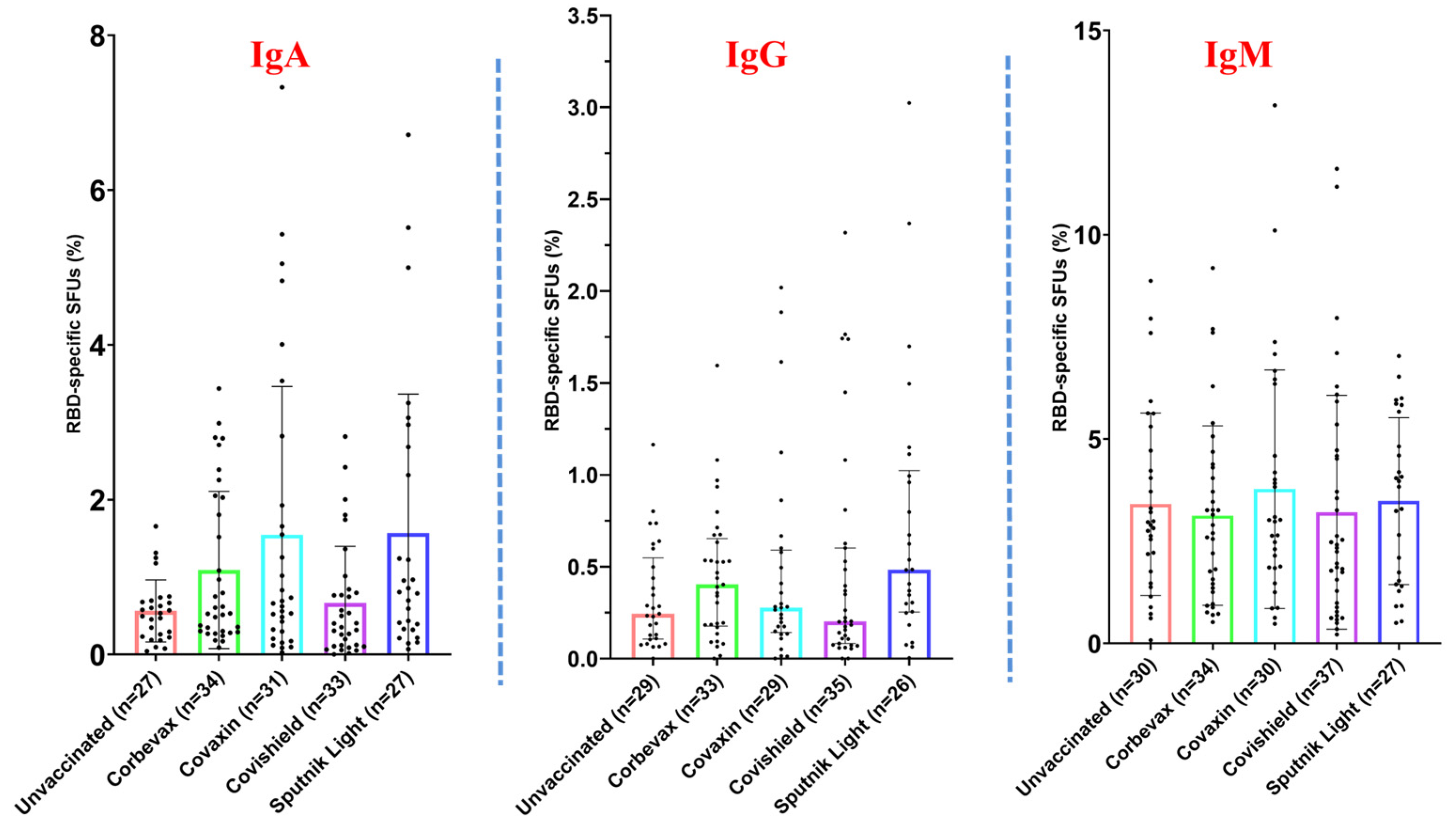
3.3. Magnitude of the RBD-Specific Antibody-Secreting B Cells Among Vaccinees from Different Vaccine Groups
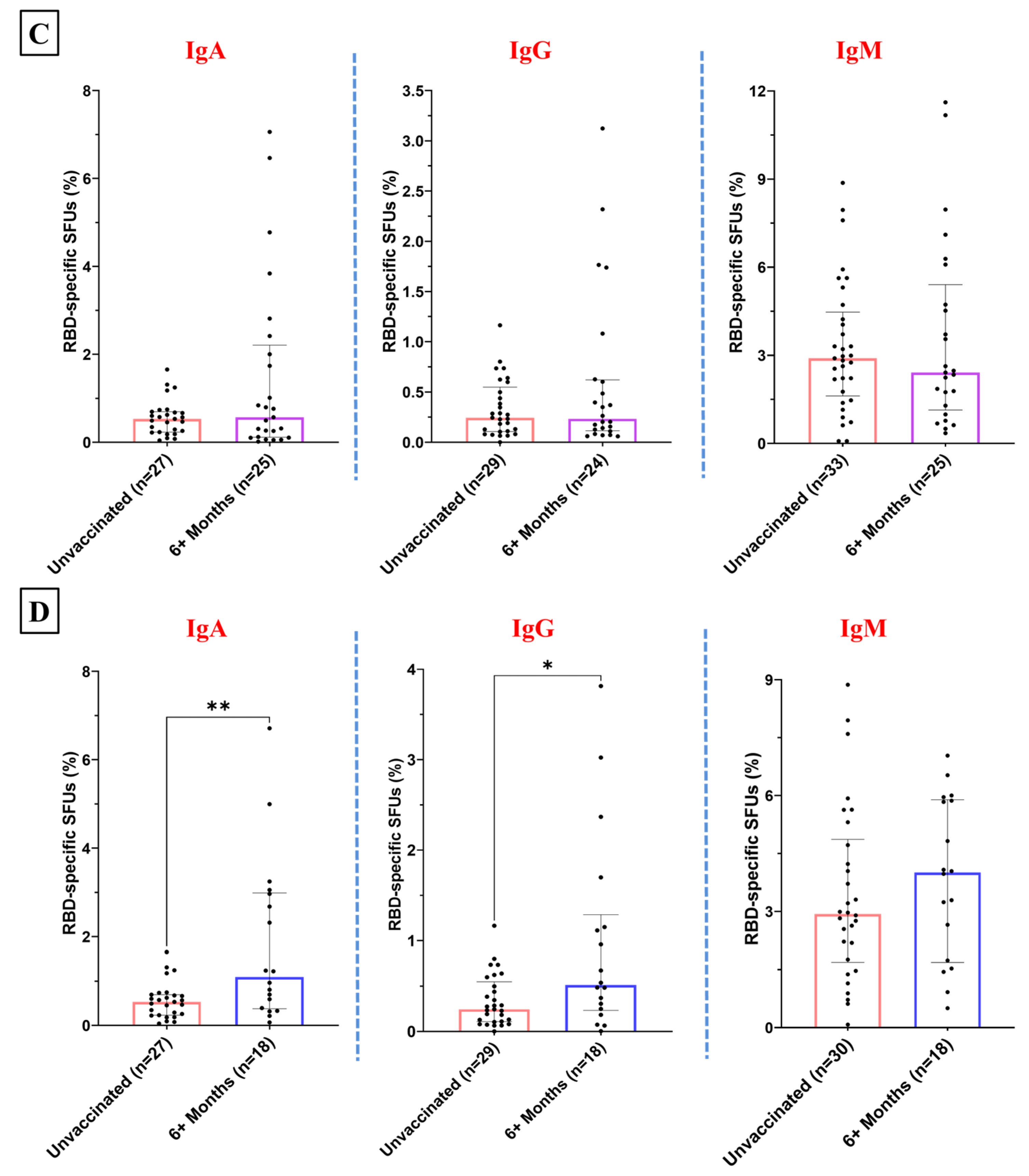
3.4. Temporal Patterns in the RBD-Specific Antibody-Secreting B Cells Post-Vaccination
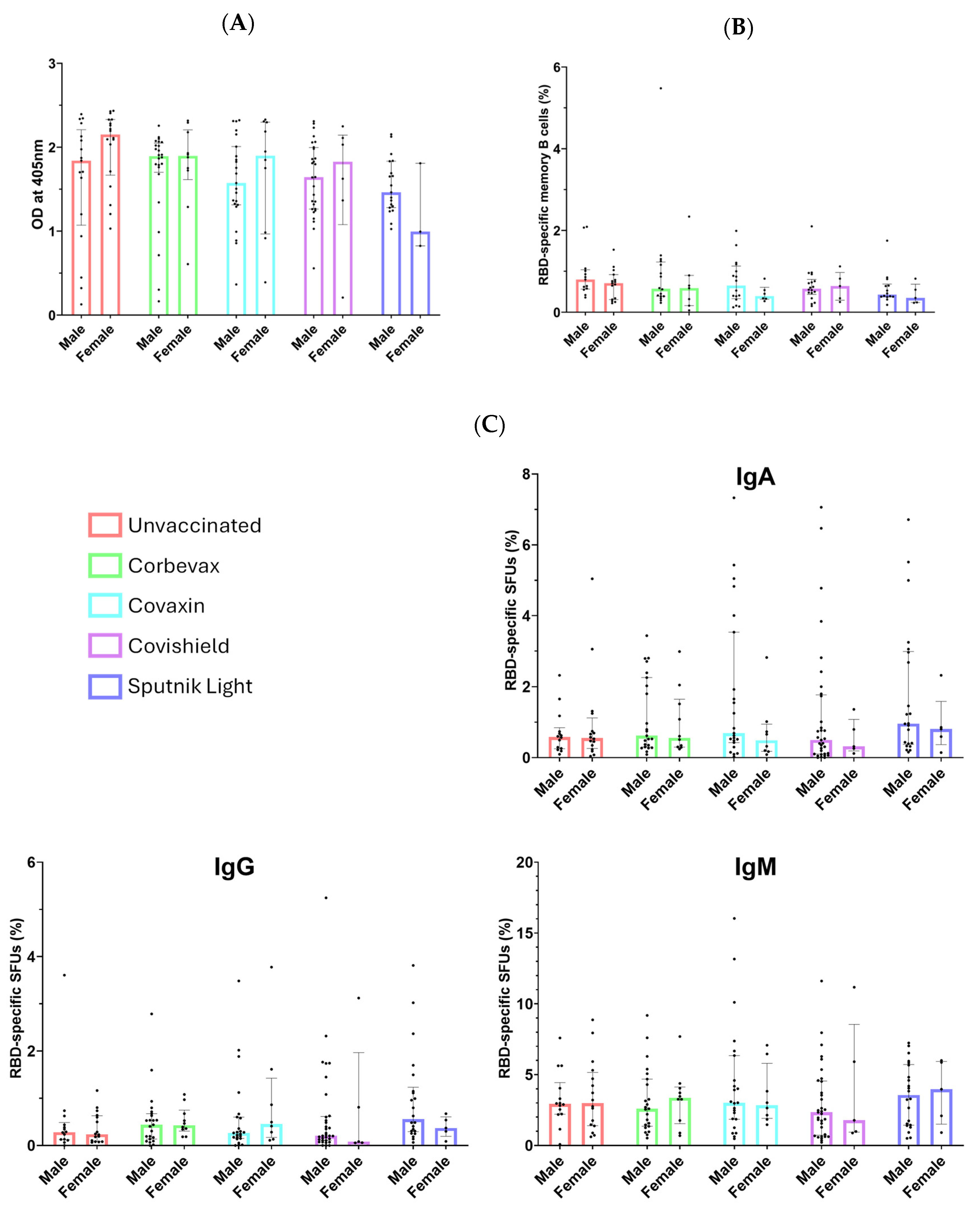
3.5. No Sex-Specific Disparities Observed in Serological or Memory Immune Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Subbarao, K. The Success of SARS-CoV-2 Vaccines and Challenges Ahead. Cell Host Microbe 2021, 29, 1111–1123. [Google Scholar] [CrossRef]
- Ciabattini, A.; Pastore, G.; Lucchesi, S.; Montesi, G.; Costagli, S.; Polvere, J.; Fiorino, F.; Pettini, E.; Lippi, A.; Ancillotti, L.; et al. Trajectory of Spike-Specific B Cells Elicited by Two Doses of BNT162b2 mRNA Vaccine. Cells 2023, 12, 1706. [Google Scholar] [CrossRef]
- Chua, C.-L.; Sam, I.-C.; Chiam, C.-W.; Chan, Y.-F. The Neutralizing Role of IgM during Early Chikungunya Virus Infection. PLoS ONE 2017, 12, e0171989. [Google Scholar] [CrossRef]
- Lizeng, Q.; Nilsson, C.; Sourial, S.; Andersson, S.; Larsen, O.; Aaby, P.; Ehnlund, M.; Björling, E. Potent Neutralizing Serum Immunoglobulin A (IgA) in Human Immunodeficiency Virus Type 2-Exposed IgG-Seronegative Individuals. J. Virol. 2004, 78, 7016–7022. [Google Scholar] [CrossRef]
- Pušnik, J.; König, J.; Mai, K.; Richter, E.; Zorn, J.; Proksch, H.; Schulte, B.; Alter, G.; Streeck, H. Persistent Maintenance of Intermediate Memory B Cells Following SARS-CoV-2 Infection and Vaccination Recall Response. J. Virol. 2022, 96, e0076022. [Google Scholar] [CrossRef]
- Quast, I.; Tarlinton, D. B Cell Memory: Understanding COVID-19. Immunity 2021, 54, 205–210. [Google Scholar] [CrossRef]
- Kurosaki, T.; Kometani, K.; Ise, W. Memory B Cells. Nat. Rev. Immunol. 2015, 15, 149–159. [Google Scholar] [CrossRef]
- Inoue, T.; Moran, I.; Shinnakasu, R.; Phan, T.G.; Kurosaki, T. Generation of Memory B Cells and Their Reactivation. Immunol. Rev. 2018, 283, 138–149. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The Multifaceted Role of CD4+ T Cells in CD8+ T Cell Memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.-K.E.; Henry, C. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 2019, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, D.; Good-Jacobson, K. Diversity among Memory B Cells: Origin, Consequences, and Utility. Science 2013, 341, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, M.; Obara, M.; Chiyyeadu, A.; Costa, B.; Salam, A.; Ziegler, A.; Waltl, I.; Pavlou, A.; Bonifacius, A.; Hoffmann, M.; et al. Memory B Cells Anticipate SARS-CoV-2 Variants through Somatic Hypermutation. J. Infect. 2024, 88, 57–60. [Google Scholar] [CrossRef]
- Crotty, S.; Aubert, R.D.; Glidewell, J.; Ahmed, R. Tracking Human Antigen-Specific Memory B Cells: A Sensitive and Generalized ELISPOT System. J. Immunol. Methods 2004, 286, 111–122. [Google Scholar] [CrossRef]
- Hartley, G.E.; Edwards, E.S.J.; O’Hehir, R.E.; Van Zelm, M.C. New Insights into Human Immune Memory from SARS-CoV-2 Infection and Vaccination. Allergy 2022, 77, 3553–3566. [Google Scholar] [CrossRef]
- Nayak, K.; Gottimukkala, K.; Kumar, S.; Reddy, E.S.; Edara, V.V.; Kauffman, R.; Floyd, K.; Mantus, G.; Savargaonkar, D.; Goel, P.K.; et al. Characterization of Neutralizing versus Binding Antibodies and Memory B Cells in COVID-19 Recovered Individuals from India. Virology 2021, 558, 13–21. [Google Scholar] [CrossRef]
- Subramaniam, A. Targeting Spike Protein: Modified Antibody for Broad-Spectrum Binding to Coronaviruses: An In Silico Study. Virol. Immunol. J. 2023, 7, 000324. [Google Scholar] [CrossRef]
- Ejemel, M.; Li, Q.; Hou, S.; Schiller, Z.A.; Tree, J.A.; Wallace, A.; Amcheslavsky, A.; Kurt Yilmaz, N.; Buttigieg, K.R.; Elmore, M.J.; et al. A Cross-Reactive Human IgA Monoclonal Antibody Blocks SARS-CoV-2 Spike-ACE2 Interaction. Nat. Commun. 2020, 11, 4198. [Google Scholar] [CrossRef]
- Hajilooi, M.; Keramat, F.; Moazenian, A.; Rastegari-Pouyani, M.; Solgi, G. The Quantity and Quality of Anti-SARS-CoV-2 Antibodies Show Contrariwise Association with COVID-19 Severity: Lessons Learned from IgG Avidity. Med. Microbiol. Immunol. 2023, 212, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Vadrevu, K.M.; Ganneru, B.; Reddy, S.; Jogdand, H.; Raju, D.; Sapkal, G.; Yadav, P.; Reddy, P.; Verma, S.; Singh, C.; et al. Persistence of Immunity and Impact of Third Dose of Inactivated COVID-19 Vaccine against Emerging Variants. Sci. Rep. 2022, 12, 12038. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Athavale, A.; Tripathi, A.H.; Subramaniam, A.; Upadhyay, S.K.; Pandey, A.K.; Rai, R.C.; Awasthi, A. To Be Remembered: B Cell Memory Response against SARS-CoV-2 and Its Variants in Vaccinated and Unvaccinated Individuals. Scand. J. Immunol. 2024, 99, e13345. [Google Scholar] [CrossRef] [PubMed]
- Thuluva, S.; Paradkar, V.; Gunneri, S.; Yerroju, V.; Mogulla, R.; Suneetha, P.V.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Adabala, S.; et al. Immunogenicity and Safety of Biological E’s CORBEVAXTM Vaccine Compared to COVISHIELDTM (ChAdOx1 nCoV-19) Vaccine Studied in a Phase-3, Single Blind, Multicentre, Randomized Clinical Trial. Hum. Vaccin. Immunother. 2023, 19, 2203632. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G.; et al. An Open, Non-Randomised, Phase 1/2 Trial on the Safety, Tolerability, and Immunogenicity of Single-Dose Vaccine “Sputnik Light” for Prevention of Coronavirus Infection in Healthy Adults. Lancet Reg. Health Eur. 2021, 11, 100241. [Google Scholar] [CrossRef]
- Russian Direct Investment Fund. Sputnik Light: A Single-Component Stand-Alone Vaccine Against COVID-19 and a Perfect Booster; Russian Direct Investment Fund: Moscow, Russia, 2021. [Google Scholar]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBV152: A Double-Blind, Randomised, Phase 1 Trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 nCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and Immunogenicity of an rAd26 and rAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine in Two Formulations: Two Open, Non-Randomised Phase 1/2 Studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Biological, E. Limited. CORBEVAX Is India’s 1st Indigenously Developed Protein Sub-Unit COVID-19 Vaccine; Biological E. Limited: Hyderabad, India, 2021. [Google Scholar]
- Krishna, B.; Gupta, A.; Meena, K.; Gaba, A.; Krishna, S.; Jyoti, R.; Aeron, N.; Prashanth, S.; Samriti; Ganapathy, U. Prevalence, Severity, and Risk Factor of Breakthrough Infection after Vaccination with Either the Covaxin or the Covishield among Healthcare Workers: A Nationwide Cross-Sectional Study. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, S66–S78. [Google Scholar] [CrossRef]
- Malhotra, A. Curing the Pandemic of Misinformation on COVID-19 mRNA Vaccines through Real Evidence-Based Medicine—Part 2. J. Insul. Resist. 2022, 5, 72. [Google Scholar] [CrossRef]
- Vallejo, A.; Vizcarra, P.; Martín-Hondarza, A.; Gómez-Maldonado, S.; Haemmerle, J.; Velasco, H.; Casado, J.L. Impact of SARS-CoV-2-Specific Memory B Cells on the Immune Response after mRNA-Based Comirnaty Vaccine in Seronegative Health Care Workers. Front. Microbiol. 2022, 13, 1002748. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J.; Brokstad, K.A. Not Just Antibodies: B Cells and T Cells Mediate Immunity to COVID-19. Nat. Rev. Immunol. 2020, 20, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Kudriavtsev, A.V.; Vakhrusheva, A.V.; Novoseletsky, V.N.; Bozdaganyan, M.E.; Shaitan, K.V.; Kirpichnikov, M.P.; Sokolova, O.S. Immune Escape Associated with RBD Omicron Mutations and SARS-CoV-2 Evolution Dynamics. Viruses 2022, 14, 1603. [Google Scholar] [CrossRef]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.-M.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 Neutralizing Antibody Structures Inform Therapeutic Strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, Q.; Guo, B.; Mu, D.; Lu, X.; Ma, Q.; Guo, Y.; Fang, L.; Zhang, B.; Zhang, G.; et al. A Method To Prevent SARS-CoV-2 IgM False Positives in Gold Immunochromatography and Enzyme-Linked Immunosorbent Assays. J. Clin. Microbiol. 2020, 58, e00375-20. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, Y.; Wang, J.; Liu, J.; Liu, C.; Liu, D.; Yang, S. Distinct Variations of Antibody Secreting Cells and Memory B Cells during the Course of Kawasaki Disease. BMC Immunol. 2019, 20, 16. [Google Scholar] [CrossRef]
- Berkowska, M.A.; Driessen, G.J.A.; Bikos, V.; Grosserichter-Wagener, C.; Stamatopoulos, K.; Cerutti, A.; He, B.; Biermann, K.; Lange, J.F.; van der Burg, M.; et al. Human Memory B Cells Originate from Three Distinct Germinal Center-Dependent and -Independent Maturation Pathways. Blood 2011, 118, 2150–2158. [Google Scholar] [CrossRef]
- Mehdi, F.; Chattopadhyay, S.; Thiruvengadam, R.; Yadav, S.; Kumar, M.; Sinha, S.K.; Goswami, S.; Kshetrapal, P.; Wadhwa, N.; Chandramouli Natchu, U.; et al. Development of a Fast SARS-CoV-2 IgG ELISA, Based on Receptor-Binding Domain, and Its Comparative Evaluation Using Temporally Segregated Samples From RT-PCR Positive Individuals. Front. Microbiol. 2020, 11, 618097. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Khatri, R.; Parray, H.A.; Siddiqui, G.; Chiranjivi, A.K.; Raj, S.; Kaul, R.; Maithil, V.; Samal, S.; Ahmed, S. Biophysical and Biochemical Characterization of the Receptor Binding Domain of SARS-CoV-2 Variants. Protein J. 2022, 41, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, R.; Paul, J.S.; Babji, S.; Thamizh, I.; Kumar, D.; Khakha, S.A.; Rennie, A.; Kumar, K.; Dhanapal, P.; Saravanan, P.; et al. SARS-CoV-2 Infections before, during, and after the Omicron Wave: A 2-Year Indian Community Cohort Study. Lancet Reg. Health Southeast Asia 2024, 28, 100470. [Google Scholar] [CrossRef] [PubMed]
- Skountzou, I.; Satyabhama, L.; Stavropoulou, A.; Ashraf, Z.; Esser, E.S.; Vassilieva, E.; Koutsonanos, D.; Compans, R.; Jacob, J. Influenza Virus-Specific Neutralizing IgM Antibodies Persist for a Lifetime. Clin. Vaccine Immunol. 2014, 21, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Takemori, T.; Kaji, T.; Takahashi, Y.; Shimoda, M.; Rajewsky, K. Generation of Memory B Cells inside and Outside Germinal Centers. Eur. J. Immunol. 2014, 44, 1258–1264. [Google Scholar] [CrossRef]
- Shlomchik, M.J.; Weisel, F. Germinal Center Selection and the Development of Memory B and Plasma Cells. Immunol. Rev. 2012, 247, 52–63. [Google Scholar] [CrossRef]
- Brown, E.L.; Essigmann, H.T. Original Antigenic Sin: The Downside of Immunological Memory and Implications for COVID-19. mSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Sasikala, M.; Shashidhar, J.; Deepika, G.; Ravikanth, V.; Krishna, V.V.; Sadhana, Y.; Pragathi, K.; Reddy, D.N. Immunological Memory and Neutralizing Activity to a Single Dose of COVID-19 Vaccine in Previously Infected Individuals. Int. J. Infect. Dis. 2021, 108, 183–186. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Yasuhara, A.; Ito, M.; Akasaka, O.; Nakamura, M.; Nakachi, I.; Koga, M.; Mitamura, K.; Yagi, K.; Maeda, K.; et al. Antibody Titers against SARS-CoV-2 Decline, but Do Not Disappear for Several Months. EClinicalMedicine 2021, 32, 100734. [Google Scholar] [CrossRef]
- Jo, D.-H.; Minn, D.; Lim, J.; Lee, K.-D.; Kang, Y.-M.; Choe, K.-W.; Kim, K.-N. Rapidly Declining SARS-CoV-2 Antibody Titers within 4 Months after BNT162b2 Vaccination. Vaccines 2021, 9, 1145. [Google Scholar] [CrossRef]
- Choudhary, H.R.; Parai, D.; Chandra Dash, G.; Kshatri, J.S.; Mishra, N.; Choudhary, P.K.; Pattnaik, D.; Panigrahi, K.; Behera, S.; Ranjan Sahoo, N.; et al. Persistence of Antibodies Against Spike Glycoprotein of SARS-CoV-2 in Healthcare Workers Post Double Dose of BBV-152 and AZD1222 Vaccines. Front. Med. 2021, 8, 778129. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Kaku, C.I.; Bergeron, A.J.; Ahlm, C.; Normark, J.; Sakharkar, M.; Forsell, M.N.E.; Walker, L.M. Recall of Preexisting Cross-Reactive B Cell Memory after Omicron BA.1 Breakthrough Infection. Sci. Immunol. 2022, 7, eabq3511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Raspe, R.; Johannsen, F.; Turroja, M.; Canis, M.; ElTanbouly, M.A.; Santos, G.S.S.; Johnson, B.; Baharani, V.A.; et al. Memory B Cell Development Elicited by mRNA Booster Vaccinations in the Elderly. J. Exp. Med. 2023, 220, e20230668. [Google Scholar] [CrossRef] [PubMed]
- Morales-Núñez, J.J.; García-Chagollán, M.; Muñoz-Valle, J.F.; Díaz-Pérez, S.A.; Torres-Hernández, P.C.; Rodríguez-Reyes, S.C.; Santoscoy-Ascencio, G.; Sierra García de Quevedo, J.J.; Hernández-Bello, J. Differences in B-Cell Immunophenotypes and Neutralizing Antibodies Against SARS-CoV-2 After Administration of BNT162b2 (Pfizer-BioNTech) Vaccine in Individuals with and without Prior COVID-19—A Prospective Cohort Study. J. Inflamm. Res. 2022, 15, 4449–4466. [Google Scholar] [CrossRef]
- Terreri, S.; Piano Mortari, E.; Vinci, M.R.; Russo, C.; Alteri, C.; Albano, C.; Colavita, F.; Gramigna, G.; Agrati, C.; Linardos, G.; et al. Persistent B Cell Memory after SARS-CoV-2 Vaccination Is Functional during Breakthrough Infections. Cell Host Microbe 2022, 30, 400–408.e4. [Google Scholar] [CrossRef]
- Geropeppa, M.; Papadatou, I.; Sarantis, P.; Tzanoudaki, M.; Ntanasis-Stathopoulos, I.; Bagratuni, T.; Terpos, E.; Spoulou, V. Receptor-Binding-Domain-Specific B Cell Responses Induced by mRNA Immunization against SARS-CoV-2. Vaccines 2023, 11, 1148. [Google Scholar] [CrossRef]
- Busà, R.; Miele, M.; Sorrentino, M.C.; Amico, G.; Timoneri, F.; Miceli, V.; Di Bella, M.; Russelli, G.; Gallo, A.; Zito, G.; et al. Long-Term Effectiveness of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine on B Cell Compartment: Efficient Recall of SARS-CoV-2-Specific Memory B Cells. Int. J. Mol. Sci. 2022, 23, 15046. [Google Scholar] [CrossRef]
- Brewer, R.C.; Ramadoss, N.S.; Lahey, L.J.; Jahanbani, S.; Robinson, W.H.; Lanz, T.V. BNT162b2 Vaccine Induces Divergent B Cell Responses to SARS-CoV-2 S1 and S2. Nat. Immunol. 2022, 23, 33–39. [Google Scholar] [CrossRef]
- Mise-Omata, S.; Ikeda, M.; Takeshita, M.; Uwamino, Y.; Wakui, M.; Arai, T.; Yoshifuji, A.; Murano, K.; Siomi, H.; Nakagawara, K.; et al. Memory B Cells and Memory T Cells Induced by SARS-CoV-2 Booster Vaccination or Infection Show Different Dynamics and Responsiveness to the Omicron Variant. J. Immunol. 2022, 209, 2104–2113. [Google Scholar] [CrossRef]
- Weisel, N.M.; Joachim, S.M.; Smita, S.; Callahan, D.; Elsner, R.A.; Conter, L.J.; Chikina, M.; Farber, D.L.; Weisel, F.J.; Shlomchik, M.J. Surface Phenotypes of Naive and Memory B Cells in Mouse and Human Tissues. Nat. Immunol. 2022, 23, 135–145. [Google Scholar] [CrossRef]
- Grimsholm, O. CD27 on Human Memory B Cells-More than Just a Surface Marker. Clin. Exp. Immunol. 2023, 213, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Wu, Q.; Shen, J.; Wu, C. CD27 Is Not an Ideal Marker for Human Memory B Cells and Can Be Modulated by IL-21 upon Stimulated by Anti-CD40. Sci. Rep. 2024, 14, 23742. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, E.; Del Rio Estrada, P.M.; DaSilva, J.; Boukadida, C.; Zhang, F.; Luna-Villalobos, Y.A.; Rodríguez-Rangel, X.; Pitén-Isidro, E.; Luna-García, E.; Díaz Rivera, D.; et al. Antibody and Memory B-Cell Immunity in a Heterogeneously SARS-CoV-2-Infected and -Vaccinated Population. mBio 2022, 13, e00840-22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and Cellular Immune Memory to Four COVID-19 Vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- Fryer, H.A.; Hartley, G.E.; Edwards, E.S.J.; O’Hehir, R.E.; van Zelm, M.C. Humoral Immunity and B-Cell Memory in Response to SARS-CoV-2 Infection and Vaccination. Biochem. Soc. Trans. 2022, 50, 1643–1658. [Google Scholar] [CrossRef]

| (A) | ||||||
|---|---|---|---|---|---|---|
| Sr. No. | Status of Vaccination | Number | ||||
| 1 | Unvaccinated | 35 | ||||
| 2 | Vaccinated (Corbevax) | 34 | ||||
| 3 | Vaccinated (Covaxin) | 33 | ||||
| 4 | Vaccinated (Covishield) | 40 | ||||
| 5 | Vaccinated (Sputnik Light) | 29 | ||||
| (B) | ||||||
| Unvaccinated | Corbevax | Covishield | Covaxin | Sputnik Light | ||
| Sex | Male | 17 | 24 | 33 | 24 | 25 |
| Female | 18 | 10 | 7 | 9 | 4 | |
| Age | Range | 37 (18–55) | 26 (20–46) | 34 (19–53) | 33 (21–54) | 39 (20–59) |
| Median | 28 | 29 | 26 | 26 | 30 | |
| Months since vaccination | Range | - | 5 (3–8) | 12 (3–15) | 12 (3–15) | 5 (6–11) |
| Median | - | 6 | 8 | 11 | 8 | |
| SN. | Molecular Marker | Fluorophore | Make and Catalogue Number |
|---|---|---|---|
| 1 | Fixable viability dye | Violet Dye | Invitrogen, Waltham, MA, USA #L34964 |
| 2 | CD3 | PerCP | BioLegend, San Diego, CA, USA #344814 |
| 3 | CD19 | PE | BioLegend, San Diego, CA, USA #302208 |
| 4 | CD20 | APC | BioLegend, San Diego, CA, USA #302310 |
| 5 | CD27 | PE-Cy7 | BioLegend, San Diego, CA, USA #356412 |
| 6 | IgD | APC-Cy7 | BioLegend, San Diego, CA, USA #348218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athavale, A.; Gaur, A.; Ahmed, N.; Subramaniam, A.; Dandotiya, J.; Raj, S.; Upadhyay, S.K.; Samal, S.; Pandey, A.K.; Rai, R.C.; et al. Receptor Binding Domain-Specific B Cell Memory Responses Among Individuals Vaccinated Against SARS-CoV-2. Vaccines 2024, 12, 1396. https://doi.org/10.3390/vaccines12121396
Athavale A, Gaur A, Ahmed N, Subramaniam A, Dandotiya J, Raj S, Upadhyay SK, Samal S, Pandey AK, Rai RC, et al. Receptor Binding Domain-Specific B Cell Memory Responses Among Individuals Vaccinated Against SARS-CoV-2. Vaccines. 2024; 12(12):1396. https://doi.org/10.3390/vaccines12121396
Chicago/Turabian StyleAthavale, Atharv, Anmol Gaur, Nafees Ahmed, Adarsh Subramaniam, Jyotsna Dandotiya, Sneha Raj, Santosh Kumar Upadhyay, Sweety Samal, Anil Kumar Pandey, Ramesh Chandra Rai, and et al. 2024. "Receptor Binding Domain-Specific B Cell Memory Responses Among Individuals Vaccinated Against SARS-CoV-2" Vaccines 12, no. 12: 1396. https://doi.org/10.3390/vaccines12121396
APA StyleAthavale, A., Gaur, A., Ahmed, N., Subramaniam, A., Dandotiya, J., Raj, S., Upadhyay, S. K., Samal, S., Pandey, A. K., Rai, R. C., & Awasthi, A. (2024). Receptor Binding Domain-Specific B Cell Memory Responses Among Individuals Vaccinated Against SARS-CoV-2. Vaccines, 12(12), 1396. https://doi.org/10.3390/vaccines12121396







