Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Study Population Demographics and Baseline Characteristics
3.2. Reactogenicity and Safety
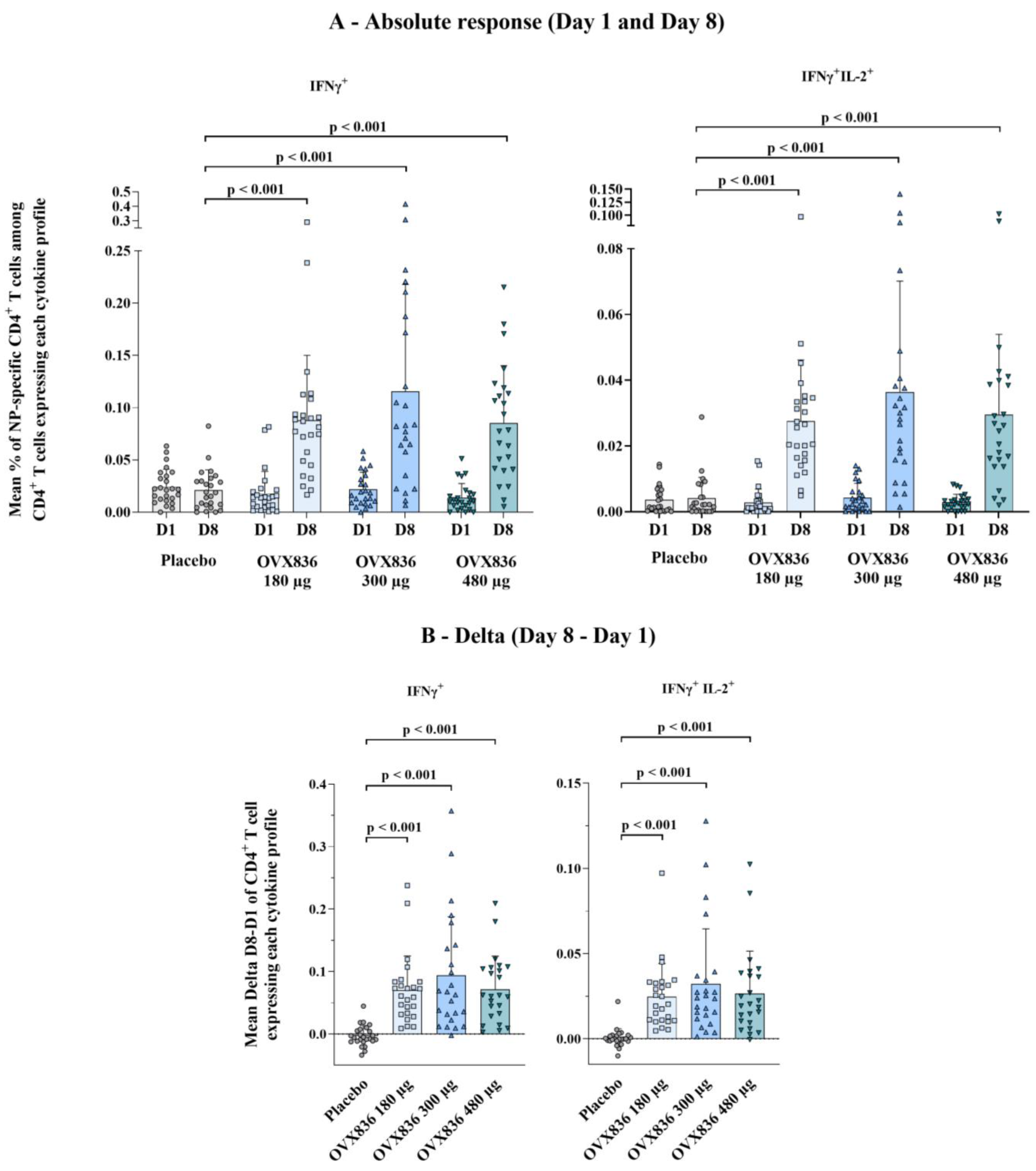
3.3. Cell-Mediated Immune Response
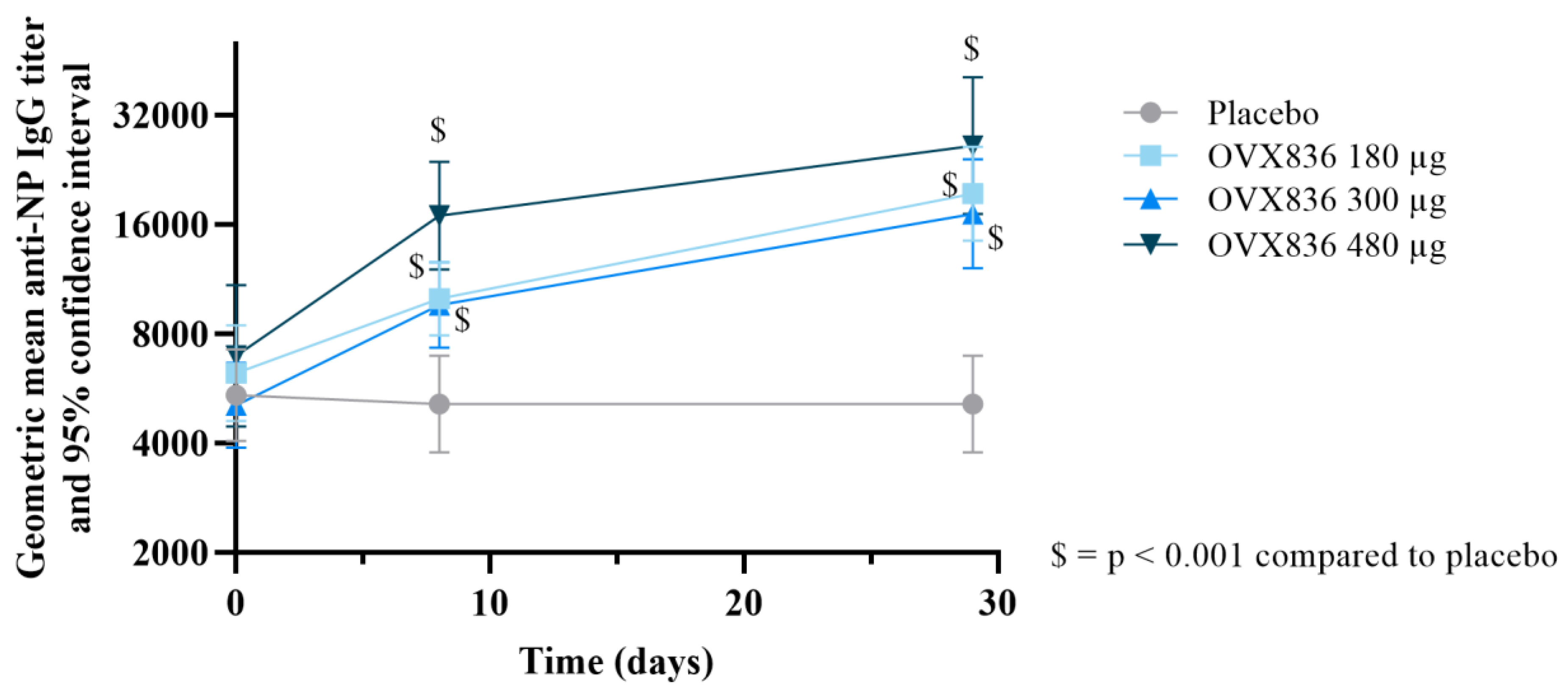
3.4. Humoral Immune Response
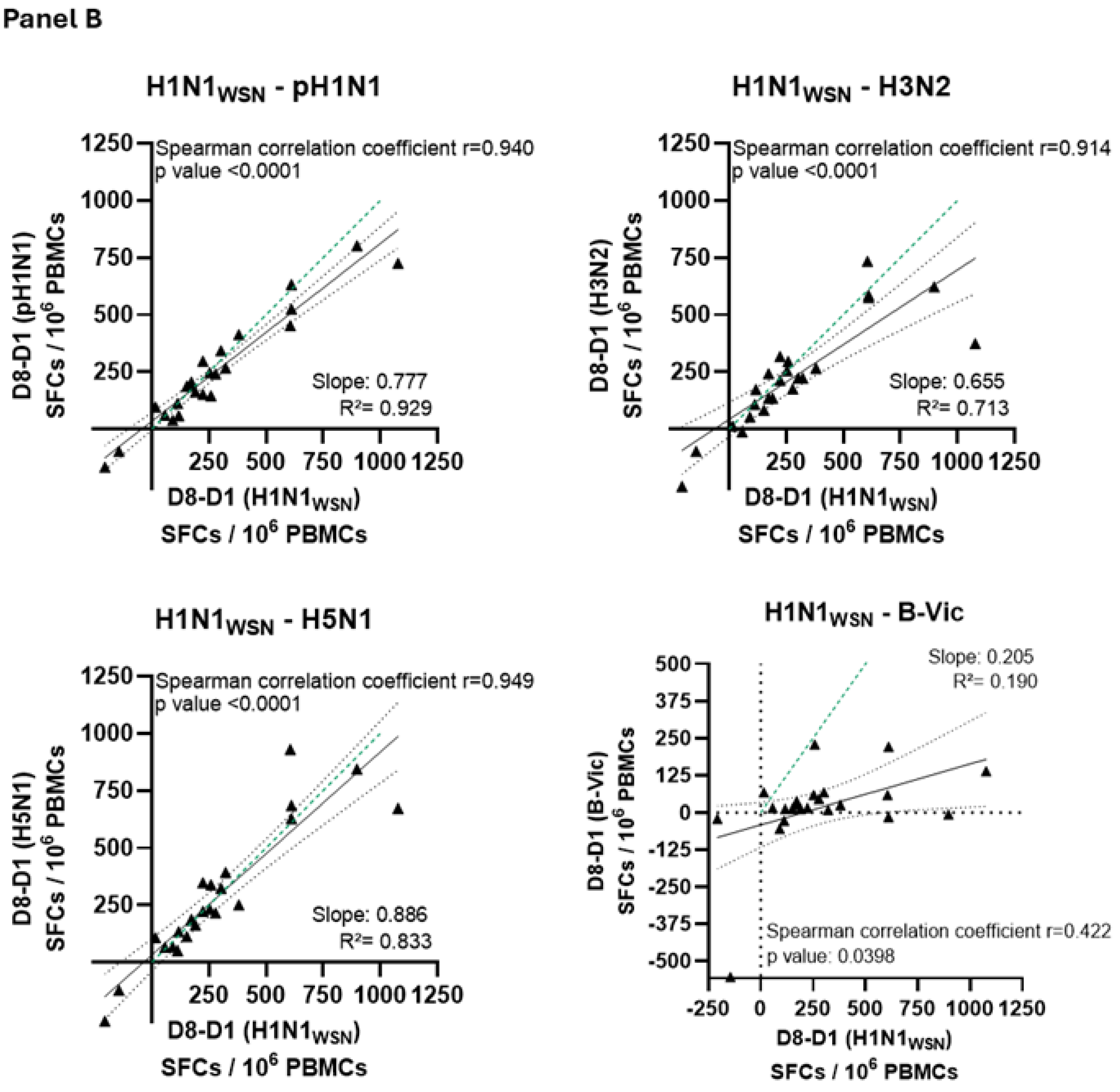
3.5. Cross-Reactive Immunity
3.6. Parameters Influencing the Response to OVX836 Vaccination
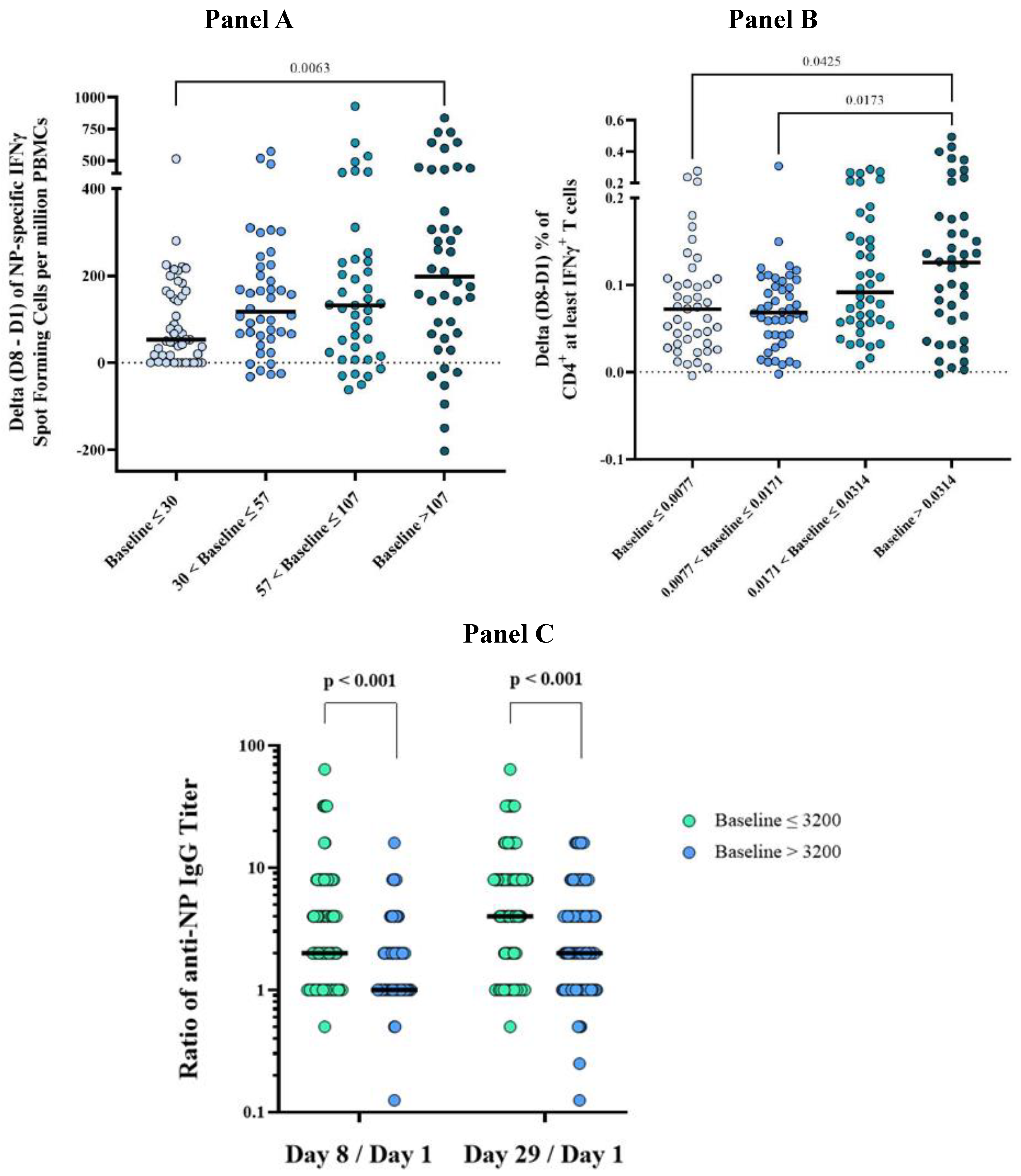
3.6.1. Influence of Pre-Vaccination Immunological Values on the Immune Response to OVX836
3.6.2. Effect of OVX836 Dose Levels, Sex and Age on the Immune Response to OVX836
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of Global Seasonal Influenza-Associated Respiratory Mortality: A Modelling Study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; McMichael, A.J.; LeVert, A.; McCauley, J.W.; Almond, J.W. Opportunities and Challenges for T Cell-Based Influenza Vaccines. Nat. Rev. Immunol. 2024, 24, 736–752. [Google Scholar] [CrossRef] [PubMed]
- Lafond, K.E.; Porter, R.M.; Whaley, M.J.; Suizan, Z.; Ran, Z.; Aleem, M.A.; Thapa, B.; Sar, B.; Proschle, V.S.; Peng, Z.; et al. Global Burden of Influenza-Associated Lower Respiratory Tract Infections and Hospitalizations among Adults: A Systematic Review and Meta-Analysis. PLoS Med. 2021, 18, e1003550. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Kissling, E.; Emborg, H.-D.; Larrauri, A.; McMenamin, J.; Pozo, F.; Trebbien, R.; Mazagatos, C.; Whitaker, H.; Valenciano, M.; et al. Interim 2019/20 Influenza Vaccine Effectiveness: Six European Studies, September 2019 to January 2020. Eurosurveillance 2020, 25, 2000153. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cowling, B.J.; Kelly, H.; Sullivan, S.G. Estimating Influenza Vaccine Effectiveness with the Test-Negative Design Using Alternative Control Groups: A Systematic Review and Meta-Analysis. Am. J. Epidemiol. 2018, 187, 389–397. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Kuchel, G.A.; Zhou, X.; Swain, S.L.; Haynes, L. T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines. Front. Immunol. 2016, 7, 41. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Torelli, A.; Montomoli, E. The Use of Cell-Mediated Immunity for the Evaluation of Influenza Vaccines: An Upcoming Necessity. Hum. Vaccines Immunother. 2019, 15, 1021–1030. [Google Scholar] [CrossRef]
- Janssens, Y.; Joye, J.; Waerlop, G.; Clement, F.; Leroux-Roels, G.; Leroux-Roels, I. The Role of Cell-Mediated Immunity against Influenza and Its Implications for Vaccine Evaluation. Front. Immunol. 2022, 13, 959379. [Google Scholar] [CrossRef]
- Wilkinson, T.M.; Li, C.K.F.; Chui, C.S.C.; Huang, A.K.Y.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting Influenza-Specific CD4+ T Cells Correlate with Disease Protection against Influenza Challenge in Humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef]
- Hayward, A.C.; Wang, L.; Goonetilleke, N.; Fragaszy, E.B.; Bermingham, A.; Copas, A.; Dukes, O.; Millett, E.R.; Nazareth, I.; Nguyen-Van-Tam, J.S.; et al. Natural T Cell-Mediated Protection against Seasonal and Pandemic Influenza. Results of the Flu Watch Cohort Study. Am. J. Respir. Crit. Care Med. 2015, 191, 1422–1431. [Google Scholar] [CrossRef]
- Tsang, T.K.; Lam, K.-T.; Liu, Y.; Fang, V.J.; Mu, X.; Leung, N.H.L.; Peiris, J.S.M.; Leung, G.M.; Cowling, B.J.; Tu, W. Investigation of CD4 and CD8 T Cell-Mediated Protection against Influenza A Virus in a Cohort Study. BMC Med. 2022, 20, 230. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyer, T.; Schmelz, S.; Degiacomi, M.T.; Dal, P.M.; Daneschdar, M.; Scrima, A.; van den Heuvel, J.; Heinz, D.W.; Kolmar, H. Arranged Sevenfold: Structural Insights into the C-Terminal Oligomerization Domain of Human C4b-Binding Protein. J. Mol. Biol. 2013, 425, 1302–1317. [Google Scholar] [CrossRef]
- Del Campo, J.; Pizzorno, A.; Djebali, S.; Bouley, J.; Haller, M.; Perez-Vargas, J.; Lina, B.; Boivin, G.; Hamelin, M.E.; Nicolas, F.; et al. OVX836 a Recombinant Nucleoprotein Vaccine Inducing Cellular Responses and Protective Efficacy against Multiple Influenza A Subtypes. NPJ Vaccines 2019, 4, 4. [Google Scholar] [CrossRef]
- Hu, Y.; Sneyd, H.; Dekant, R.; Wang, J. Influenza A Virus Nucleoprotein: A Highly Conserved Multi-Functional Viral Protein as a Hot Antiviral Drug Target. Curr. Top. Med. Chem. 2017, 17, 2271–2285. [Google Scholar] [CrossRef]
- Babar, M.M.; Zaidi, N.U. Protein Sequence Conservation and Stable Molecular Evolution Reveals Influenza Virus Nucleoprotein as a Universal Druggable Target. Infect. Genet. Evol. 2015, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.P.; Tan, W.S.; Mohamed Alitheen, N.B.; Yap, W.B. M2e-Based Influenza Vaccines with Nucleoprotein: A Review. Vaccines 2021, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Willems, P.; Waerlop, G.; Janssens, Y.; Tourneur, J.; De Boever, F.; Bruhwyler, J.; Alhatemi, A.; Jacobs, B.; Nicolas, F.; et al. Immunogenicity, Safety, and Preliminary Efficacy Evaluation of OVX836, a Nucleoprotein-Based Universal Influenza A Vaccine Candidate: A Randomised, Double-Blind, Placebo-Controlled, Phase 2a Trial. Lancet Infect. Dis. 2023, 23, 1360–1369. [Google Scholar] [CrossRef]
- Leroux-Roels, I.; Waerlop, G.; Tourneur, J.; De Boever, F.; Maes, C.; Bruhwyler, J.; Guyon-Gellin, D.; Moris, P.; Del Campo, J.; Willems, P.; et al. Randomized, Double-Blind, Reference-Controlled, Phase 2a Study Evaluating the Immunogenicity and Safety of OVX836, A Nucleoprotein-Based Influenza Vaccine. Front. Immunol. 2022, 13, 852904. [Google Scholar] [CrossRef]
- Jang, Y.H.; Seong, B.L. The Quest for a Truly Universal Influenza Vaccine. Front. Cell. Infect. Microbiol. 2019, 9, 344. [Google Scholar] [CrossRef]
- Lin, X.; Lin, F.; Liang, T.; Ducatez, M.F.; Zanin, M.; Wong, S.-S. Antibody Responsiveness to Influenza: What Drives It? Viruses 2021, 13, 1400. [Google Scholar] [CrossRef]
- Ng, T.W.Y.; Perera, R.A.P.M.; Fang, V.J.; Yau, E.M.; Peiris, J.S.M.; Tam, Y.H.; Cowling, B.J. The Effect of Influenza Vaccination History on Changes in Hemagglutination Inhibition Titers After Receipt of the 2015-2016 Influenza Vaccine in Older Adults in Hong Kong. J. Infect. Dis. 2020, 221, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Tadount, F.; Kiely, M.; Assi, A.; Rafferty, E.; Sadarangani, M.; MacDonald, S.E.; Quach, C. Sex Differences in the Immunogenicity and Efficacy of Seasonal Influenza Vaccines: A Meta-Analysis of Randomized Controlled Trials. Open Forum Infect. Dis. 2024, 11, ofae222. [Google Scholar] [CrossRef] [PubMed]
- Denly, L. The Effect of Sex on Responses to Influenza Vaccines. Hum. Vaccines Immunother. 2021, 17, 1396–1402. [Google Scholar] [CrossRef]
- Klein, S.L.; Hodgson, A.; Robinson, D.P. Mechanisms of Sex Disparities in Influenza Pathogenesis. J. Leukoc. Biol. 2012, 92, 67–73. [Google Scholar] [CrossRef]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-Based Differences in Immune Function and Responses to Vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef]
- Ruggieri, A.; Anticoli, S.; D’Ambrosio, A.; Giordani, L.; Viora, M. The Influence of Sex and Gender on Immunity, Infection and Vaccination. Ann. Dell’istituto Super. Sanita 2016, 52, 198–204. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R. Insights into Vaccines for Elderly Individuals: From the Impacts of Immunosenescence to Delivery Strategies. NPJ Vaccines 2024, 9, 77. [Google Scholar] [CrossRef]
- Prelog, M. Differential Approaches for Vaccination from Childhood to Old Age. Gerontology 2013, 59, 230–239. [Google Scholar] [CrossRef]
- Grubeck-Loebenstein, B. Fading Immune Protection in Old Age: Vaccination in the Elderly. J. Comp. Pathol. 2010, 142 (Suppl. 1), S116–S119. [Google Scholar] [CrossRef]
- Wagner, A.; Garner-Spitzer, E.; Jasinska, J.; Kollaritsch, H.; Stiasny, K.; Kundi, M.; Wiedermann, U. Age-Related Differences in Humoral and Cellular Immune Responses after Primary Immunisation: Indications for Stratified Vaccination Schedules. Sci. Rep. 2018, 8, 9825. [Google Scholar] [CrossRef]
- Gustafson, C.E.; Weyand, C.M.; Goronzy, J.J. T Follicular Helper Cell Development and Functionality in Immune Ageing. Clin. Sci. 2018, 132, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Clave, E.; Wanke, K.; von Braun, A.; Bondet, V.; Alanio, C.; Douay, C.; Baque, M.; Lependu, C.; Marconi, P.; et al. Primary Immune Responses Are Negatively Impacted by Persistent Herpesvirus Infections in Older People: Results from an Observational Study on Healthy Subjects and a Vaccination Trial on Subjects Aged More than 70 Years Old. EBioMedicine 2022, 76, 103852. [Google Scholar] [CrossRef]
- McMichael, A.J.; Gotch, F.M.; Noble, G.R.; Beare, P.A. Cytotoxic T-Cell Immunity to Influenza. N. Engl. J. Med. 1983, 309, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.; Kar, S.; Ung, S.K.; Gardener, Z.; Bergstrom, E.; Ascough, S.; Kalyan, M.; Zyla, J.; Maertzdorf, J.; Mollenkopf, H.-J.; et al. Innate-like Gene Expression of Lung-Resident Memory CD8+ T Cells during Experimental Human Influenza: A Clinical Study. Am. J. Respir. Crit. Care Med. 2021, 204, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Von Holle, T.A.; Moody, M.A. Influenza and Antibody-Dependent Cellular Cytotoxicity. Front. Immunol. 2019, 10, 1457. [Google Scholar] [CrossRef]
- Jegaskanda, S.; Co, M.D.T.; Cruz, J.; Subbarao, K.; Ennis, F.A.; Terajima, M. Induction of H7N9-Cross-Reactive Antibody-Dependent Cellular Cytotoxicity Antibodies by Human Seasonal Influenza A Viruses That Are Directed Toward the Nucleoprotein. J. Infect. Dis. 2017, 215, 818–823. [Google Scholar] [CrossRef]
- Vanderven, H.A.; Ana-Sosa-Batiz, F.; Jegaskanda, S.; Rockman, S.; Laurie, K.; Barr, I.; Chen, W.; Wines, B.; Hogarth, P.M.; Lambe, T.; et al. What Lies Beneath: Antibody Dependent Natural Killer Cell Activation by Antibodies to Internal Influenza Virus Proteins. EBioMedicine 2016, 8, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-U.; Jeong, Y.-J.; Lee, P.; Lee, M.-S.; Park, J.-H.; Kim, Y.-S.; Kim, D.-J. Extracellular Nucleoprotein Exacerbates Influenza Virus Pathogenesis by Activating Toll-like Receptor 4 and the NLRP3 Inflammasome. Cell. Mol. Immunol. 2022, 19, 715–725. [Google Scholar] [CrossRef]
- Caddy, S.L.; Vaysburd, M.; Papa, G.; Wing, M.; O’Connell, K.; Stoycheva, D.; Foss, S.; Terje Andersen, J.; Oxenius, A.; James, L.C. Viral Nucleoprotein Antibodies Activate TRIM21 and Induce T Cell Immunity. EMBO J. 2021, 40, e106228. [Google Scholar] [CrossRef]
- López-Muñoz, A.D.; Yewdell, J.W. Cell Surface RNA Virus Nucleocapsid Proteins: A Viral Strategy for Immunosuppression? NPJ Viruses 2024, 2, 41. [Google Scholar] [CrossRef]
- Del Campo, J.; Bouley, J.; Chevandier, M.; Rousset, C.; Haller, M.; Indalecio, A.; Guyon-Gellin, D.; Le Vert, A.; Hill, F.; Djebali, S.; et al. OVX836 Heptameric Nucleoprotein Vaccine Generates Lung Tissue-Resident Memory CD8+ T-Cells for Cross-Protection against Influenza. Front. Immunol. 2021, 12, 678483. [Google Scholar] [CrossRef] [PubMed]
- Lamere, M.W.; Lam, H.T.; Moquin, A.; Haynes, L.; Lund, F.E.; Randall, T.D.; Kaminski, D.A. Contributions of Antinucleoprotein IgG to Heterosubtypic Immunity against Influenza Virus. J. Immunol. 2011, 186, 4331–4339. [Google Scholar] [CrossRef] [PubMed]
- Carragher, D.M.; Kaminski, D.A.; Moquin, A.; Hartson, L.; Randall, T.D. A Novel Role for Non-Neutralizing Antibodies against Nucleoprotein in Facilitating Resistance to Influenza Virus. J. Immunol. 2008, 181, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- Moris, P.; van der Most, R.; Leroux-Roels, I.; Clement, F.; Dramé, M.; Hanon, E.; Leroux-Roels, G.G.; Van Mechelen, M. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J. Clin. Immunol. 2011, 31, 443–454. [Google Scholar] [CrossRef]
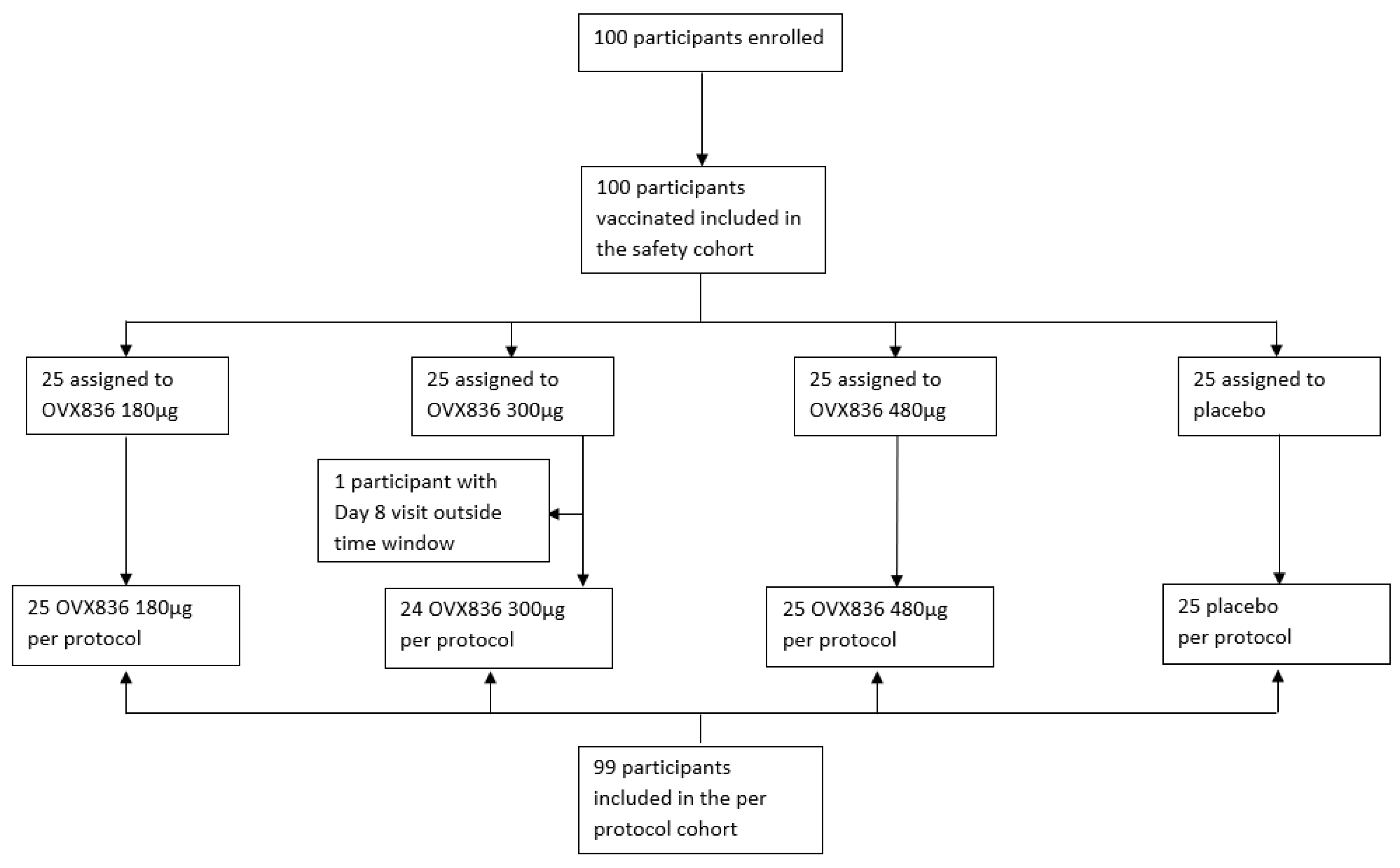


| Placebo | OVX836 180 µg | OVX836 300 µg | OVX836 480 µg | All Participants | |
|---|---|---|---|---|---|
| N = 25 | N = 25 | N = 25 | N = 25 | N = 100 | |
| Age (year) | 70.9 ± 5.6 | 70.4 ± 4.6 | 71.0 ± 4.1 | 71.8 ± 5.8 | 71.0 ± 5.0 |
| Weight (kg) | 75.9 ± 15.1 | 73.3 ± 13.7 | 75.2 ± 14.0 | 73.4 ± 13.5 | 74.4 ± 13.9 |
| Height (cm) | 172 ± 9 | 171 ± 8 | 167 ± 9 | 167 ± 8 | 169 ± 9 |
| BMI (kg/m2) | 25.3 ± 3.2 | 25.0 ± 2.9 | 26.9 ± 3.3 | 26.3 ± 3.5 | 25.9 ± 3.3 |
| Sex (female) | 10 (40.0) | 12 (48.0) | 14 (56.0) | 13 (52.0) | 49 (49.0) |
| Race (White) | 25 (100.0) | 25 (100.0) | 25 (100.0) | 25 (100.0) | 100 (100.0) |
| Smoking status | |||||
| Non-smoker | 9 (36.0) | 16 (64.0) | 16 (64.0) | 14 (56.0) | 55 (55.0) |
| Current smoker | 2 (8.0) | 3 (12.0) | 2 (8.0) | 1 (4.0) | 8 (8.0) |
| Former smoker | 14 (56.0) | 6 (24.0) | 7 (28.0) | 10 (40.0) | 37 (37.0) |
| Influenza vaccination (season 2021–2022) | 20 (80.0) | 19 (76.0) | 21 (84.0) | 20 (80.0) | 80 (80.0) |
| Intensity | OVX836 180 µg (N = 25) | OVX836 300 µg (N = 25) | OVX836 480 µg (N = 25) | Placebo (N = 25) | |
|---|---|---|---|---|---|
| Local symptoms | |||||
| Pain | All | 10 (40.0) | 6 (24.0) | 15 (60.0) | 1 (4.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Redness | All | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Swelling | All | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Systemic symptoms | |||||
| Arthralgia | All | 2 (8.0) | 2 (8.0) | 0 (0.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Fatigue | All | 7 (28.0) | 3 (12.0) | 6 (24.0) | 4 (16.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Fever | All | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Headache | All | 4 (16.0) | 3 (12.0) | 2 (8.0) | 5 (20.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Malaise | All | 2 (8.0) | 4 (16.0) | 0 (0.0) | 1 (4.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Myalgia | All | 2 (8.0) | 2 (8.0) | 1 (4.0) | 2 (8.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Unsolicited adverse events | |||||
| All | 7 (28.0) | 6 (24.0) | 11 (44.0) | 11 (44.0) | |
| Severe | 1 (4.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | |
| Related to the IP | 0 (0.0) | 1 (4.0) | 2 (8.0) | 0 (0.0) | |
| Related to the IP and severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Serious adverse events | |||||
| All | 3 (12.0) | 1 (4.0) | 1 (4.0) | 0 (0.0) | |
| Related to the IP | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, B.; Leroux-Roels, I.; Bruhwyler, J.; Groth, N.; Waerlop, G.; Janssens, Y.; Tourneur, J.; De Boever, F.; Alhatemi, A.; Moris, P.; et al. Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults. Vaccines 2024, 12, 1391. https://doi.org/10.3390/vaccines12121391
Jacobs B, Leroux-Roels I, Bruhwyler J, Groth N, Waerlop G, Janssens Y, Tourneur J, De Boever F, Alhatemi A, Moris P, et al. Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults. Vaccines. 2024; 12(12):1391. https://doi.org/10.3390/vaccines12121391
Chicago/Turabian StyleJacobs, Bart, Isabel Leroux-Roels, Jacques Bruhwyler, Nicola Groth, Gwenn Waerlop, Yorick Janssens, Jessika Tourneur, Fien De Boever, Azhar Alhatemi, Philippe Moris, and et al. 2024. "Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults" Vaccines 12, no. 12: 1391. https://doi.org/10.3390/vaccines12121391
APA StyleJacobs, B., Leroux-Roels, I., Bruhwyler, J., Groth, N., Waerlop, G., Janssens, Y., Tourneur, J., De Boever, F., Alhatemi, A., Moris, P., Le Vert, A., Leroux-Roels, G., & Nicolas, F. (2024). Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults. Vaccines, 12(12), 1391. https://doi.org/10.3390/vaccines12121391






