Protein Expression Platforms and the Challenges of Viral Antigen Production
Abstract
:1. Introduction
2. Bacterial Expression Systems
Applications of Bacterial Expression Systems
3. Insect Cell (Baculovirus) Expression Systems
Applications of Baculovirus Expression System
4. Mammalian Expression Systems
Applications of Mammalian Expression System
5. Yeast Expression Systems
Applications of Yeast Expression System
6. Algal Expression Systems
Applications of Algal Expression System
7. Plant Expression Systems
Applications of Plant Expression System
8. Cell-Free Expression Systems
| System | Advantages | Disadvantages | Reference |
|---|---|---|---|
| E. coli | High protein yield. Simple cultivation, rapid cell growth and easy lysate preparation. Cost-efficient. Well-established genetic engineering methods. High levels of VLP production. | Post-translational modification issues. Lack of endogenous membrane structures for integral membrane protein synthesis. Only prokaryotic chaperones. Eukaryotic proteins often incorrectly folded. | [306,307,329,330,331,332,336,337,338,339] |
| Yeast | Post-translational modifications possible. Rapid, easy propagation of cells and lysate preparation. Well-established genetic engineering methods. | Low protein yield. No mammalian-like post-translational modifications. Slightly more costly than E. coli. Moderate levels of VLP production. | [328,333,335,340,341,342] |
| Wheat germ | High yield of complex proteins. Synthesis of disulphide bridged proteins. Correct protein folding and high solubility. Well-established genetic engineering methods. | Labour-intensive and expensive lysate preparation. Limited post-translational modifications. No endogenous membrane structures. Low protein yield compared to prokaryotic and cell-based wheat germ systems. | [319,334,343] |
| Tobacco | High yield of complex proteins. Rapid, simple lysate preparation. Glycosylation and disulphide bridge formation possible. | Few studies on tobacco CFSs | [320,325,344] |
| Insect cell | Rapid, simple lysate preparation. Post-translational modifications possible. Endogenous microsomes available. Direct synthesis and integration of membrane proteins. | High cost of cell propagation. Moderate levels of VLP production. | [299,345,346] |
| CHO cell | Contains endogenous microsomes. Mammalian PTMs. Direct production of membrane proteins. Well-established cell lines. IRES-mediated translation initiation allows high protein yield. | Low yield compared to prokaryotic CFSs. High cost of cell propagation. Low levels of VLP production. | [347,348] |
| Human cell | Optimal environment for native protein folding and assembly of viral membrane proteins. Contain endogenous microsomes. Human post-translational modifications. Codon manipulation allows the synthesis of high molecular weight proteins. | Low protein yield compared to prokaryotic CFS. High cell propagation costs. Labour intensive cell culture technologies needed as human cells are sensitive. | [323,349,350] |
Applications of Cell-Free Expression System
9. Future Directions
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chadd, H.E.; Chamow, S.M. Therapeutic antibody expression technology. Curr. Opin. Biotechnol. 2001, 12, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.C.; Krummen, L. Recombinant protein expression for therapeutic applications. Curr. Opin. Biotechnol. 2002, 13, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P. Hepatitis B Vaccines BT—Pediatric Vaccines and Vaccinations: A European Textbook; Vesikari, T., Van Damme, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 109–116. [Google Scholar]
- McNeil, C. Who invented the VLP cervical cancer vaccines? J. Natl. Cancer Inst. 2006, 98, 433. [Google Scholar] [CrossRef] [PubMed]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.T.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Crisci, E.; Bárcena, J.; Montoya, M. Virus-like particles: The new frontier of vaccines for animal viral infections. Vet. Immunol. Immunopathol. 2012, 148, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Teffera, M.; Babiuk, S. Potential of Using Capripoxvirus Vectored Vaccines Against Arboviruses in Sheep, Goats, and Cattle. Front. Vet. Sci. 2019, 6, 450. Available online: https://www.frontiersin.org/article/10.3389/fvets.2019.00450 (accessed on 1 June 2024). [CrossRef]
- Singh, A. Modern Vaccines with DIVA Capability: An Overview. Res. Rev. A J. Immunol. 2019, 5, 195–205. [Google Scholar]
- Chu, L.; Robinson, D.K. Industrial choices for protein production by large-scale cell culture. Curr. Opin. Biotechnol. 2001, 12, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.R.; Fauci, A.S. Novel vaccine technologies for the 21st century. Nat. Rev. Immunol. 2020, 20, 87–88. [Google Scholar] [CrossRef]
- Chan, C.Y.-Y.; Tambyah, P.A. Preflucel®: A Vero-cell culture-derived trivalent influenza vaccine. Expert Rev. Vaccines 2012, 11, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Manini, I.; Domnich, A.; Amicizia, D.; Rossi, S. Flucelvax (Optaflu) for seasonal influenza. Expert Rev. Vaccines 2015, 14, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Yoon, I.-K. A review of Dengvaxia®: Development to deployment. Hum. Vaccines Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Andréll, J.; Tate, C.G. Overexpression of membrane proteins in mammalian cells for structural studies. Mol. Membr. Biol. 2013, 30, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Shukla, P. Glycosylation control technologies for recombinant therapeutic proteins. Appl. Microbiol. Biotechnol. 2018, 102, 10457–10468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liang, L.; Wang, S.; Nakao, T.; Li, Y.; Liu, L.; Guan, Y.; Fukuyama, S.; Bu, Z.; Kawaoka, Y.; et al. Glycosylation of the Hemagglutinin Protein of H5N1 Influenza Virus Increases Its Virulence in Mice by Exacerbating the Host Immune Response. J. Virol. 2017, 91, e02215-16. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.C.; Barton, W.A. Over-expression of secreted proteins from mammalian cell lines. Protein Sci. 2014, 23, 517–525. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H. Essentials of Glycobiology [Internet]. 2015. Available online: https://pubmed.ncbi.nlm.nih.gov/27010055/ (accessed on 1 June 2024).
- Qian, H.; Wu, Y.; Bian, G.; Zhang, Q.; Xu, Y.; Bai, Z.; Wu, S.; Che, Y.; Lv, Y.; Zha, Y. Porcine Epidemic Diarrhea Virus S Protein and Subunit Vaccine Thereof as Well as Method for Preparing Subunit Vaccine and Application Thereof. U.S. Patent App. 16/600,334, 18 June 2020. [Google Scholar]
- Fattom, A.I.; Hamouda, T.; Bitko, V.; Baker, J.R., Jr. Nanoemulsion Respiratory Syncytial Virus (RSV) Subunit Vaccine. U.S. Patent 10,596,251, 24 March 2020. [Google Scholar]
- Eldemery, F.; Joiner, K.S.; Toro, H.; Van Santen, V.L. Protection against infectious bronchitis virus by spike ectodomain subunit vaccine. Vaccine 2017, 35, 5864–5871. [Google Scholar] [CrossRef]
- Li, D.; von Schaewen, M.; Wang, X.; Tao, W.; Zhang, Y.; Li, L.; Heller, B.; Hrebikova, G.; Deng, Q.; Ploss, A. Altered glycosylation patterns increase immunogenicity of a subunit hepatitis C virus vaccine, inducing neutralizing antibodies which confer protection in mice. J. Virol. 2016, 90, 10486–10498. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, A.T.; Wong, T.-A.S.; Lieberman, M.M.; Johns, L.; Medina, L.; Feldmann, F.; Feldmann, H.; Marzi, A. Recombinant subunit vaccines protect guinea pigs from lethal Ebola virus challenge. Vaccine 2019, 37, 6942–6950. [Google Scholar] [CrossRef] [PubMed]
- Morcol, T.; Nagappan, P.; Bell, S.J.D.; Cawthon, A.G. Influenza A (H5N1) virus subunit vaccine administered with CaPNP adjuvant induce high virus neutralization antibody titers in mice. AAPS PharmSciTech 2019, 20, 315. [Google Scholar] [CrossRef]
- Medina, L.O.; To, A.; Lieberman, M.M.; Wong, T.A.S.; Namekar, M.; Nakano, E.; Andersen, H.; Yalley-Ogunro, J.; Greenhouse, J.; Higgs, S. A recombinant subunit based zika virus vaccine is efficacious in non-human primates. Front. Immunol. 2018, 9, 2464. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.P.; Pierce, K.K.; Kirkpatrick, B.D.; Grier, P.; Sabundayo, B.P.; He, H.; Sausser, M.; Russell, A.F.; Martin, J.; Hyatt, D. Immunogenicity and Safety of a Tetravalent Recombinant Subunit Dengue Vaccine in Adults Previously Vaccinated with a Live Attenuated Tetravalent Dengue Vaccine: Results of a Phase-I Randomized Clinical Trial. Am. J. Trop. Med. Hyg. 2020, 103, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Bourgeois, L.; Carlin, N.; Clements, J.; Gustafsson, B.; Lundgren, A.; Nygren, E.; Tobias, J.; Walker, R.; Svennerholm, A.-M. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered al. Vaccine 2013, 31, 2457–2464. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Zhang, J.; Xia, N.; Zhao, Q. Escherichia coli-derived virus-like particles in vaccine development. npj Vaccines 2017, 2, 3. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, L.; Li, S.; Fang, M.; Zhang, J.; Xia, N.; Zhao, Q. Lessons learned from successful human vaccines: Delineating key epitopes by dissecting the capsid proteins. Hum. Vaccines Immunother. 2015, 11, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-W.; Zhao, Q.; Wu, T.; Chen, S.; Zhang, J.; Xia, N.-S. The development of a recombinant hepatitis E vaccine HEV 239. Hum. Vaccines Immunother. 2015, 11, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Babiuk, S.; Asper, D.J.; Rogan, D.; Mutwiri, G.K.; Potter, A.A. Subcutaneous and intranasal immunization with type III secreted proteins can prevent colonization and shedding of Escherichia coli O157:H7 in mice. Microb. Pathog. 2008, 45, 7–11. [Google Scholar] [CrossRef]
- Lobstein, J.; Emrich, C.A.; Jeans, C.; Faulkner, M.; Riggs, P.; Berkmen, M. SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb. Cell Fact. 2012, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Dopp, J.L.; Reuel, N.F. Simple, functional, inexpensive cell extract for in vitro prototyping of proteins with disulfide bonds. Biochem. Eng. J. 2020, 164, 107790. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2014.00172 (accessed on 1 June 2024). [CrossRef]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Mamat, U.; Wilke, K.; Bramhill, D.; Schromm, A.B.; Lindner, B.; Kohl, T.A.; Corchero, J.L.; Villaverde, A.; Schaffer, L.; Head, S.R.; et al. Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb. Cell Fact. 2015, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Mamat, U.; Woodard, R.W.; Wilke, K.; Souvignier, C.; Mead, D.; Steinmetz, E.; Terry, K.; Kovacich, C.; Zegers, A.; Knox, C. Endotoxin-Free Protein Production—ClearColiTM Technology; Nature Publishing Group: New York, NY, USA, 2013. [Google Scholar]
- Schwarz, H.; Schmittner, M.; Duschl, A.; Horejs-Hoeck, J. Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells. PLoS ONE 2014, 9, e113840. [Google Scholar] [CrossRef]
- Palmer, I.; Wingfield, P.T. Preparation and extraction of insoluble (inclusion-body) proteins from Escherichia coli. Curr. Protoc. Protein Sci. 2012, 70, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, L.; Martín, L.; Mangues, R.; Ferrer-Miralles, N.; Vázquez, E.; Villaverde, A. Recombinant pharmaceuticals from microbial cells: A 2015 update. Microb. Cell Fact. 2016, 15, 33. [Google Scholar] [CrossRef]
- Kyriakopoulos, S.; Kontoravdi, C. Analysis of the landscape of biologically-derived pharmaceuticals in Europe: Dominant production systems, molecule types on the rise and approval trends. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Proffitt, A. First HEV vaccine approved. Nat. Biotechnol. 2012, 30, 300. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, M.; Pan, H.; Lin, Z.; Wang, K.; Weng, Z.; Zhu, Y.; Xin, L.; Zhang, J.; Li, S.; et al. Robust manufacturing and comprehensive characterization of recombinant hepatitis E virus-like particles in Hecolin(®). Vaccine 2014, 32, 4039–4050. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-C.; Sun, S.-Q.; Jin, Y.; Yang, S.-L.; Wei, Y.-Q.; Sun, D.-H.; Yin, S.-H.; Ma, J.-W.; Liu, Z.-X.; Guo, J.-H.; et al. Foot-and-mouth disease virus-like particles produced by a SUMO fusion protein system in Escherichia coli induce potent protective immune responses in guinea pigs, swine and cattle. Vet. Res. 2013, 44, 48. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, H.-Y.; Wang, Y.; Yin, B.; Lv, C.; Mo, X.; Yan, H.; Xuan, Y.; Huang, Y.; Pang, W.; et al. Large-scale production of foot-and-mouth disease virus (serotype Asia1) VLP vaccine in Escherichia coli and protection potency evaluation in cattle. BMC Biotechnol. 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, R.; Song, H.; Pan, S.; Zhang, Y.; Dong, H.; Bai, M.; Sun, S.; Guo, H.; Yin, S. Local and systemic immune responses induced by intranasal immunization with biomineralized foot-and-mouth disease virus-like particles. Front. Microbiol. 2023, 14, 1112641. [Google Scholar] [CrossRef]
- Chathuranga, W.A.G.; Hewawaduge, C.; Nethmini, N.A.N.; Kim, T.-H.; Kim, J.H.; Ahn, Y.-H.; Yoon, I.-J.; Yoo, S.-S.; Park, J.-H.; Lee, J.-S. Efficacy of a Novel Multiepitope Vaccine Candidate against Foot-and-Mouth Disease Virus Serotype O and A. Vaccines 2022, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, N.; Hughes, F.K.; Fairmaid, E.J.; Lua, L.H.L.; Brown, L.E.; Middelberg, A.P.J. Protective efficacy of a bacterially produced modular capsomere presenting M2e from influenza: Extending the potential of broadly cross-protecting epitopes. Vaccine 2014, 32, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Cho, K.J.; Fiers, W.; Saelens, X. M2e-based universal influenza a vaccines. Vaccines 2015, 3, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Watkins, H.C.; Pagan, C.L.; Childs, H.R.; Posada, S.; Chau, A.; Rios, J.; Guarino, C.; DeLisa, M.P.; Whittaker, G.R.; Putnam, D. A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine 2017, 35, 5373–5380. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, X.; Chang, C.; Hua, T.; Wang, J.; Tang, B.; Zhang, D. Reduction of Postweaning Multisystemic Wasting Syndrome-Associated Clinical Symptoms by Virus-Like Particle Vaccine Against Porcine Parvovirus and Porcine Circovirus Type 2. Viral Immunol. 2020, 33, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Sun, P.; Wang, T.; Mi, T.; Xu, H.; Wu, J.; Liu, B. Non-glycosylated SARS-CoV-2 RBD elicited a robust neutralizing antibody response in mice. J. Immunol. Methods 2022, 506, 113279. [Google Scholar] [CrossRef]
- Liu, X.; Chang, X.; Rothen, D.; Derveni, M.; Krenger, P.; Roongta, S.; Wright, E.; Vogel, M.; Tars, K.; Mohsen, M.O. AP205 VLPs based on dimerized capsid proteins accommodate RBM domain of SARS-CoV-2 and serve as an attractive vaccine candidate. Vaccines 2021, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kim, J.H.; Lee, J.; Ka, S.Y.; Chae, H.D.; Jung, I.; Jung, S.T.; Na, J.-H. Functional expression of the recombinant spike receptor binding domain of SARS-CoV-2 Omicron in the periplasm of Escherichia coli. Bioengineering 2022, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- McGuire, B.E.; Mela, J.E.; Thompson, V.C.; Cucksey, L.R.; Stevens, C.E.; McWhinnie, R.L.; Winkler, D.F.H.; Pelech, S.; Nano, F.E. Escherichia coli recombinant expression of SARS-CoV-2 protein fragments. Microb. Cell Fact. 2022, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, G.A.; Komarov, A.; Kaznadzey, A.; Mazo, I.; Kireeva, M.L. Expression of SARS-CoV-2 surface glycoprotein fragment 319–640 in E. coli, and its refolding and purification. Protein Expr. Purif. 2021, 183, 105861. [Google Scholar] [CrossRef]
- He, Y.; Qi, J.; Xiao, L.; Shen, L.; Yu, W.; Hu, T. Purification and characterization of the receptor-binding domain of SARS-CoV-2 spike protein from Escherichia coli. Eng. Life Sci. 2021, 21, 453–460. [Google Scholar] [CrossRef]
- Clem, R.J.; Passarelli, A.L. Baculoviruses: Sophisticated pathogens of insects. PLoS Pathog. 2013, 9, e1003729. [Google Scholar] [CrossRef] [PubMed]
- Pennock, G.D.; Shoemaker, C.; Miller, L.K. Strong and regulated expression of Escherichia coli beta-galactosidase in insect cells with a baculovirus vector. Mol. Cell. Biol. 1984, 4, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.E.; Summers, M.D.; Fraser, M.J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983, 3, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Hitchman, R.B.; Possee, R.D.; King, L.A. High-throughput baculovirus expression in insect cells. Methods Mol. Biol. 2012, 824, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Hitchman, R.B.; Locanto, E.; Possee, R.D.; King, L.A. Optimizing the baculovirus expression vector system. Methods 2011, 55, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kitts, P.A.; Ayres, M.D.; Possee, R.D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990, 18, 5667–5672. [Google Scholar] [CrossRef] [PubMed]
- Kitts, P.A.; Possee, R.D. A method for producing recombinant baculovirus expression vectors at high frequency. Biotechniques 1993, 14, 810–817. [Google Scholar]
- Luckow, V.A.; Lee, S.C.; Barry, G.F.; Olins, P.O. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993, 67, 4566–4579. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Poterszman, A. Baculovirus expression: Old dog, new tricks. Bioengineered 2015, 6, 316–322. [Google Scholar] [CrossRef]
- Shi, X.; Jarvis, D.L. Protein N-glycosylation in the baculovirus-insect cell system. Curr. Drug Targets 2007, 8, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, T.; Xue, W.; Zhang, S.; Cui, L.; Wang, H.; Zhang, Y.; Zhou, L.; Gu, Y.; Xia, N. Genetic engineering of baculovirus-insect cell system to improve protein production. Front. Bioeng. Biotechnol. 2022, 10, 994743. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.; Mabashi-Asazuma, H.; Jarvis, D.L. An overview and history of glyco-engineering in insect expression systems. Glyco-Eng. Methods Protoc. 2015, 1321, 131–152. [Google Scholar]
- Palomares, L.A.; Srivastava, I.K.; Ramírez, O.T.; Cox, M.M.J. Glycobiotechnology of the insect cell-baculovirus expression system technology. In Advances in Glycobiotechnology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 71–92. [Google Scholar]
- Palmberger, D.; Ashjaei, K.; Strell, S.; Hoffmann-Sommergruber, K.; Grabherr, R. Minimizing fucosylation in insect cell-derived glycoproteins reduces binding to IgE antibodies from the sera of patients with allergy. Biotechnol. J. 2014, 9, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.L. Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology 2003, 310, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Peleg, Y. Recombinant protein expression in the baculovirus-infected insect cell system. In Chemical Genomics and Proteomics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 187–199. [Google Scholar]
- Rendić, D.; Wilson, I.B.H.; Paschinger, K. The glycosylation capacity of insect cells. Croat. Chem. Acta 2008, 81, 7–21. [Google Scholar]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of recombinant antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Futatsumori-Sugai, M.; Tsumoto, K. Signal peptide design for improving recombinant protein secretion in the baculovirus expression vector system. Biochem. Biophys. Res. Commun. 2010, 391, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Soejima, Y.; Lee, J.M.; Nagata, Y.; Mon, H.; Iiyama, K.; Kitano, H.; Matsuyama, M.; Kusakabe, T. Comparison of signal peptides for efficient protein secretion in the baculovirus-silkworm system. Cent. Eur. J. Biol. 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Mori, K.; Hamada, H.; Ogawa, T.; Ohmuro-Matsuyama, Y.; Katsuda, T.; Yamaji, H. Efficient production of antibody Fab fragment by transient gene expression in insect cells. J. Biosci. Bioeng. 2017, 124, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, M.; Schürig, M.; Endres, M.; Samuels, A.; Gebauer, D.; Konisch, N.; van den Heuvel, J. Identifying parameters to improve the reproducibility of transient gene expression in High Five cells. PLoS ONE 2019, 14, e0217878. [Google Scholar] [CrossRef]
- Bleckmann, M.; Schmelz, S.; Schinkowski, C.; Scrima, A.; van den Heuvel, J. Fast plasmid based protein expression analysis in insect cells using an automated SplitGFP screen. Biotechnol. Bioeng. 2016, 113, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Pitol, A.K.; Bachmann, V.; Hacker, D.L.; Baldi, L.; Wurm, F.M. A simple plasmid-based transient gene expression method using High Five cells. J. Biotechnol. 2015, 216, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Hacker, D.L.; Baldi, L.; Wurm, F.M. Virus-free transient protein production in Sf9 cells. J. Biotechnol. 2014, 171, 61–70. [Google Scholar] [CrossRef]
- Korn, J.; Schäckermann, D.; Kirmann, T.; Bertoglio, F.; Steinke, S.; Heisig, J.; Ruschig, M.; Rojas, G.; Langreder, N.; Wenzel, E.V.; et al. Baculovirus-free insect cell expression system for high yield antibody and antigen production. Sci. Rep. 2020, 10, 21393. [Google Scholar] [CrossRef]
- Ogay, I.D.; Lihoradova, O.A.; Azimova, S.S.; Abdukarimov, A.A.; Slack, J.M.; Lynn, D.E. Transfection of insect cell lines using polyethylenimine. Cytotechnology 2006, 51, 89–98. [Google Scholar]
- Puente-Massaguer, E.; Lecina, M.; Gòdia, F. Nanoscale characterization coupled to multi-parametric optimization of Hi5 cell transient gene expression. Appl. Microbiol. Biotechnol. 2018, 102, 10495–10510. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pandey, D.; Halder, A. Preventive, Diagnostic and Therapeutic Applications of Baculovirus Expression Vector System BT—Trends in Insect Molecular Biology and Biotechnology; Kumar, D., Gong, C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 163–191. [Google Scholar]
- Felberbaum, R.S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015, 10, 702–714. [Google Scholar] [CrossRef] [PubMed]
- To, A.; Lai, C.-Y.; Wong, T.A.; Namekar, M.; Lehrer, A.T. A recombinant subunit Lassa virus vaccine elicits strong antibody responses. J. Immunol. 2020, 204, 167.30. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, W.; Li, D.; Zhang, C.; Liu, Q.; Zhang, X.; Wang, X.; Dai, W.; Xu, Y.; Leng, Q.; et al. Insect cell-produced recombinant protein subunit vaccines protect against Zika virus infection. Antivir. Res. 2018, 154, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Meindl-Böhmer, A.; Loeffen, W.; Moennig, V. Assessment of classical swine fever diagnostics and vaccine performance. Int. Off. Epizoot. 2006, 25, 1025–1038. [Google Scholar]
- Monie, A.; Hung, C.-F.; Roden, R.; Wu, T.-C. Cervarix: A vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics 2008, 2, 97–105. [Google Scholar] [CrossRef]
- Cox, M.M.J.; Patriarca, P.A.; Treanor, J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respi. Viruses 2008, 2, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Sibila, M.; Pérez-Martín, E.; Nofrarías, M.; Mateu, E.; Segalés, J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef]
- Coll, T.; Villalba, D.; Maass, P. Meta-Analysis of Globally Published Results on the Efficacy of Ingelvac CircoFLEX® Vaccination. 2010. Available online: https://hdl.handle.net/11299/135564 (accessed on 1 June 2024).
- Sno, M.; Cox, E.; Holtslag, H.; Nell, T.; Pel, S.; Segers, R.; Fachinger, V.; Witvliet, M. Efficacy and safety of a new intradermal PCV2 vaccine in pigs. Trials Vaccinol. 2016, 5, 24–31. [Google Scholar] [CrossRef]
- Metz, S.W.; Gardner, J.; Geertsema, C.; Le, T.T.; Goh, L.; Vlak, J.M.; Suhrbier, A.; Pijlman, G.P. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS Negl. Trop. Dis. 2013, 7, e2124. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.W.; Martina, B.E.; van den Doel, P.; Geertsema, C.; Osterhaus, A.D.; Vlak, J.M.; Pijlman, G.P. Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits. Vaccine 2013, 31, 6092–6096. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.T.; Truong, D.A.; Ly, V.D.; Vu, H.T.; Van Hoang, T.; Nguyen, C.T.; Chu, N.T.; Nguyen, V.T.; Nguyen, D.T.; Miyazawa, K.; et al. The potential efficacy of the E2-subunit vaccine to protect pigs against different genotypes of classical swine fever virus circulating in Vietnam. Clin. Exp. Vaccine Res. 2020, 9, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wen, Z.; Carrion, R.J.; Nunneley, J.; Staples, H.; Ticer, A.; Patterson, J.L.; Compans, R.W.; Ye, L.; Yang, C. Intradermal Immunization of EBOV VLPs in Guinea Pigs Induces Broader Antibody Responses Against GP Than Intramuscular Injection. Front. Microbiol. 2020, 11, 304. [Google Scholar] [CrossRef]
- Sunwoo, S.Y.; Noronha, L.E.; Morozov, I.; Trujillo, J.D.; Kim, I.J.; Schirtzinger, E.E.; Faburay, B.; Drolet, B.S.; Urbaniak, K.; Scott Mcvey, D.; et al. Evaluation of a baculovirus-expressed VP2 subunit vaccine for the protection of white-tailed deer (Odocoileus virginianus) from epizootic hemorrhagic disease. Vaccines 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Pyo, H.-M.; Masic, A.; Woldeab, N.; Embury-Hyatt, C.; Lin, L.; Shin, Y.-K.; Song, J.-Y.; Babiuk, S.; Zhou, Y. Pandemic H1N1 influenza virus-like particles are immunogenic and provide protective immunity to pigs. Vaccine 2012, 30, 1297–1304. [Google Scholar] [CrossRef]
- Zhu, W.-Z.; Wen, Y.-C.; Lin, S.-Y.; Chen, T.-C.; Chen, H.-W. Anti-influenza protective efficacy of a H6 virus-like particle in chickens. Vaccines 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Wilson, W.C.; Gaudreault, N.N.; Davis, A.S.; Shivanna, V.; Bawa, B.; Sunwoo, S.Y.; Ma, W.; Drolet, B.S.; Morozov, I.; et al. A Recombinant Rift Valley Fever Virus Glycoprotein Subunit Vaccine Confers Full Protection against Rift Valley Fever Challenge in Sheep. Sci. Rep. 2016, 6, 27719. [Google Scholar] [CrossRef]
- Faburay, B.; Lebedev, M.; McVey, D.S.; Wilson, W.; Morozov, I.; Young, A.; Richt, J.A. A Glycoprotein Subunit Vaccine Elicits a Strong Rift Valley Fever Virus Neutralizing Antibody Response in Sheep. Vector-Borne Zoonotic Dis. 2014, 14, 746–756. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H.; Oh, H.; Park, Y.W.; Kwak, H.W.; Oh, E.Y.; Park, H.-J.; Kang, K.W.; Kim, G.; Koo, B.-S.; Hwang, E.-H.; et al. Immunization with RBD-P2 and N protects against SARS-CoV-2 in nonhuman primates. Sci. Adv. 2023, 7, eabg7156. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Castro, R.; Bhoelan, F.; Bemelman, D.; Gomes, R.A.; Costa, J.; Gomes-Alves, P.; Stegmann, T.; Amacker, M.; Alves, P.M. Insect cells for high-yield production of SARS-CoV-2 spike protein: Building a virosome-based COVID-19 vaccine candidate. Pharmaceutics 2022, 14, 854. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Xie, T.; Zhu, B.; Tan, J.; Li, X.; Luo, Y.; Li, F.; Niu, H.; Han, J.; Lv, W. Production of SARS-CoV-2 virus-like particles in insect cells. Vaccines 2021, 9, 554. [Google Scholar] [CrossRef]
- Jaron, M.; Lehky, M.; Zarà, M.; Zaydowicz, C.N.; Lak, A.; Ballmann, R.; Heine, P.A.; Wenzel, E.V.; Schneider, K.-T.; Bertoglio, F. Baculovirus-Free SARS-CoV-2 Virus-like Particle Production in Insect Cells for Rapid Neutralization Assessment. Viruses 2022, 14, 2087. [Google Scholar] [CrossRef] [PubMed]
- Struble, L.R.; Smith, A.L.; Lutz, W.E.; Grubbs, G.; Sagar, S.; Bayles, K.W.; Radhakrishnan, P.; Khurana, S.; El-Gamal, D.; Borgstahl, G.E.O. Insect cell expression and purification of recombinant SARS-COV-2 spike proteins that demonstrate ACE2 binding. Protein Sci. 2022, 31, e4300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Z.; Hu, W.; Hao, P.; Yang, S. Impact of expressing cells on glycosylation and glycan of the SARS-CoV-2 spike glycoprotein. ACS Omega 2021, 6, 15988–15999. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, Q.; Yu, H.; Wu, D.; Xue, W.; Xiong, H.; Huang, X.; Nie, M.; Yue, M.; Rong, R.; et al. SARS-CoV-2 spike produced in insect cells elicits high neutralization titres in non-human primates. Emerg. Microbes Infect. 2020, 9, 2076–2090. [Google Scholar] [CrossRef] [PubMed]
- Bandaranayake, A.D.; Almo, S.C. Recent advances in mammalian protein production. FEBS Lett. 2014, 588, 253–260. [Google Scholar] [CrossRef]
- Zhu, J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol. Adv. 2012, 30, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.; Meleady, P.; Tyther, R.; Murphy, L. Strategies for analysing and improving the expression and quality of recombinant proteins made in mammalian cells. Biotechnol. Appl. Biochem. 2009, 53, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Butler, M. Animal cell cultures: Recent achievements and perspectives in the production of biopharmaceuticals. Appl. Microbiol. Biotechnol. 2005, 68, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Wiznerowicz, M.; Trono, D. Harnessing HIV for therapy, basic research and biotechnology. Trends Biotechnol. 2005, 23, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Giry-Laterrière, M.; Verhoeyen, E.; Salmon, P. Lentiviral vectors. Methods Mol. Biol. 2011, 737, 183–209. [Google Scholar] [CrossRef]
- Ramezani, A.; Hawley, R.G. Overview of the HIV-1 Lentiviral Vector System. Curr. Protoc. Mol. Biol. 2002, 60, 16–21. [Google Scholar] [CrossRef]
- Kafri, T.; van Praag, H.; Gage, F.H.; Verma, I.M. Lentiviral Vectors: Regulated Gene Expression. Mol. Ther. 2000, 1, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Tiscornia, G.; Singer, O.; Verma, I.M. Production and purification of lentiviral vectors. Nat. Protoc. 2006, 1, 241–245. [Google Scholar] [CrossRef]
- Rueckert, C.; Guzmán, C.A. Vaccines: From empirical development to rational design. PLoS Pathog. 2012, 8, e1003001. [Google Scholar] [CrossRef] [PubMed]
- Geisse, S. Reflections on more than 10 years of TGE approaches. Protein Expr. Purif. 2009, 64, 99–107. [Google Scholar] [CrossRef]
- Liu, C.; Dalby, B.; Chen, W.; Kilzer, J.M.; Chiou, H.C. Transient transfection factors for high-level recombinant protein production in suspension cultured mammalian cells. Mol. Biotechnol. 2008, 39, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Baldi, L.; Hacker, D.L.; Adam, M.; Wurm, F.M. Recombinant protein production by large-scale transient gene expression in mammalian cells: State of the art and future perspectives. Biotechnol. Lett. 2007, 29, 677–684. [Google Scholar] [CrossRef]
- Rozkov, A.; Larsson, B.; Gillström, S.; Björnestedt, R.; Schmidt, S.R. Large-scale production of endotoxin-free plasmids for transient expression in mammalian cell culture. Biotechnol. Bioeng. 2008, 99, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Yarranton, G.T. Inducible vectors for expression in mammalian cells. Curr. Opin. Biotechnol. 1992, 3, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Fike, R. Nutrient supplementation strategies for biopharmaceutical production, Part 3. Bioproc. Int. 2010, 8, 24–31. [Google Scholar]
- Lufino, M.M.P.; Edser, P.A.H.; Wade-Martins, R. Advances in high-capacity extrachromosomal vector technology: Episomal maintenance, vector delivery, and transgene expression. Mol. Ther. 2008, 16, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, J.J.; Chasin, L.A.; Leonard, E.F. Gene amplification and vector engineering to achieve rapid and high-level therapeutic protein production using the Dhfr-based CHO cell selection system. Biotechnol. Adv. 2010, 28, 673–681. [Google Scholar] [CrossRef]
- Barnes, L.M.; Bentley, C.M.; Dickson, A.J. Advances in animal cell recombinant protein production: GS-NS0 expression system. Cytotechnology 2000, 32, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Hackl, M.; Druz, A.; Shridhar, S.; Chung, C.-Y.; Heffner, K.M.; Kreil, D.P.; Betenbaugh, M.; Shiloach, J.; Barron, N. CHO microRNA engineering is growing up: Recent successes and future challenges. Biotechnol. Adv. 2013, 31, 1501–1513. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.R.; Rodrigues, M.E.; Henriques, M.; Oliveira, R.; Azeredo, J. Glycosylation: Impact, control and improvement during therapeutic protein production. Crit. Rev. Biotechnol. 2014, 34, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Wagner-Rousset, E.; Bussat, M.-C.; Lokteff, M.; Klinguer-Hamour, C.; Haeuw, J.-F.; Goetsch, L.; Wurch, T.; Dorsselaer, A.V.; Corvaïa, N. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr. Pharm. Biotechnol. 2008, 9, 482–501. [Google Scholar] [CrossRef]
- Bosques, C.J.; Collins, B.E.; Meador, J.W.; Sarvaiya, H.; Murphy, J.L.; DelloRusso, G.; Bulik, D.A.; Hsu, I.-H.; Washburn, N.; Sipsey, S.F. Chinese hamster ovary cells can produce galactose-α-1, 3-galactose antigens on proteins. Nat. Biotechnol. 2010, 28, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.E.; Patel, A.; Boyd, P.; James, D.C. Metabolic control of recombinant monoclonal antibody N-glycosylation in GS-NS0 cells. Biotechnol. Bioeng. 2001, 75, 239–251. [Google Scholar] [CrossRef]
- Baker, K.N.; Rendall, M.H.; Hills, A.E.; Hoare, M.; Freedman, R.B.; James, D.C. Metabolic control of recombinant protein N-glycan processing in NS0 and CHO cells. Biotechnol. Bioeng. 2001, 73, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Sordo-Puga, Y.; Suárez-Pedroso, M.; Naranjo-Valdéz, P.; Pérez-Pérez, D.; Santana-Rodríguez, E.; Sardinas-Gonzalez, T.; Mendez-Orta, M.K.; Duarte-Cano, C.A.; Estrada-Garcia, M.P.; Rodríguez-Moltó, M.P. Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus. Vaccines 2021, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Pérez Rubio, A.; Eiros, J.M. Cell culture-derived flu vaccine: Present and future. Hum. Vaccines Immunother. 2018, 14, 1874–1882. [Google Scholar] [CrossRef]
- Partridge, J.; Kieny, M.P. Global production capacity of seasonal influenza vaccine in 2011. Vaccine 2013, 31, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Cell-Based Quadrivalent Inactivated InfluenzaVirus Vaccine (Flucelvax® Tetra/FlucelvaxQuadrivalent®): A Review in the Prevention ofInfluenza. Drugs 2019, 79, 1337–1348. [Google Scholar] [CrossRef]
- Bouzya, B.; Rouxel, R.N.; Sacconnay, L.; Mascolo, R.; Nols, L.; Quique, S.; François, L.; Atas, A.; Warter, L.; Dezutter, N.; et al. Immunogenicity of an AS01-adjuvanted respiratory syncytial virus prefusion F (RSVPreF3) vaccine in animal models. npj Vaccines 2023, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Kotb, S.; Haranaka, M.; Folschweiller, N.; Nakanwagi, P.; Verheust, C.; De Schrevel, N.; David, M.-P.; Mesaros, N.; Hulstrøm, V. Safety and immunogenicity of a respiratory syncytial virus prefusion F protein (RSVPreF3) candidate vaccine in older Japanese adults: A phase I, randomized, observer-blind clinical trial. Respir. Investig. 2023, 61, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kopera, E.; Czajka, H.; Zapolnik, P.; Mazur, A. New Insights on Respiratory Syncytial Virus Prevention. Vaccines 2023, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, I.; Davis, M.G.; Steenackers, K.; Essink, B.; Vandermeulen, C.; Fogarty, C.; Andrews, C.P.; Kerwin, E.; David, M.-P.; Fissette, L.; et al. Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F (RSVPreF3) Candidate Vaccine in Older Adults: Phase 1/2 Randomized Clinical Trial. J. Infect. Dis. 2023, 227, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Bebia, Z.; Reyes, O.; Jeanfreau, R.; Kantele, A.; De Leon, R.G.; Sánchez, M.G.; Banooni, P.; Gardener, G.J.; Rasero, J.L.B.; Pardilla, M.B.E.; et al. Safety and Immunogenicity of an Investigational Respiratory Syncytial Virus Vaccine (RSVPreF3) in Mothers and Their Infants: A Phase 2 Randomized Trial. J. Infect. Dis. 2023, 228, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: Recommendations of the Advisory Committee on Immunization Practices—United Stat. MMWR. Morb. Mortal. Wkly. Rep. 2023, 72, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Gribenko, A.V.; Song, X.; Handke, L.D.; Efferen, K.S.; Tompkins, K.; Kodali, S.; Nunez, L.; Prasad, A.K.; Phelan, L.M.; et al. Rational design of a highly immunogenic prefusion-stabilized F glycoprotein antigen for a respiratory syncytial virus vaccine. Sci. Transl. Med. 2024, 15, eade6422. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Falsey, A.R.; Scott, D.A.; Gurtman, A.; Zareba, A.M.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Radley, D.; et al. A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine. J. Infect. Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Britton, A. Evidence to Recommendations Framework: Respiratory Syncytial Virus (RSV) in Adults Pfizer bivalent RSVpreF vaccine in older adults GSK adjuvanted RSVPreF3 vaccine in older adults. In Proceedings of the ACIP Meeting, Atlanta, GA, USA, 21–23 June 2023. [Google Scholar]
- Li, J.; Li, X.; Ma, H.; Ren, X.; Hao, G.; Zhang, H.; Zhao, Z.; Fang, K.; Li, X.; Rong, Z.; et al. Efficient mucosal vaccination of a novel classical swine fever virus E2-Fc fusion protein mediated by neonatal Fc receptor. Vaccine 2020, 38, 4574–4583. [Google Scholar] [CrossRef] [PubMed]
- Bossart, K.N.; Crameri, G.; Dimitrov, A.S.; Mungall, B.A.; Feng, Y.-R.; Patch, J.R.; Choudhary, A.; Wang, L.-F.; Eaton, B.T.; Broder, C.C. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J. Virol. 2005, 79, 6690–6702. [Google Scholar] [CrossRef] [PubMed]
- Bossart, K.N.; Rockx, B.; Feldmann, F.; Brining, D.; Scott, D.; LaCasse, R.; Geisbert, J.B.; Feng, Y.-R.; Chan, Y.-P.; Hickey, A.C.; et al. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 2012, 4, 146ra107. [Google Scholar] [CrossRef] [PubMed]
- McEachern, J.A.; Bingham, J.; Crameri, G.; Green, D.J.; Hancock, T.J.; Middleton, D.; Feng, Y.-R.; Broder, C.C.; Wang, L.-F.; Bossart, K.N. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine 2008, 26, 3842–3852. [Google Scholar] [CrossRef] [PubMed]
- Pallister, J.; Middleton, D.; Wang, L.-F.; Klein, R.; Haining, J.; Robinson, R.; Yamada, M.; White, J.; Payne, J.; Feng, Y.-R.; et al. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine 2011, 29, 5623–5630. [Google Scholar] [CrossRef] [PubMed]
- Middleton, D.; Pallister, J.; Klein, R.; Feng, Y.-R.; Haining, J.; Arkinstall, R.; Frazer, L.; Huang, J.-A.; Edwards, N.; Wareing, M.; et al. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis. 2014, 20, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Pallister, J.A.; Klein, R.; Arkinstall, R.; Haining, J.; Long, F.; White, J.R.; Payne, J.; Feng, Y.-R.; Wang, L.-F.; Broder, C.C.; et al. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol. J. 2013, 10, 237. [Google Scholar] [CrossRef]
- Young-Il, K.; Dokyun, K.; Kwang-Min, Y.; David, S.H.; Shin-Ae, L.; Casel, M.A.B.; Seung-Gyu, J.; Stephanie, K.; WooRam, J.; Chih-Jen, L.; et al. Development of Spike Receptor-Binding Domain Nanoparticles as a Vaccine Candidate against SARS-CoV-2 Infection in Ferrets. MBio 2021, 12, e00230-21. [Google Scholar] [CrossRef]
- Kassardjian, A.; Sun, E.; Sookhoo, J.; Muthuraman, K.; Boligan, K.F.; Kucharska, I.; Rujas, E.; Jetha, A.; Branch, D.R.; Babiuk, S. Modular adjuvant-free pan-HLA-DR-immunotargeting subunit vaccine against SARS-CoV-2 elicits broad sarbecovirus-neutralizing antibody responses. Cell Rep. 2023, 42, 112391. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Snelling, H.J.; Shiell, B.J.; Feng, Y.-R.; Chan, Y.-P.; Bossart, K.N.; Xu, K.; Nikolov, D.B.; Broder, C.C.; Michalski, W.P. Site occupancy and glycan compositional analysis of two soluble recombinant forms of the attachment glycoprotein of Hendra virus. Glycobiology 2012, 22, 572–584. [Google Scholar] [CrossRef]
- Gstöttner, C.; Zhang, T.; Resemann, A.; Ruben, S.; Pengelley, S.; Suckau, D.; Welsink, T.; Wuhrer, M.; Domínguez-Vega, E. Structural and Functional Characterization of SARS-CoV-2 RBD Domains Produced in Mammalian Cells. Anal. Chem. 2021, 93, 6839–6847. [Google Scholar] [CrossRef] [PubMed]
- Arbeitman, C.R.; Auge, G.; Blaustein, M.; Bredeston, L.; Corapi, E.S.; Craig, P.O.; Cossio, L.A.; Dain, L.; D’Alessio, C.; Elias, F.; et al. Structural and functional comparison of SARS-CoV-2-spike receptor binding domain produced in Pichia pastoris and mammalian cells. Sci. Rep. 2020, 10, 21779. [Google Scholar] [CrossRef]
- Brindha, S.; Kuroda, Y. A Multi-Disulfide Receptor-Binding Domain (RBD) of the SARS-CoV-2 Spike Protein Expressed in E. coli Using a SEP-Tag Produces Antisera Interacting with the Mammalian Cell Expressed Spike (S1) Protein. Int. J. Mol. Sci. 2022, 23, 1703. [Google Scholar] [CrossRef]
- Merkuleva, I.A.; Shcherbakov, D.N.; Borgoyakova, M.B.; Shanshin, D.V.; Rudometov, A.P.; Karpenko, L.I.; Belenkaya, S.V.; Isaeva, A.A.; Nesmeyanova, V.S.; Kazachinskaia, E.I.; et al. Comparative Immunogenicity of the Recombinant Receptor-Binding Domain of Protein S SARS-CoV-2 Obtained in Prokaryotic and Mammalian Expression Systems. Vaccines 2022, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Arias-Arias, J.L.; Molina-Castro, S.E.; Monturiol-Gross, L.; Lomonte, B.; Corrales-Aguilar, E. Stable production of recombinant SARS-CoV-2 receptor-binding domain in mammalian cells with co-expression of a fluorescent reporter and its validation as antigenic target for COVID-19 serology testing. Biotechnol. Rep. 2023, 37, e00780. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shi, M.; Li, J.; Song, P.; Li, N. Construction of SARS-CoV-2 virus-like particles by mammalian expression system. Front. Bioeng. Biotechnol. 2020, 8, 862. [Google Scholar]
- García-Cordero, J.; Mendoza-Ramírez, J.; Fernández-Benavides, D.; Roa-Velazquez, D.; Filisola-Villaseñor, J.; Martínez-Frías, S.P.; Sanchez-Salguero, E.S.; Miguel-Rodríguez, C.E.; Maravillas Montero, J.L.; Torres-Ruiz, J.J.; et al. Recombinant Protein Expression and Purification of N, S1, and RBD of SARS-CoV-2 from Mammalian Cells and Their Potential Applications. Diagnostics 2021, 11, 1808. [Google Scholar] [CrossRef] [PubMed]
- Shouval, D. Hepatitis B vaccines. J. Hepatol. 2003, 39 (Suppl. 1), S70–S76. [Google Scholar] [CrossRef]
- Bryan, J.T. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine 2007, 25, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, L.M.; Huang, C.-J.; Batt, C.A. Protein secretion in Pichia pastoris and advances in protein production. Appl. Microbiol. Biotechnol. 2012, 93, 31–39. [Google Scholar] [CrossRef]
- Jeong, K.J.; Jang, S.H.; Velmurugan, N. Recombinant antibodies: Engineering and production in yeast and bacterial hosts. Biotechnol. J. 2011, 6, 16–27. [Google Scholar] [CrossRef]
- Cregg, J.M.; Tolstorukov, I.; Kusari, A.; Sunga, J.; Madden, K.; Chappell, T. Expression in the yeast Pichia pastoris. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 463, pp. 169–189. [Google Scholar]
- Jahic, M.; Veide, A.; Charoenrat, T.; Teeri, T.; Enfors, S. Process technology for production and recovery of heterologous proteins with Pichia pastoris. Biotechnol. Prog. 2006, 22, 1465–1473. [Google Scholar] [CrossRef]
- Tomimoto, K.; Fujita, Y.; Iwaki, T.; Chiba, Y.; Jigami, Y.; Nakayama, K.; Nakajima, Y.; Abe, H. Protease-deficient Saccharomyces cerevisiae strains for the synthesis of human-compatible glycoproteins. Biosci. Biotechnol. Biochem. 2013, 77, 2461–2466. [Google Scholar] [CrossRef]
- Celik, E.; Calık, P. Production of recombinant proteins by yeast cells. Biotechnol. Adv. 2012, 30, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Laurent, J.M.; Young, J.H.; Kachroo, A.H.; Marcotte, E.M. Efforts to make and apply humanized yeast. Brief. Funct. Genom. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Wildt, S.; Gerngross, T.U. The humanization of N-glycosylation pathways in yeast. Nat. Rev. Microbiol. 2005, 3, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, J.; Chen, X.; Chen, L.; Wan, S.; Qiu, X.; Chen, K.; Chen, C.; Tan, H. Humanization of yeasts for glycan-type end-products. Front. Microbiol. 2022, 13, 930658. [Google Scholar] [CrossRef] [PubMed]
- Bobrowicz, P.; Davidson, R.C.; Li, H.; Potgieter, T.I.; Nett, J.H.; Hamilton, S.R.; Stadheim, T.A.; Miele, R.G.; Bobrowicz, B.; Mitchell, T. Engineering of an artificial glycosylation pathway blocked in core oligosaccharide assembly in the yeast Pichia pastoris: Production of complex humanized glycoproteins with terminal galactose. Glycobiology 2004, 14, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.B.; Bhalla, P.L. Recombinant expression systems for allergen vaccines. Inflamm. Allergy-Drug Targets (Formerly Curr. Drug Targets-Inflamm. Allergy) 2006, 5, 53–59. [Google Scholar] [CrossRef]
- Bernstein, M.B.; Chakraborty, M.; Wansley, E.K.; Guo, Z.; Franzusoff, A.; Mostböck, S.; Sabzevari, H.; Schlom, J.; Hodge, J.W. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine 2008, 26, 509–521. [Google Scholar] [CrossRef]
- Munson, S.; Parker, J.; King, T.H.; Lu, Y.; Kelley, V.; Guo, Z.; Borges, V.; Franzusoff, A. Coupling innate and adaptive immunity with yeast-based cancer immunotherapy. In Cancer Vaccines and Tumor Immunity; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 131–149. [Google Scholar]
- Remondo, C.; Cereda, V.; Mostböck, S.; Sabzevari, H.; Franzusoff, A.; Schlom, J.; Tsang, K.-Y. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine 2009, 27, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Okawara, A.; Yamagoe, S.; Naka, T.; Umeyama, T.; Utena-Abe, Y.; Tarumoto, N.; Niimi, M.; Ohno, H.; Doe, M.; et al. The mannan of Candida albicans lacking β-1,2-linked oligomannosides increases the production of inflammatory cytokines by dendritic cells. Med. Mycol. 2013, 51, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Roohvand, F.; Shokri, M.; Abdollahpour-Alitappeh, M.; Ehsani, P. Biomedical applications of yeast- a patent view, part one: Yeasts as workhorses for the production of therapeutics and vaccines. Expert Opin. Ther. Pat. 2017, 27, 929–951. [Google Scholar] [CrossRef] [PubMed]
- Schellenbacher, C.; Roden, R.B.S.; Kirnbauer, R. Developments in L2-based human papillomavirus (HPV) vaccines. Virus Res. 2017, 231, 166–175. [Google Scholar] [CrossRef]
- Liu, C.; Yao, Y.; Yang, X.; Bai, H.; Huang, W.; Xia, Y.; Ma, Y. Production of Recombinant Human Papillomavirus Type 52 L1 Protein in Hansenula polymorpha Formed Virus-Like Particles. J. Microbiol. Biotechnol. 2015, 25, 936–940. [Google Scholar] [CrossRef]
- Caetano, K.A.A.; Del-Rios, N.H.A.; Pinheiro, R.S.; Bergamaschi, F.P.R.; Carneiro, M.A.D.S.; Teles, S.A. Low Immunogenicity of Recombinant Hepatitis B Vaccine Derived from Hansenula polymorpha in Adults Aged Over 40 Years. Am. J. Trop. Med. Hyg. 2017, 96, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhao, P.; Du, J.; Li, X.; Lu, W.; Hao, X.; Dong, B.; Yu, Y.; Wang, L. High-level expression and immunogenicity of porcine circovirus type 2b capsid protein without nuclear localization signal expressed in Hansenula polymorpha. Biologicals 2018, 51, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, V.; Arora, U.; Shukla, R.; Poddar, A.; Shanmugam, R.K.; White, L.J.; Mattocks, M.M.; Raut, R.; Perween, A.; Tyagi, P.; et al. A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice. PLoS Negl. Trop. Dis. 2018, 12, e0006191. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, R.K.; Shukla, R.; Arora, U.; Swaminathan, S.; Khanna, N. Dengue envelope-based “four-in-one” virus-like particles produced using Pichia pastoris induce enhancement-lacking, domain III-directed tetravalent neutralising antibodies in mice. Sci. Rep. 2018, 8, 8643. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Yu, Z.; Fang, D.; Fu, C.; Zhu, X.; He, Z.; Yan, H.; Jiang, L. Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: Immunological properties. BMC Microbiol. 2014, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Poddar, A.; Ramasamy, V.; Shukla, R.; Rajpoot, R.K.; Arora, U.; Jain, S.K.; Swaminathan, S.; Khanna, N. Virus-like particles derived from Pichia pastoris-expressed dengue virus type 1 glycoprotein elicit homotypic virus-neutralizing envelope domain III-directed antibodies. BMC Biotechnol. 2016, 16, 50. [Google Scholar] [CrossRef]
- Khetarpal, N.; Shukla, R.; Rajpoot, R.K.; Poddar, A.; Pal, M.; Swaminathan, S.; Arora, U.; Khanna, N. Recombinant Dengue Virus 4 Envelope Glycoprotein Virus-Like Particles Derived from Pichia pastoris are Capable of Eliciting Homotypic Domain III-Directed Neutralizing Antibodies. Am. J. Trop. Med. Hyg. 2017, 96, 126–134. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Zhang, W.; Dai, W.; Xie, J.; Ye, L.; Wang, H.; Chen, H.; Liu, Q.; Gong, S.; et al. Enterovirus D68 virus-like particles expressed in Pichia pastoris potently induce neutralizing antibody responses and confer protection against lethal viral infection in mice. Emerg. Microbes Infect. 2018, 7, 3. [Google Scholar] [CrossRef]
- Zahid, M.; Lünsdorf, H.; Rinas, U. Assessing stability and assembly of the hepatitis B surface antigen into virus-like particles during down-stream processing. Vaccine 2015, 33, 3739–3745. [Google Scholar] [CrossRef]
- Fazlalipour, M.; Keyvani, H.; Monavari, S.H.R.; Mollaie, H.R. Expression, Purification and Immunogenic Description of a Hepatitis C Virus Recombinant CoreE1E2 Protein Expressed by Yeast Pichia pastoris. Jundishapur J. Microbiol. 2015, 8, e17157. [Google Scholar] [CrossRef] [PubMed]
- Bredell, H.; Smith, J.J.; Görgens, J.F.; van Zyl, W.H. Expression of unique chimeric human papilloma virus type 16 (HPV-16) L1-L2 proteins in Pichia pastoris and Hansenula polymorpha. Yeast 2018, 35, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Glueck, R.; Rishi, N. Physicochemical characterization and immunological properties of Pichia pastoris based HPV16L1 and 18L1 virus like particles. Biologicals 2017, 46, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Athmaram, T.N.; Singh, A.K.; Saraswat, S.; Srivastava, S.; Misra, P.; Kameswara Rao, M.; Gopalan, N.; Rao, P.V.L. A simple Pichia pastoris fermentation and downstream processing strategy for making recombinant pandemic Swine Origin Influenza a virus Hemagglutinin protein. J. Ind. Microbiol. Biotechnol. 2013, 40, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Liao, H.-Y.; Zhang, J.-Y.; Cheng, T.-J.R.; Wong, C.-H. Development of a universal influenza vaccine using hemagglutinin stem protein produced from Pichia pastoris. Virology 2019, 526, 125–137. [Google Scholar] [CrossRef]
- Pietrzak, M.; Macioła, A.; Zdanowski, K.; Protas-Klukowska, A.M.; Olszewska, M.; Śmietanka, K.; Minta, Z.; Szewczyk, B.; Kopera, E. An avian influenza H5N1 virus vaccine candidate based on the extracellular domain produced in yeast system as subviral particles protects chickens from lethal challenge. Antivir. Res. 2016, 133, 242–249. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Wu, C.-W.; Lin, G.-J.; Lee, W.-C.; Chien, M.-S.; Huang, C. Enhancing expression of the classical swine fever virus glycoprotein E2 in yeast and its application to a blocking ELISA. J. Biotechnol. 2014, 174, 1–6. [Google Scholar] [CrossRef]
- Lin, G.-J.; Deng, M.-C.; Chen, Z.-W.; Liu, T.-Y.; Wu, C.-W.; Cheng, C.-Y.; Chien, M.-S.; Huang, C. Yeast expressed classical swine fever E2 subunit vaccine candidate provides complete protection against lethal challenge infection and prevents horizontal virus transmission. Vaccine 2012, 30, 2336–2341. [Google Scholar] [CrossRef]
- Saraswat, S.; Athmaram, T.N.; Parida, M.; Agarwal, A.; Saha, A.; Dash, P.K. Expression and Characterization of Yeast Derived Chikungunya Virus Like Particles (CHIK-VLPs) and Its Evaluation as a Potential Vaccine Candidate. PLoS Negl. Trop. Dis. 2016, 10, e0004782. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Zhu, Y.; Zhou, Y.; Gu, C.; Yi, Y.; Wang, S.; Xu, S.; Hu, G.; Du, S.; Yin, Y.; et al. Yeast-produced RBD-based recombinant protein vaccines elicit broadly neutralizing antibodies and durable protective immunity against SARS-CoV-2 infection. Cell Discov. 2021, 7, 71. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.Y.; Kang, H.A.; Lee, Y.; Park, E.-J.; Kim, H.-J. Oral immunization with whole yeast producing viral capsid antigen provokes a stronger humoral immune response than purified viral capsid antigen. Lett. Appl. Microbiol. 2014, 58, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hadiji-Abbes, N.; Martin, M.; Benzina, W.; Karray-Hakim, H.; Gergely, C.; Gargouri, A.; Mokdad-Gargouri, R. Extraction and purification of hepatitis B virus-like M particles from a recombinant Saccharomyces cerevisiae strain using alumina powder. J. Virol. Methods 2013, 187, 132–137. [Google Scholar] [CrossRef]
- Pleckaityte, M.; Bremer, C.M.; Gedvilaite, A.; Kucinskaite-Kodze, I.; Glebe, D.; Zvirbliene, A. Construction of polyomavirus-derived pseudotype virus-like particles displaying a functionally active neutralizing antibody against hepatitis B virus surface antigen. BMC Biotechnol. 2015, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- King, T.H.; Kemmler, C.B.; Guo, Z.; Mann, D.; Lu, Y.; Coeshott, C.; Gehring, A.J.; Bertoletti, A.; Ho, Z.Z.; Delaney, W.; et al. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and Core specific T cells in mice and activates human T cells recognizing epitopes linked to viral clearance. PLoS ONE 2014, 9, e101904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Lu, J.; Yan, B.; Song, L.; Li, L.; Cui, F.; Zhang, G.; Wang, F.; Liang, X. Antibody response to revaccination among adult non-responders to primary Hepatitis B vaccination in China. Hum. Vaccines Immunother. 2015, 11, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Gaggar, A.; Coeshott, C.; Apelian, D.; Rodell, T.; Armstrong, B.R.; Shen, G.; Subramanian, G.M.; McHutchison, J.G. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: A randomized study. Vaccine 2014, 32, 4925–4931. [Google Scholar] [CrossRef]
- Lin, T.; Xianyu, L.; Lyu, S. Monoclonal neutralizing antibodies against EV71 screened from mice immunized with yeast-produced virus-like particles. Virol. Sin. 2015, 30, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, X.; Zhao, M.; Liu, W.; Pang, L.; Sun, X.; Cen, S.; Yang, B.B.; Huang, Y.; Sheng, W. EV71 virus-like particles produced by co-expression of capsid proteins in yeast cells elicit humoral protective response against EV71 lethal challenge. BMC Res. Notes 2016, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Penkert, R.R.; Young, N.S.; Surman, S.L.; Sealy, R.E.; Rosch, J.; Dormitzer, P.R.; Settembre, E.C.; Chandramouli, S.; Wong, S.; Hankins, J.S.; et al. Saccharomyces cerevisiae-derived virus-like particle parvovirus B19 vaccine elicits binding and neutralizing antibodies in a mouse model for sickle cell disease. Vaccine 2017, 35, 3615–3620. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, S.; Medina-Selby, A.; Coit, D.; Schaefer, M.; Spencer, T.; Brito, L.A.; Zhang, P.; Otten, G.; Mandl, C.W.; Mason, P.W.; et al. Generation of a parvovirus B19 vaccine candidate. Vaccine 2013, 31, 3872–3878. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-L.; Kim, J.-M.; Park, J.-A.; Park, S.-M.; Jang, Y.-S.; Yang, M.-S.; Kim, D.-H. Expression and purification of an immunogenic dengue virus epitope using a synthetic consensus sequence of envelope domain III and Saccharomyces cerevisiae. Protein Expr. Purif. 2013, 88, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.; Wang, D.; Yao, C.; Wang, Q.; Xie, J.; Shi, X.; Xiang, Y.; Liu, W.; Zhang, L. Epitope-focused immunogens against the CD4-binding site of HIV-1 envelope protein induce neutralizing antibodies against auto-and heterologous viruses. J. Biol. Chem. 2018, 293, 830–846. [Google Scholar] [CrossRef]
- Sablon, E.; Van Broekhoven, A.; Bosman, F.; Depla, E.; Deschamps, G. Constructs and Methods for Expression of Recombinant HCV Envelope Proteins. U.S. Patent 7,314,925, 1 January 2008. [Google Scholar]
- Evans, J.D.; Beatty, N.M. Yeast Expression of Flavivirus Virus-Like Particles and Use Thereof. U.S. Patent 4/241,530, 7 August 2014. [Google Scholar]
- Liu, Y.; Zhao, D.; Wang, Y.; Chen, Z.; Yang, L.; Li, W.; Gong, Y.; Gan, C.; Tang, J.; Zhang, T. A vaccine based on the yeast-expressed receptor-binding domain (RBD) elicits broad immune responses against SARS-CoV-2 variants. Front. Immunol. 2022, 13, 1011484. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Abid, T.; Pereira Ribeiro, S.; Edara, V.V.; Floyd, K.; Smith, J.C.; Latif, M.B.; Pacheco-Sanchez, G.; Dutta, D.; Wang, S.; et al. A yeast-expressed RBD-based SARS-CoV-2 vaccine formulated with 3M-052-alum adjuvant promotes protective efficacy in non-human primates. Sci. Immunol. 2021, 6, eabh3634. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Chen, W.-H.; Versteeg, L.; Keegan, B.; Zhan, B.; Wei, J.; Liu, Z.; Lee, J.; Kundu, R.; Adhikari, R.; et al. SARS-CoV-2 RBD219-N1C1: A yeast-expressed SARS-CoV-2 recombinant receptor-binding domain candidate vaccine stimulates virus neutralizing antibodies and T-cell immunity in mice. Hum. Vaccines Immunother. 2021, 17, 2356–2366. [Google Scholar] [CrossRef]
- Chen, W.-H.; Pollet, J.; Strych, U.; Lee, J.; Liu, Z.; Kundu, R.T.; Versteeg, L.; Villar, M.J.; Adhikari, R.; Wei, J.; et al. Yeast-expressed recombinant SARS-CoV-2 receptor binding domain RBD203-N1 as a COVID-19 protein vaccine candidate. Protein Expr. Purif. 2022, 190, 106003. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Du, L.; Chag, S.M.; Ma, C.; Tricoche, N.; Tao, X.; Seid, C.A.; Hudspeth, E.M.; Lustigman, S.; Tseng, C.-T.K.; et al. Yeast-expressed recombinant protein of the receptor-binding domain in SARS-CoV spike protein with deglycosylated forms as a SARS vaccine candidate. Hum. Vaccines Immunother. 2014, 10, 648–658. [Google Scholar] [CrossRef]
- Dai, L.; Zheng, T.; Xu, K.; Han, Y.; Xu, L.; Huang, E.; An, Y.; Cheng, Y.; Li, S.; Liu, M. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell 2020, 182, 722–733. [Google Scholar] [CrossRef]
- Thak, E.J.; Kim, J.; Lee, D.-J.; Kim, J.Y.; Kang, H.A. Structural analysis of N-/O-glycans assembled on proteins in yeasts. J. Microbiol. 2018, 56, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.R.; Cook, W.J.; Gomathinayagam, S.; Burnina, I.; Bukowski, J.; Hopkins, D.; Schwartz, S.; Du, M.; Sharkey, N.J.; Bobrowicz, P. Production of sialylated O-linked glycans in Pichia pastoris. Glycobiology 2013, 23, 1192–1203. [Google Scholar] [CrossRef]
- Shajahan, A.; Supekar, N.T.; Gleinich, A.S.; Azadi, P. Deducing the N-and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology 2020, 30, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Han, J.-F.; Qin, C.-F.; Chen, R. Virus-like particles for enterovirus 71 produced from Saccharomyces cerevisiae potently elicits protective immune responses in mice. Vaccine 2013, 31, 3281–3287. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.-Y.; Han, J.-F.; Deng, Y.-Q.; Li, Y.-X.; Zhu, S.-Y.; He, Y.-L.; Qin, E.-D.; Chen, R.; Qin, C.-F. Virus-like particles produced in Saccharomyces cerevisiae elicit protective immunity against Coxsackievirus A16 in mice. Appl. Microbiol. Biotechnol. 2013, 97, 10445–10452. [Google Scholar] [CrossRef] [PubMed]
- Bill, R.M. Recombinant protein subunit vaccine synthesis in microbes: A role for yeast? J. Pharm. Pharmacol. 2015, 67, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S.; Paz-Maldonado, L.M.T.; Soria-Guerra, R.E. Chlamydomonas reinhardtii as a viable platform for the production of recombinant proteins: Current status and perspectives. Plant Cell Rep. 2012, 31, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Lauersen, K.J.; Kruse, O.; Berger, H.; Mussgnug, J.H. Efficient recombinant protein production and secretion from nuclear transgenes in Chlamydomonas reinhardtii. J. Biotechnol. 2013, 167, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Lauersen, K.J. Gene delivery technologies with applications in microalgal genetic engineering. Biology 2021, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, T.M.; Kopera, H.C.; Saunders, T.L. Principles of genetic engineering. Genes 2020, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of cell-penetrating peptides with nanoparticles for therapeutic application: A review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef]
- Gregory, J.A.; Topol, A.B.; Doerner, D.Z.; Mayfield, S. Alga-Produced Cholera Toxin-Pfs25 Fusion Proteins as Oral Vaccines. Appl. Environ. Microbiol. 2013, 79, 3917–3925. [Google Scholar] [CrossRef]
- Dreesen, I.A.J.; Hamri, G.C.-E.; Fussenegger, M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J. Biotechnol. 2010, 145, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Specht, E.; Mayfield, S. Algae-based oral recombinant vaccines. Front. Microbiol. 2014, 5, 60. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2014.00060 (accessed on 1 June 2024). [CrossRef] [PubMed]
- Lössl, A.G.; Waheed, M.T. Chloroplast-derived vaccines against human diseases: Achievements, challenges and scopes. Plant Biotechnol. J. 2011, 9, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.; Ngo, B.; Efuet, E.; Mayfield, S.P. Development of a GFP reporter gene for Chlamydomonas reinhardtii chloroplast. Plant J. 2002, 30, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, S.P.; Franklin, S.E.; Lerner, R.A. Expression and assembly of a fully active antibody in algae. Proc. Natl. Acad. Sci. USA 2003, 100, 438–442. [Google Scholar] [CrossRef]
- Butinar, L.; Sonjak, S.; Zalar, P.; Plemenitaš, A.; Gunde-Cimerman, N. Melanized halophilic fungi are eukaryotic members of microbial communities in hypersaline waters of solar salterns. Bot. Mar. 2005, 48, 73–79. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. The biotechnology of cultivating the halotolerant algaDunaliella. Trends Biotechnol. 1990, 8, 121–126. [Google Scholar] [CrossRef]
- Qin, S.; Lin, H.; Jiang, P. Advances in genetic engineering of marine algae. Biotechnol. Adv. 2012, 30, 1602–1613. [Google Scholar] [CrossRef]
- Georgianna, D.R.; Mayfield, S.P. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 2012, 488, 329–335. [Google Scholar] [CrossRef]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hu, H.; Gao, Y.; Xu, X.; Gao, H. Microalgae as platforms for production of recombinant proteins and valuable compounds: Progress and prospects. J. Ind. Microbiol. Biotechnol. 2011, 38, 1879–1890. [Google Scholar] [CrossRef]
- Berndt, A.J.; Smalley, T.N.; Ren, B.; Simkovsky, R.; Badary, A.; Sproles, A.E.; Fields, F.J.; Torres-Tiji, Y.; Heredia, V.; Mayfield, S.P. Recombinant production of a functional SARS-CoV-2 spike receptor binding domain in the green algae Chlamydomonas reinhardtii. PLoS ONE 2021, 16, e0257089. [Google Scholar] [CrossRef] [PubMed]
- Govea-Alonso, D.O.; Malla, A.; Bolaños-Martínez, O.C.; Vimolmangkang, S.; Rosales-Mendoza, S. An Algae-Made RBD from SARS-CoV-2 Is Immunogenic in Mice. Pharmaceuticals 2022, 15, 1298. [Google Scholar] [CrossRef]
- Malla, A.; Rosales-Mendoza, S.; Phoolcharoen, W.; Vimolmangkang, S. Efficient transient expression of recombinant proteins using DNA viral vectors in freshwater microalgal species. Front. Plant Sci. 2021, 12, 650820. [Google Scholar] [CrossRef] [PubMed]
- Slattery, S.S.; Giguere, D.J.; Stuckless, E.E.; Shrestha, A.; Briere, L.-A.K.; Galbraith, A.; Reaume, S.; Boyko, X.; Say, H.H.; Browne, T.S.; et al. Phosphate-regulated expression of the SARS-CoV-2 receptor-binding domain in the diatom Phaeodactylum tricornutum for pandemic diagnostics. Sci. Rep. 2022, 12, 7010. [Google Scholar] [CrossRef] [PubMed]
- Gimpel, J.A.; Hyun, J.S.; Schoepp, N.G.; Mayfield, S.P. Production of recombinant proteins in microalgae at pilot greenhouse scale. Biotechnol. Bioeng. 2015, 112, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Rattanapisit, K.; Shanmugaraj, B.; Manopwisedjaroen, S.; Purwono, P.B.; Siriwattananon, K.; Khorattanakulchai, N.; Hanittinan, O.; Boonyayothin, W.; Thitithanyanont, A.; Smith, D.R. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 2020, 10, 17698. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Rabindran, S. Recent progress in the development of plant derived vaccines. Expert Rev. Vaccines 2008, 7, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Streatfield, S.J.; Kushnir, N. Clinical development of plant-produced recombinant pharmaceuticals: Vaccines, antibodies and beyond. Hum. Vaccines 2011, 7, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Egelkrout, E.; Hayden, C.; Fake, G.; Keener, T.; Arruda, P.; Saltzman, R.; Walker, J.; Howard, J. Oral delivery of maize-produced porcine epidemic diarrhea virus spike protein elicits neutralizing antibodies in pigs. Plant Cell. Tissue Organ Cult. 2020, 142, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Hayden, C.A.; Egelkrout, E.M.; Moscoso, A.M.; Enrique, C.; Keener, T.K.; Jimenez-Flores, R.; Wong, J.C.; Howard, J.A. Production of highly concentrated, heat-stable hepatitis B surface antigen in maize. Plant Biotechnol. J. 2012, 10, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Hayden, C.A.; Smith, E.M.; Turner, D.D.; Keener, T.K.; Wong, J.C.; Walker, J.H.; Tizard, I.R.; Jimenez-Flores, R.; Howard, J.A. Supercritical fluid extraction provides an enhancement to the immune response for orally-delivered hepatitis B surface antigen. Vaccine 2014, 32, 1240–1246. [Google Scholar] [CrossRef]
- Shahid, N.; Daniell, H. Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant Biotechnol. J. 2016, 14, 2079–2099. [Google Scholar] [CrossRef]
- Gehl, C.; Li, G.; Serek, M. An efficient protocol for Agrobacterium-mediated transformation and regeneration of Campanula medium (Canterbury bells) based on leaf disc explants. Plant Cell Tissue Organ Cult. 2020, 140, 635–645. [Google Scholar] [CrossRef]
- Ariga, H.; Toki, S.; Ishibashi, K. Potato virus X vector-mediated DNA-free genome editing in plants. Plant Cell Physiol. 2020, 61, 1946–1953. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the spotlight back on plant suspension cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef]
- Kolotilin, I.; Topp, E.; Cox, E.; Devriendt, B.; Conrad, U.; Joensuu, J.; Stöger, E.; Warzecha, H.; McAllister, T.; Potter, A.; et al. Plant-based solutions for veterinary immunotherapeutics and prophylactics. Vet. Res. 2014, 45, 117. [Google Scholar] [CrossRef] [PubMed]
- Floss, D.M.; Falkenburg, D.; Conrad, U. Production of vaccines and therapeutic antibodies for veterinary applications in transgenic plants: An overview. Transgenic Res. 2007, 16, 315–332. [Google Scholar] [CrossRef]
- Oh, Y.; Park, Y.; Choi, B.-H.; Park, S.; Gu, S.; Park, J.; Kim, J.-K.; Sohn, E.-J. Field Application of a New CSF Vaccine Based on Plant-Produced Recombinant E2 Marker Proteins on Pigs in Areas with Two Different Control Strategies. Vaccines 2021, 9, 537. [Google Scholar] [CrossRef]
- Landry, N.; Pillet, S.; Favre, D.; Poulin, J.-F.; Trépanier, S.; Yassine-Diab, B.; Ward, B.J. Influenza virus-like particle vaccines made in Nicotiana benthamiana elicit durable, poly-functional and cross-reactive T cell responses to influenza HA antigens. Clin. Immunol. 2014, 154, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Racine, T.; Nfon, C.; Di Lenardo, T.Z.; Babiuk, S.; Ward, B.J.; Kobinger, G.P.; Landry, N. Plant-derived H7 VLP vaccine elicits protective immune response against H7N9 influenza virus in mice and ferrets. Vaccine 2015, 33, 6282–6289. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Chichester, J.A.; Jones, M.; Manceva, S.D.; Damon, E.; Mett, V.; Musiychuk, K.; Bi, H.; Farrance, C.; Shamloul, M.; et al. Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum. Vaccines 2011, 7 (Suppl. 1), 41–50. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; O’Kennedy, M.M.; Wandrag, D.B.R.; Adeyemi, M.; Abolnik, C. Efficacy of a plant-produced virus-like particle vaccine in chickens challenged with Influenza A H6N2 virus. Plant Biotechnol. J. 2020, 18, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Sepotokele, K.M.; O’Kennedy, M.M.; Wandrag, D.B.R.; Abolnik, C. Optimization of infectious bronchitis virus-like particle expression in Nicotiana benthamiana as potential poultry vaccines. PLoS ONE 2023, 18, e0288970. [Google Scholar] [CrossRef] [PubMed]
- Siriwattananon, K.; Manopwisedjaroen, S.; Shanmugaraj, B.; Rattanapisit, K.; Phumiamorn, S.; Sapsutthipas, S.; Trisiriwanich, S.; Prompetchara, E.; Ketloy, C.; Buranapraditkun, S. Plant-produced receptor-binding domain of SARS-CoV-2 elicits potent neutralizing responses in mice and non-human primates. Front. Plant Sci. 2021, 12, 682953. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, S.; Kang, H.; Park, M.; Min, K.; Kim, N.H.; Gu, S.; Kim, J.K.; An, D.-J.; Choe, S. A classical swine fever virus E2 fusion protein produced in plants elicits a neutralizing humoral immune response in mice and pigs. Biotechnol. Lett. 2020, 42, 1247–1261. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hoshikawa, K.; Ezura, K.; Okazawa, R.; Fujita, S.; Takaoka, M.; Mason, H.S.; Ezura, H.; Miura, K. Improvement of the transient expression system for production of recombinant proteins in plants. Sci. Rep. 2018, 8, 4755. [Google Scholar] [CrossRef]
- Garabagi, F.; McLean, M.D.; Hall, J.C. Transient and stable expression of antibodies in Nicotiana species. Methods Mol. Biol. 2012, 907, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Chilton, P.M. Naturally occurring low biological reactivity lipopolysaccharides as vaccine adjuvants. Expert Rev. Vaccines 2013, 12, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Global Biodefense Staff. DARPA Hits Milestone in Plant-Based Vaccines for Pandemics. Glob. Biodefense. Available online: https://globalbiodefense.com/2012/07/28/darpa-program-hits-milestone-in-plant-based-vaccines-for-pandemics/ (accessed on 3 June 2020).
- Potera, C. Vaccine Manufacturing Gets Boost from Tobacco Plants. Genet. Eng. Biotechnol. News 2012, 32, 8–10. [Google Scholar] [CrossRef]
- Shin, Y.-J.; König-Beihammer, J.; Vavra, U.; Schwestka, J.; Kienzl, N.F.; Klausberger, M.; Laurent, E.; Grünwald-Gruber, C.; Vierlinger, K.; Hofner, M. N-glycosylation of the SARS-CoV-2 receptor binding domain is important for functional expression in plants. Front. Plant Sci. 2021, 12, 689104. [Google Scholar] [CrossRef] [PubMed]
- Makatsa, M.S.; Tincho, M.B.; Wendoh, J.M.; Ismail, S.D.; Nesamari, R.; Pera, F.; De Beer, S.; David, A.; Jugwanth, S.; Gededzha, M.P. SARS-CoV-2 antigens expressed in plants detect antibody responses in COVID-19 patients. Front. Plant Sci. 2021, 12, 589940. [Google Scholar] [CrossRef]
- Jung, J.; Zahmanova, G.; Minkov, I.; Lomonossoff, G.P. Plant-based expression and characterization of SARS-CoV-2 virus-like particles presenting a native spike protein. Plant Biotechnol. J. 2022, 20, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.-C.; Schillberg, S.; Christou, P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.M.; Cheon, J.; Jung, J.; Kim, H.; Lee, J.; Song, M.; Jeong, G.U.; Kwon, Y.; Shim, B.; Choe, S. Plant-expressed receptor binding domain of the SARS-CoV-2 spike protein elicits Humoral immunity in mice. Vaccines 2021, 9, 978. [Google Scholar] [CrossRef]
- Moon, K.-B.; Jeon, J.-H.; Choi, H.; Park, J.-S.; Park, S.-J.; Lee, H.-J.; Park, J.M.; Cho, H.S.; Moon, J.S.; Oh, H. Construction of SARS-CoV-2 virus-like particles in plant. Sci. Rep. 2022, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, N.E.; Levine, M.Z.; Oza, J.P. A User’s Guide to Cell-Free Protein Synthesis. Methods Protoc. 2019, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Hong, S.H. Cell-free protein synthesis for producing ‘difficult-to-express’ proteins. Biochem. Eng. J. 2018, 138, 156–164. [Google Scholar] [CrossRef]
- Rosenblum, G.; Cooperman, B.S. Engine out of the chassis: Cell-free protein synthesis and its uses. FEBS Lett. 2014, 588, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, R.K.; Marcus, L.; Chaloupka, J.; Halvorson, H.O.; Bock, R.M. Amino acid incorporation into protein by cell-free extracts of yeast. Biochemistry 1963, 2, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Zubay, G. In Vitro Synthesis of Protein in Microbial Systems. Annu. Rev. Genet. 1973, 7, 267–287. [Google Scholar] [CrossRef]
- Shin, J.; Noireaux, V. An E. coli Cell-Free Expression Toolbox: Application to Synthetic Gene Circuits and Artificial Cells. ACS Synth. Biol. 2012, 1, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Thomas, C.J.; Sprang, S.R.; Tall, G.G. Molecular chaperoning function of Ric-8 is to fold nascent heterotrimeric G protein α subunits. Proc. Natl. Acad. Sci. USA 2013, 110, 3794–3799. [Google Scholar] [CrossRef]
- Li, J.; Gu, L.; Aach, J.; Church, G.M. Improved Cell-Free RNA and Protein Synthesis System. PLoS ONE 2014, 9, e106232. [Google Scholar] [CrossRef] [PubMed]
- Tinafar, A.; Jaenes, K.; Pardee, K. Synthetic Biology Goes Cell-Free. BMC Biol. 2019, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Berkheimer, S.D.; Werner, C.J.; Bundy, B.C. Lyophilized Escherichia coli-based cell-free systems for robust, high-density, long-term storage. Biotechniques 2014, 56, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pardee, K.; Slomovic, S.; Nguyen, P.Q.; Lee, J.W.; Donghia, N.; Burrill, D.; Ferrante, T.; McSorley, F.R.; Furuta, Y.; Vernet, A.; et al. Portable, On-Demand Biomolecular Manufacturing. Cell 2016, 167, 248–259.e12. [Google Scholar] [CrossRef]
- Stech, M.; Quast, R.B.; Sachse, R.; Schulze, C.; Wüstenhagen, D.A.; Kubick, S. A Continuous-Exchange Cell-Free Protein Synthesis System Based on Extracts from Cultured Insect Cells. PLoS ONE 2014, 9, e96635. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Emileh, A.; Brideau, N.; Britton, J. Cell-free reactions in continuous manufacturing systems. Curr. Opin. Green Sustain. Chem. 2020, 25, 100380. [Google Scholar] [CrossRef]
- Quast, R.B.; Kortt, O.; Henkel, J.; Dondapati, S.K.; Wüstenhagen, D.A.; Stech, M.; Kubick, S. Automated production of functional membrane proteins using eukaryotic cell-free translation systems. J. Biotechnol. 2015, 203, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.D.; Karim, A.S.; Jewett, M.C. Cell-free gene expression: An expanded repertoire of applications. Nat. Rev. Genet. 2020, 21, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Dopp, B.J.L.; Tamiev, D.D.; Reuel, N.F. Cell-free supplement mixtures: Elucidating the history and biochemical utility of additives used to support in vitro protein synthesis in E. coli extract. Biotechnol. Adv. 2019, 37, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.D.; Gan, R.; Hodgman, C.E.; Jewett, M.C. Cell-free protein synthesis: Applications come of age. Biotechnol. Adv. 2012, 30, 1185–1194. [Google Scholar] [CrossRef]
- Chiao, A.C.; Murray, R.M.; Sun, Z.Z. Development of prokaryotic cell-free systems for synthetic biology. bioRxiv 2016, 48710. [Google Scholar] [CrossRef]
- Yang, W.C.; Patel, K.G.; Wong, H.E.; Swartz, J.R. Simplifying and streamlining Escherichia coli-based cell-free protein synthesis. Biotechnol. Prog. 2012, 28, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Caschera, F.; Noireaux, V. Synthesis of 2.3 mg/ml of protein with an all Escherichia coli cell-free transcription–translation system. Biochimie 2014, 99, 162–168. [Google Scholar] [CrossRef]
- Des Soye, B.J.; Davidson, S.R.; Weinstock, M.T.; Gibson, D.G.; Jewett, M.C. Establishing a High-Yielding Cell-Free Protein Synthesis Platform Derived from Vibrio natriegens. ACS Synth. Biol. 2018, 7, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, W.-Q.; Li, J. Translation Related Factors Improve the Productivity of a Streptomyces-Based Cell-Free Protein Synthesis System. ACS Synth. Biol. 2020, 9, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Kelwick, R.; Webb, A.J.; MacDonald, J.T.; Freemont, P.S. Development of a Bacillus subtilis cell-free transcription-translation system for prototyping regulatory elements. Metab. Eng. 2016, 38, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; MacDonald, J.T.; Wienecke, S.; Ishwarbhai, A.; Tsipa, A.; Aw, R.; Kylilis, N.; Bell, D.J.; McClymont, D.W.; Jensen, K.; et al. Rapid acquisition and model-based analysis of cell-free transcription–translation reactions from nonmodel bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E4340–E4349. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Jewett, M.C. Development of a Pseudomonas putida cell-free protein synthesis platform for rapid screening of gene regulatory elements. Synth. Biol. 2018, 3, ysy003. [Google Scholar] [CrossRef]
- Tran, K.; Gurramkonda, C.; Cooper, M.A.; Pilli, M.; Taris, J.E.; Selock, N.; Han, T.-C.; Tolosa, M.; Zuber, A.; Peñalber-Johnstone, C.; et al. Cell-free production of a therapeutic protein: Expression, purification, and characterization of recombinant streptokinase using a CHO lysate. Biotechnol. Bioeng. 2018, 115, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.W.; Majewska, N.I.; Chen, C.X.; Albanetti, T.E.; Jimenez, R.B.C.; Schmelzer, A.E.; Jewett, M.C.; Roy, V. Development of a CHO-Based Cell-Free Platform for Synthesis of Active Monoclonal Antibodies. ACS Synth. Biol. 2017, 6, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hu, J. Host-regulated Hepatitis B Virus Capsid Assembly in a Mammalian Cell-free System. Bio-protocol 2018, 8, e2813. [Google Scholar] [CrossRef] [PubMed]
- Kopniczky, M.B.; Canavan, C.; McClymont, D.W.; Crone, M.A.; Suckling, L.; Goetzmann, B.; Siciliano, V.; MacDonald, J.T.; Jensen, K.; Freemont, P.S. Cell-Free Protein Synthesis as a Prototyping Platform for Mammalian Synthetic Biology. ACS Synth. Biol. 2020, 9, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Ezure, T.; Suzuki, T.; Higashide, S.; Shintani, E.; Endo, K.; Kobayashi, S.; Shikata, M.; Ito, M.; Tanimizu, K.; Nishimura, O. Cell-Free Protein Synthesis System Prepared from Insect Cells by Freeze-Thawing. Biotechnol. Prog. 2006, 22, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Ezure, T.; Suzuki, T.; Shikata, M.; Ito, M.; Ando, E. A cell-free protein synthesis system from insect cells. Methods Mol. Biol. 2010, 607, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Harbers, M. Wheat germ systems for cell-free protein expression. FEBS Lett. 2014, 588, 2762–2773. [Google Scholar] [CrossRef] [PubMed]
- Buntru, M.; Vogel, S.; Stoff, K.; Spiegel, H.; Schillberg, S. A versatile coupled cell-free transcription–translation system based on tobacco BY-2 cell lysates. Biotechnol. Bioeng. 2015, 112, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, M.; Tada, Y. Cell-free protein synthesis of plant transcription factors. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1830, pp. 337–349. [Google Scholar]
- Zemella, A.; Thoring, L.; Hoffmeister, C.; Šamalíková, M.; Ehren, P.; Wüstenhagen, D.A.; Kubick, S. Cell-free protein synthesis as a novel tool for directed glycoengineering of active erythropoietin. Sci. Rep. 2018, 8, 8514. [Google Scholar] [CrossRef] [PubMed]
- Burgenson, D.; Gurramkonda, C.; Pilli, M.; Ge, X.; Andar, A.; Kostov, Y.; Tolosa, L.; Rao, G. Rapid recombinant protein expression in cell-free extracts from human blood. Sci. Rep. 2018, 8, 9569. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Kwon, Y.-C.; Jewett, M.C. Establishing a high yielding streptomyces-based cell-free protein synthesis system. Biotechnol. Bioeng. 2017, 114, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Buntru, M.; Vogel, S.; Spiegel, H.; Schillberg, S. Tobacco BY-2 cell-free lysate: An alternative and highly-productive plant-based in vitro translation system. BMC Biotechnol. 2014, 14, 37. [Google Scholar] [CrossRef]
- Kovtun, O.; Mureev, S.; Jung, W.; Kubala, M.H.; Johnston, W.; Alexandrov, K. Leishmania cell-free protein expression system. Methods 2011, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dasgupta, A.; Shen, L.; Bell-Pedersen, D.; Sachs, M.S. The cell free protein synthesis system from the model filamentous fungus Neurospora crassa. Methods 2018, 137, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.; Jewett, M.C. A combined cell-free transcription-translation system from Saccharomyces cerevisiae for rapid and robust protein synthe. Biotechnol. J. 2014, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Jaroentomeechai, T.; Stark, J.C.; Natarajan, A.; Glasscock, C.J.; Yates, L.E.; Hsu, K.J.; Mrksich, M.; Jewett, M.C.; DeLisa, M.P. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat. Commun. 2018, 9, 2686. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.W.; Des Soye, B.J.; Kwon, Y.-C.; Kay, J.; Davis, R.G.; Thomas, P.M.; Majewska, N.I.; Chen, C.X.; Marcum, R.D.; Weiss, M.G.; et al. Cell-free protein synthesis from genomically recoded bacteria enables multisite incorporation of noncanonical amino acids. Nat. Commun. 2018, 9, 1203. [Google Scholar] [CrossRef]
- Shinoda, T.; Shinya, N.; Ito, K.; Ishizuka-Katsura, Y.; Ohsawa, N.; Terada, T.; Hirata, K.; Kawano, Y.; Yamamoto, M.; Tomita, T.; et al. Cell-free methods to produce structurally intact mammalian membrane proteins. Sci. Rep. 2016, 6, 30442. [Google Scholar] [CrossRef] [PubMed]
- Focke, P.J.; Hein, C.; Hoffmann, B.; Matulef, K.; Bernhard, F.; Dötsch, V.; Valiyaveetil, F.I. Combining in Vitro Folding with Cell Free Protein Synthesis for Membrane Protein Expression. Biochemistry 2016, 55, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.; Kerruth, S.; Fitter, J.; Büldt, G.; Heberle, J.; Schlesinger, R.; Ataka, K. In-Situ Observation of Membrane Protein Folding during Cell-Free Expression. PLoS ONE 2016, 11, e0151051. [Google Scholar] [CrossRef]
- Fogeron, M.-L.; Badillo, A.; Penin, F.; Böckmann, A. Wheat Germ Cell-Free Overexpression for the Production of Membrane Proteins. Methods Mol. Biol. 2017, 1635, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Junge, F.; Haberstock, S.; Roos, C.; Stefer, S.; Proverbio, D.; Dötsch, V.; Bernhard, F. Advances in cell-free protein synthesis for the functional and structural analysis of membrane proteins. New Biotechnol. 2011, 28, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.; Noireaux, V. Quantitative modeling of transcription and translation of an all-E. coli cell-free system. Sci. Rep. 2019, 9, 11980. [Google Scholar] [CrossRef]
- Marshall, R.; Maxwell, C.S.; Collins, S.P.; Jacobsen, T.; Luo, M.L.; Begemann, M.B.; Gray, B.N.; January, E.; Singer, A.; He, Y.; et al. Rapid and Scalable Characterization of CRISPR Technologies Using an E. coli Cell-Free Transcription-Translation System. Mol. Cell 2018, 69, 146–157.e3. [Google Scholar] [CrossRef] [PubMed]
- Des Soye, B.J.; Gerbasi, V.R.; Thomas, P.M.; Kelleher, N.L.; Jewett, M.C. A Highly Productive, One-Pot Cell-Free Protein Synthesis Platform Based on Genomically Recoded Escherichia coli. Cell Chem. Biol. 2019, 26, 1743–1754.e9. [Google Scholar] [CrossRef]
- Vilkhovoy, M.; Horvath, N.; Shih, C.-H.; Wayman, J.A.; Calhoun, K.; Swartz, J.; Varner, J.D. Sequence Specific Modeling of E. coli Cell-Free Protein Synthesis. ACS Synth. Biol. 2018, 7, 1844–1857. [Google Scholar] [CrossRef]
- Hodgman, C.E.; Jewett, M.C. Optimized extract preparation methods and reaction conditions for improved yeast cell-free protein synthesis. Biotechnol. Bioeng. 2013, 110, 2643–2654. [Google Scholar] [CrossRef]
- Hodgman, C.E.; Jewett, M.C. Characterizing IGR IRES-mediated translation initiation for use in yeast cell-free protein synthesis. New Biotechnol. 2014, 31, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Hodgman, C.E.; Anderson, M.J.; Jewett, M.C. Evaluating fermentation effects on cell growth and crude extract metabolic activity for improved yeast cell-free protein synthesis. Biochem. Eng. J. 2014, 91, 140–148. [Google Scholar] [CrossRef]
- Miura, K.; Tachibana, M.; Takashima, E.; Morita, M.; Kanoi, B.N.; Nagaoka, H.; Baba, M.; Torii, M.; Ishino, T.; Tsuboi, T. Malaria transmission-blocking vaccines: Wheat germ cell-free technology can accelerate vaccine development. Expert Rev. Vaccines 2019, 18, 1017–1027. [Google Scholar] [CrossRef]
- Das Gupta, M.; Flaskamp, Y.; Roentgen, R.; Juergens, H.; Armero-Gimenez, J.; Albrecht, F.; Hemmerich, J.; Arfi, Z.A.; Neuser, J.; Spiegel, H. Scaling eukaryotic cell-free protein synthesis achieved with the versatile and high-yielding tobacco BY-2 cell lysate. Biotechnol. Bioeng. 2023, 120, 2890–2906. [Google Scholar] [CrossRef]
- Zemella, A.; Thoring, L.; Hoffmeister, C.; Kubick, S. Cell-Free Protein Synthesis: Pros and Cons of Prokaryotic and Eukaryotic Systems. Chembiochem 2015, 16, 2420–2431. [Google Scholar] [CrossRef] [PubMed]
- Kubick, S.; Schacherl, J.; Fleischer-Notter, H.; Royall, E.; Roberts, L.O.; Stiege, W. In Vitro Translation in an Insect-Based Cell-Free System. In Cell-Free Protein Expression; Springer: Berlin/Heidelberg, Germany, 2003; pp. 209–217. [Google Scholar]
- Thoring, L.; Dondapati, S.K.; Stech, M.; Wüstenhagen, D.A.; Kubick, S. High-yield production of “difficult-to-express” proteins in a continuous exchange cell-free system based on CHO cell lysates. Sci. Rep. 2017, 7, 11710. [Google Scholar] [CrossRef]
- Thoring, L.; Wüstenhagen, D.A.; Borowiak, M.; Stech, M.; Sonnabend, A.; Kubick, S. Cell-Free Systems Based on CHO Cell Lysates: Optimization Strategies, Synthesis of “Difficult-to-Express” Proteins and Future Perspectives. PLoS ONE 2016, 11, e0163670. [Google Scholar] [CrossRef] [PubMed]
- Brödel, A.K.; Sonnabend, A.; Roberts, L.O.; Stech, M.; Wüstenhagen, D.A.; Kubick, S. IRES-Mediated Translation of Membrane Proteins and Glycoproteins in Eukaryotic Cell-Free Systems. PLoS ONE 2013, 8, e82234. [Google Scholar] [CrossRef] [PubMed]
- Burgenson, D.; Linton, J.; Ge, X.; Kostov, Y.; Tolosa, L.; Szeto, G.L.; Rao, G. A Cell-Free Protein Expression System Derived from Human Primary Peripheral Blood Mononuclear Cells. ACS Synth. Biol. 2020, 9, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.P.; Lu, Y.; He, X.-S.; Greenberg, H.B.; Swartz, J.R. Cell-free production of trimeric influenza hemagglutinin head domain proteins as vaccine antigens. Biotechnol. Bioeng. 2012, 109, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Welsh, J.P.; Swartz, J.R. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc. Natl. Acad. Sci. USA 2014, 111, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Lei, S.; Yuan, L.; Feng, X. Cell-free protein synthesis of norovirus virus-like particles. RSC Adv. 2017, 7, 28837–28840. [Google Scholar] [CrossRef]
- Brito, L.A.; Singh, M. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J. Pharm. Sci. 2011, 100, 34–37. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Matsuyama, S.; Fukushi, S.; Matsunaga, S.; Matsushima, Y.; Kuroyama, H.; Kimura, H.; Takeda, M.; Chimuro, T.; Ryo, A. Development of Monoclonal Antibody and Diagnostic Test for Middle East Respiratory Syndrome Coronavirus Using Cell-Free Synthesized Nucleocapsid Antigen. Front. Microbiol. 2016, 7, 509. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2016.00509 (accessed on 5 June 2024). [CrossRef] [PubMed]
- Spice, A.J.; Aw, R.; Bracewell, D.G.; Polizzi, K.M. Synthesis and Assembly of Hepatitis B Virus-Like Particles in a Pichia pastoris Cell-Free System. Front. Bioeng. Biotechnol. 2020, 8, 72. Available online: https://www.frontiersin.org/article/10.3389/fbioe.2020.00072 (accessed on 5 June 2024). [CrossRef] [PubMed]
- Yamaoka, Y.; Jeremiah, S.S.; Funabashi, R.; Miyakawa, K.; Morita, T.; Mihana, Y.; Kato, H.; Ryo, A. Characterization and Utilization of Disulfide-Bonded SARS-CoV-2 Receptor Binding Domain of Spike Protein Synthesized by Wheat Germ Cell-Free Production System. Viruses 2022, 14, 1461. [Google Scholar] [CrossRef]
- Takai, K.; Sawasaki, T.; Endo, Y. The wheat-germ cell-free expression system. Curr. Pharm. Biotechnol. 2010, 11, 272–278. [Google Scholar] [CrossRef]
- Kaur, J.; Kumar, A.; Kaur, J. Strategies for optimization of heterologous protein expression in E. coli: Roadblocks and reinforcements. Int. J. Biol. Macromol. 2018, 106, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Dondapati, S.K.; Wüstenhagen, D.A.; Kubick, S. Functional Analysis of Membrane Proteins Produced by Cell-Free Translation. In Protein Engineering: Methods and Protocols; Bornscheuer, U.T., Höhne, M., Eds.; Springer: New York, NY, USA, 2018; pp. 171–186. [Google Scholar]
- Venkat, S.; Chen, H.; Gan, Q.; Fan, C. The Application of Cell-Free Protein Synthesis in Genetic Code Expansion for Post-translational Modifications. Front. Pharmacol. 2019, 10, 248. Available online: https://www.frontiersin.org/article/10.3389/fphar.2019.00248 (accessed on 5 June 2024). [CrossRef] [PubMed]
- Karim, A.S.; Jewett, M.C. Chapter Two—Cell-Free Synthetic Biology for Pathway Prototyping. In Enzymes in Synthetic Biology; Scrutton, N., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 608, pp. 31–57. [Google Scholar]
- Bundy, B.C.; Hunt, J.P.; Jewett, M.C.; Swartz, J.R.; Wood, D.W.; Frey, D.D.; Rao, G. Cell-free biomanufacturing. Curr. Opin. Chem. Eng. 2018, 22, 177–183. [Google Scholar] [CrossRef]
- Lu, Y. Cell-free synthetic biology: Engineering in an open world. Synth. Syst. Biotechnol. 2017, 2, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Adiga, R.; Al-Adhami, M.; Andar, A.; Borhani, S.; Brown, S.; Burgenson, D.; Cooper, M.A.; Deldari, S.; Frey, D.D.; Ge, X.; et al. Point-of-care production of therapeutic proteins of good-manufacturing-practice quality. Nat. Biomed. Eng. 2018, 2, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Maghodia, A.B.; Geisler, C.; Jarvis, D.L. Characterization of an Sf-rhabdovirus-negative Spodoptera frugiperda cell line as an alternative host for recombinant protein production in the baculovirus-insect cell system. Protein Expr. Purif. 2016, 122, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sonnabend, A.; Spahn, V.; Stech, M.; Zemella, A.; Stein, C.; Kubick, S. Production of G protein-coupled receptors in an insect-based cell-free system. Biotechnol. Bioeng. 2017, 114, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Stech, M.; Merk, H.; Schenk, J.A.; Stöcklein, W.F.M.; Wüstenhagen, D.A.; Micheel, B.; Duschl, C.; Bier, F.F.; Kubick, S. Production of functional antibody fragments in a vesicle-based eukaryotic cell-free translation system. J. Biotechnol. 2012, 164, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Merk, H.; Gless, C.; Maertens, B.; Gerrits, M.; Stiege, W. Cell-free synthesis of functional and endotoxin-free antibody Fab fragments by translocation into microsomes. Biotechniques 2012, 53, 153–160. [Google Scholar] [CrossRef]
- Lai, T.; Yang, Y.; Ng, S. Advances in Mammalian Cell Line Development Technologies for Recombinant Protein Production. Pharmaceuticals 2013, 6, 579–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.-G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef]
- Brödel, A.K.; Sonnabend, A.; Kubick, S. Cell-free protein expression based on extracts from CHO cells. Biotechnol. Bioeng. 2014, 111, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Sachse, R.; Dondapati, S.K.; Fenz, S.F.; Schmidt, T.; Kubick, S. Membrane protein synthesis in cell-free systems: From bio-mimetic systems to bio-membranes. FEBS Lett. 2014, 588, 2774–2781. [Google Scholar] [CrossRef]
- Cole, S.D.; Beabout, K.; Turner, K.B.; Smith, Z.K.; Funk, V.L.; Harbaugh, S.V.; Liem, A.T.; Roth, P.A.; Geier, B.A.; Emanuel, P.A.; et al. Quantification of Interlaboratory Cell-Free Protein Synthesis Variability. ACS Synth. Biol. 2019, 8, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Chizzolini, F.; Forlin, M.; Yeh Martín, N.; Berloffa, G.; Cecchi, D.; Mansy, S.S. Cell-Free Translation Is More Variable than Transcription. ACS Synth. Biol. 2017, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Dopp, J.L.; Reuel, N.F. Process optimization for scalable E. coli extract preparation for cell-free protein synthesis. Biochem. Eng. J. 2018, 138, 21–28. [Google Scholar] [CrossRef]
- Dopp, J.L.; Jo, Y.R.; Reuel, N.F. Methods to reduce variability in E. Coli-based cell-free protein expression experiments. Synth. Syst. Biotechnol. 2019, 4, 204–211. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Bai, L.; Fan, J.; Liu, E. Expression systems and species used for transgenic animal bioreactors. Biomed Res. Int. 2013, 2013, 580463. [Google Scholar] [CrossRef] [PubMed]
- Shepelev, M.V.; Kalinichenko, S.V.; Deykin, A.V.; Korobko, I.V. Production of Recombinant Proteins in the Milk of Transgenic Animals: Current State and Prospects. Acta Naturae 2018, 10, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Pollock, D.P.; Kutzko, J.P.; Birck-Wilson, E.; Williams, J.L.; Echelard, Y.; Meade, H.M. Transgenic milk as a method for the production of recombinant antibodies. J. Immunol. Methods 1999, 231, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Meade, H.M.; Echelard, Y.; Ziomek, C.A.; Young, M.W.; Harvey, M.; Cole, E.S.; Groet, S.; Smith, T.E.; Curling, J.M. Expression of Recombinant Proteins in the Milk of Transgenic Animals. Gene Expr. Syst. 1999, 10, 399–427. [Google Scholar] [CrossRef]
- Lillico, S.G.; Sherman, A.; McGrew, M.J.; Robertson, C.D.; Smith, J.; Haslam, C.; Barnard, P.; Radcliffe, P.A.; Mitrophanous, K.A.; Elliot, E.A. Oviduct-specific expression of two therapeutic proteins in transgenic hens. Proc. Natl. Acad. Sci. USA 2007, 104, 1771–1776. [Google Scholar] [CrossRef]
- Kerr, D.E.; Liang, F.; Bondioli, K.R.; Zhao, H.; Kreibich, G.; Wall, R.J.; Sun, T.-T. The bladder as a bioreactor: Urothelium production and secretion of growth hormone into urine. Nat. Biotechnol. 1998, 16, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Dyck, M.K.; Lacroix, D.; Pothier, F.; Sirard, M.-A. Making recombinant proteins in animals–different systems, different applications. TRENDS Biotechnol. 2003, 21, 394–399. [Google Scholar] [CrossRef] [PubMed]
- CHRENEK, P.P.; Makarevich, A.V.; Pivko, J.; Bulla, J. Transgenic farm animal production and application. Slovak J. Anim. Sci. 2010, 43, 45–49. [Google Scholar]
- Chen, H.-L.; Huang, J.-Y.; Chu, T.-W.; Tsai, T.-C.; Hung, C.-M.; Lin, C.-C.; Liu, F.-C.; Wang, L.-C.; Chen, Y.-J.; Lin, M.-F.; et al. Expression of VP1 protein in the milk of transgenic mice: A potential oral vaccine protects against enterovirus 71 infection. Vaccine 2008, 26, 2882–2889. [Google Scholar] [CrossRef] [PubMed]
- Nakade, S.; Tsubota, T.; Sakane, Y.; Kume, S.; Sakamoto, N.; Obara, M.; Daimon, T.; Sezutsu, H.; Yamamoto, T.; Sakuma, T. Microhomology-mediated end-joining-dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9. Nat. Commun. 2014, 5, 5560. [Google Scholar] [CrossRef]
- Cristea, S.; Freyvert, Y.; Santiago, Y.; Holmes, M.C.; Urnov, F.D.; Gregory, P.D.; Cost, G.J. In vivo cleavage of transgene donors promotes nuclease-mediated targeted integration. Biotechnol. Bioeng. 2013, 110, 871–880. [Google Scholar] [CrossRef]
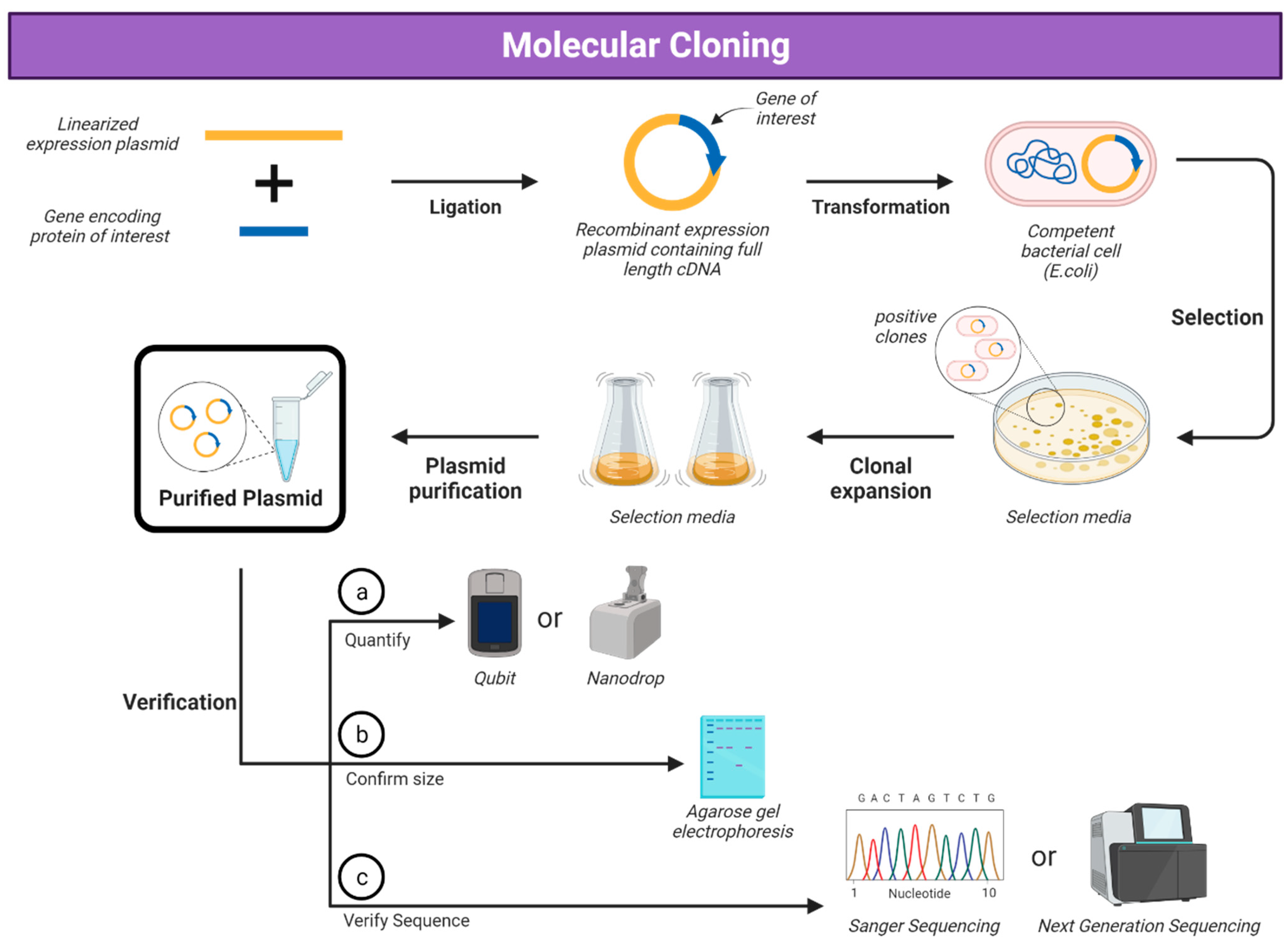
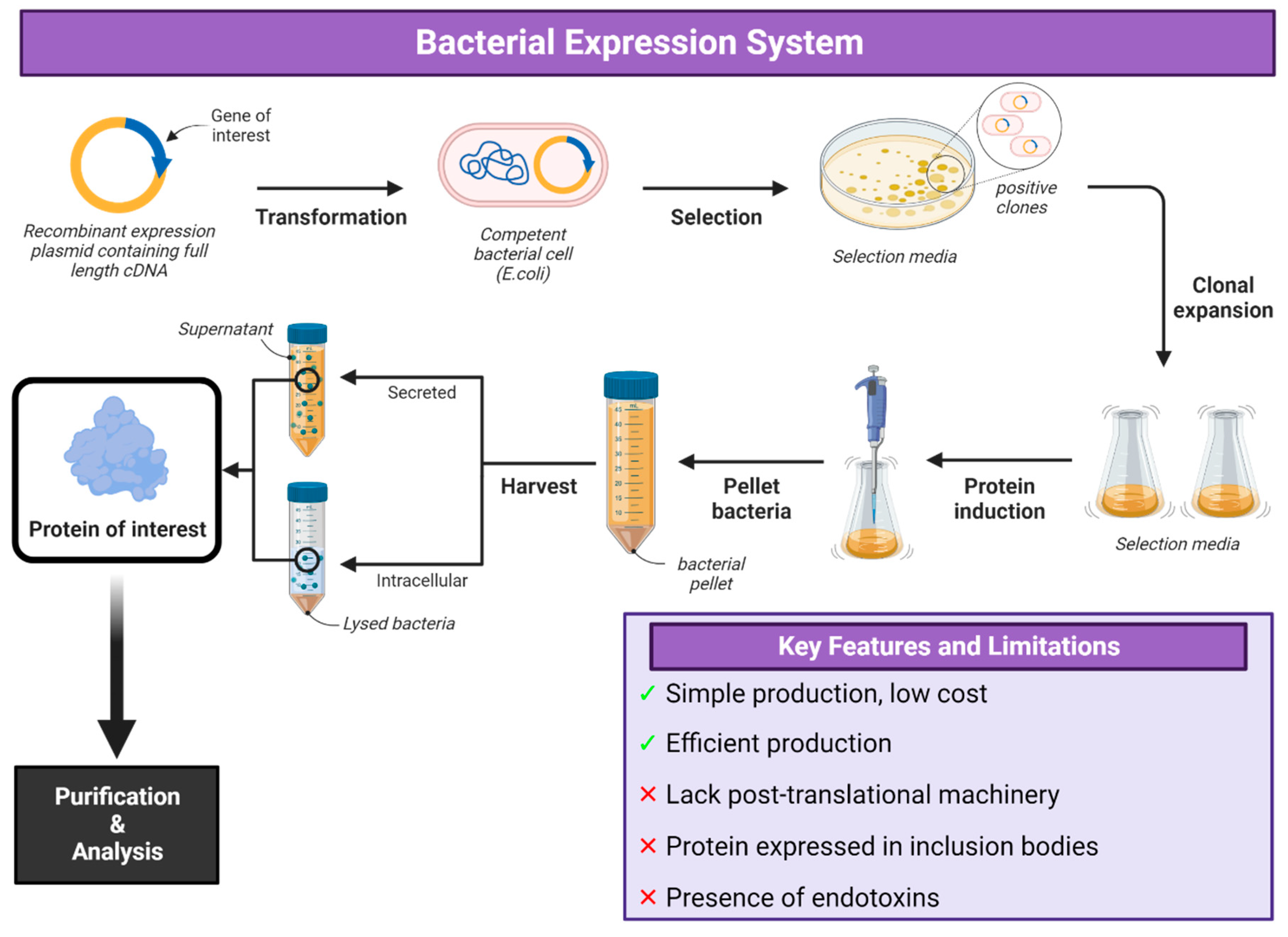


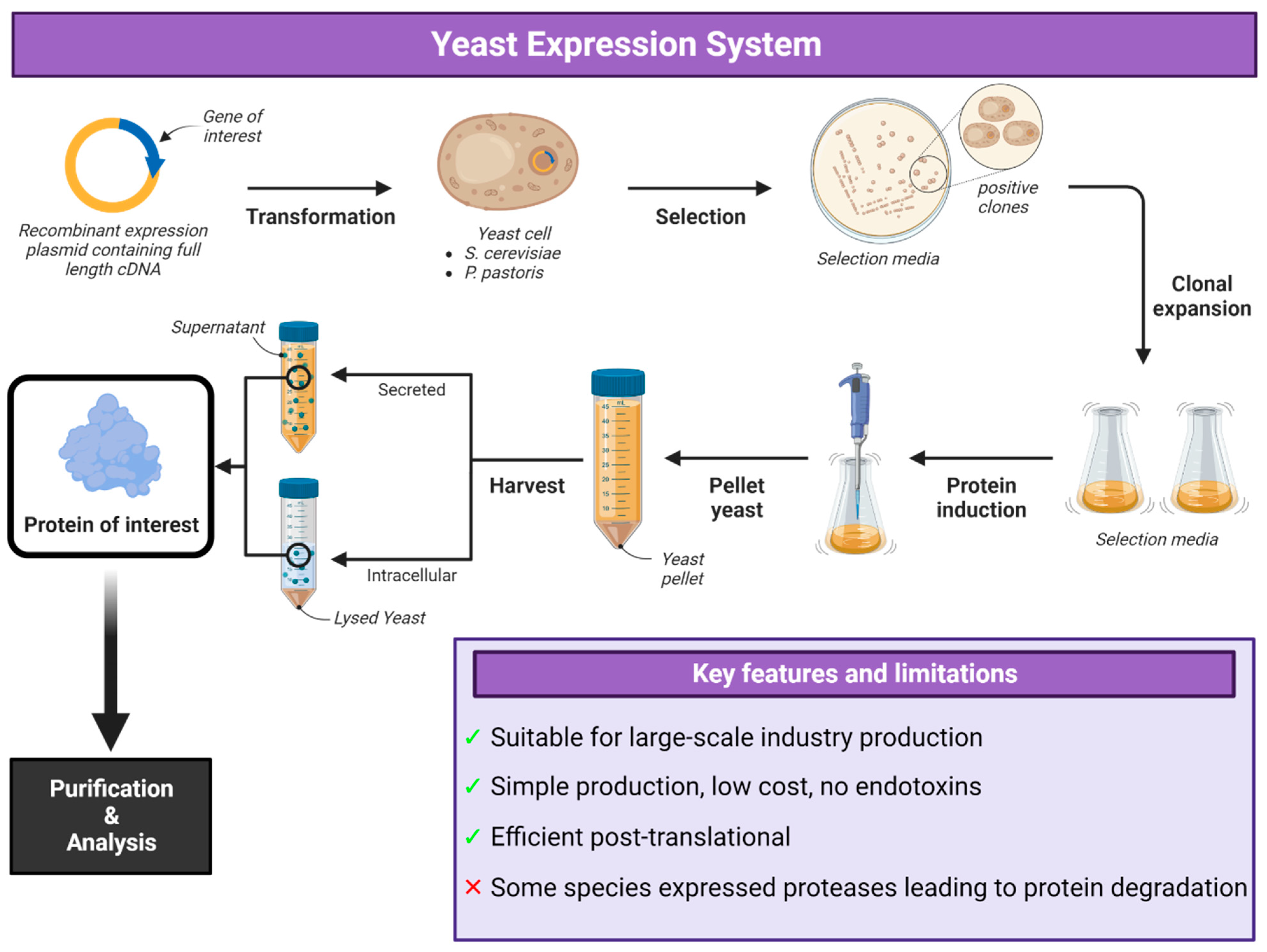
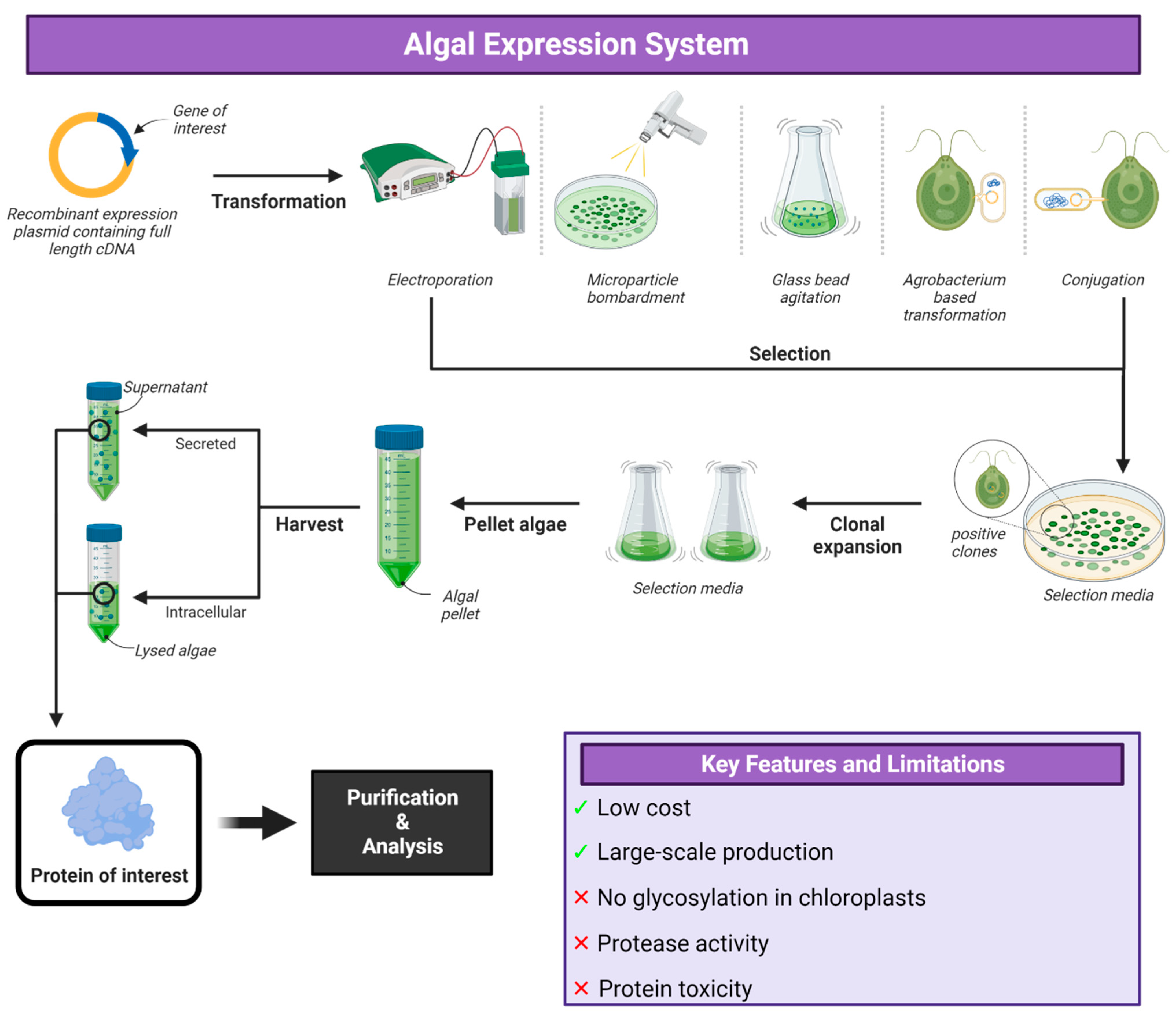
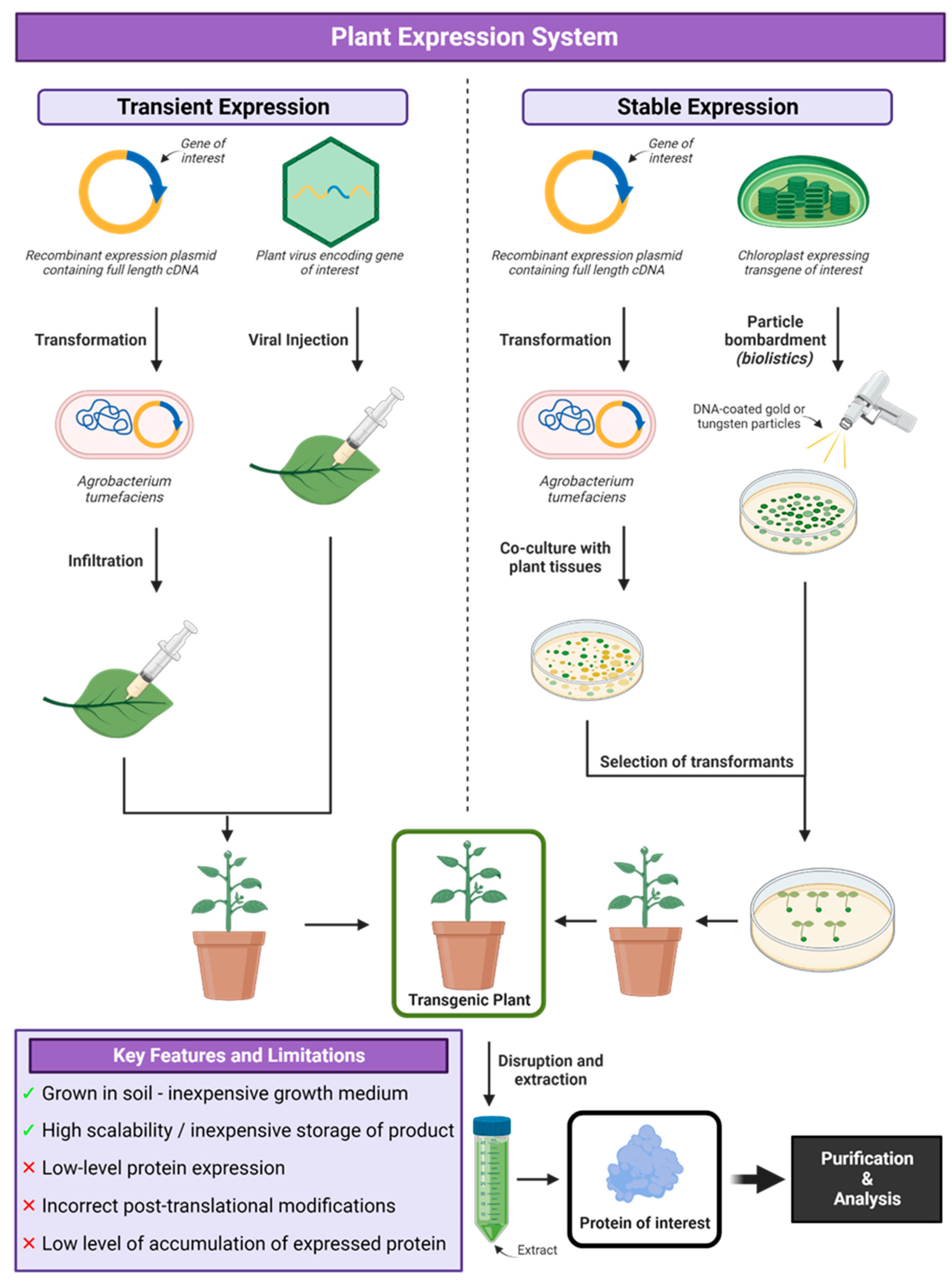
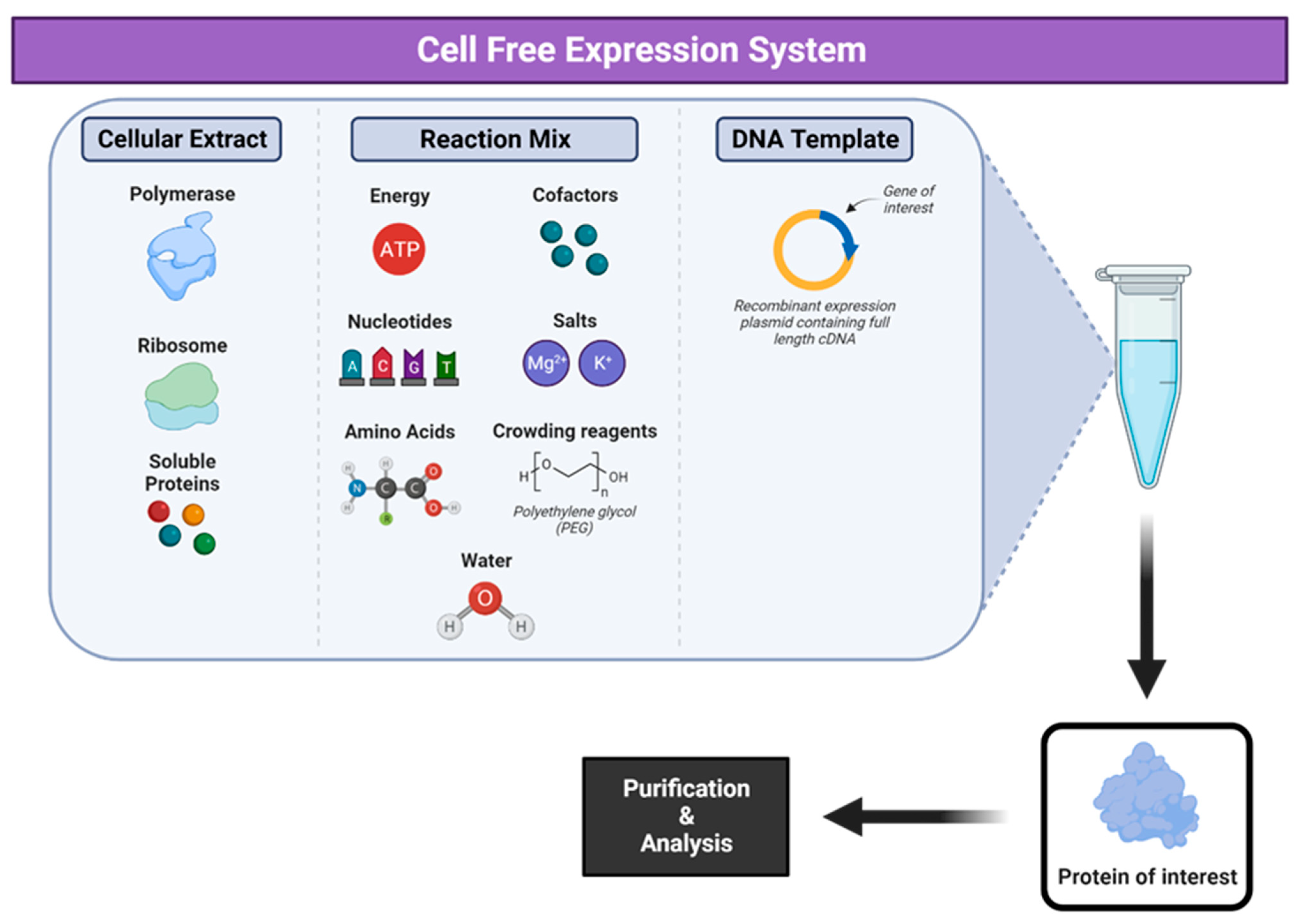
| Virus | Antigen | Findings | Reference |
|---|---|---|---|
| Foot and mouth disease virus | VP0, VP1 and VP3 VLPs | Demonstrated protection in guinea pigs, swine and cattle. Local mucosal and systemic immune responses in mice via IN immunization. | [43,44,45,46] |
| VP1 | Protected mice and pigs from challenge. | [47] | |
| Influenza A virus | ACAM-FLU-A | Phase I clinical trials. | [48,49,50] |
| Porcine circovirus type 2 | PCV2-Cap VLP | Experimental vaccine demonstrating efficacy in piglets. | [51] |
| Porcine parvovirus | PPV-VP2 VLP | Demonstrated efficacy in piglets. | [51] |
| SARS-CoV-2 | Dual-adjuvanted RBD | Demonstrated robust neutralizing antibody response in mice. | [52] |
| Virus | Vaccine | Antigen | Manufacturer | Reference |
|---|---|---|---|---|
| Classical swine fever virus | Porcilis® Pesti | E2 | MSD Animal Health (Rahway, NJ, USA) | [90] |
| BAYOVAC CSF E2® | E2 | Bayer/Pfizer Animal Health (Leverkusen, Germany) | ||
| Human papillomavirus | Cevarix® | L1 | GlaxoSmithKline (Mississauga, ON, Canada) | [91] |
| Influenza A virus | Flublok® | HA | Protein Sciences Corporation (Meriden, CT, USA) | [92] |
| Porcine circovirus type 2 | Circumvent® PCV | G2 | Merck Animal Health (Rahway, NJ, USA) | [93] |
| Ingelvac CircoFLEX® | ORF2 | Boehringer Ingelheim Vetmedica (Duluth, GA, USA) | [94] | |
| Porcilis® PCV | ORF2 | MSD Animal Health (Rahway, NJ, USA) | [95] |
| Virus | Antigen | Application | Reference |
|---|---|---|---|
| Chikungunya virus | CHIKV-S27 structural polyprotein (C, E3, E2, 6K, E1) | Experimental vaccine preventing viremia and inflammation in mice | [96] |
| Chikungunya virus | E1 and E2 proteins | Experimental vaccine demonstrating protection in mice | [97] |
| Classical swine fever virus | E2 protein | Experimental vaccine demonstrating protective immunity in pigs | [98] |
| Ebolavirus | GP and VP40 VLPs | Experimental vaccine demonstrating protection in guinea pigs | [99] |
| Epizootic hemorrhagic disease virus | VP2 protein | Experimental vaccine preventing clinical disease or viremia in deer | [100] |
| Influenza A virus | HA, NA and M1 VLPs | Experimental vaccine demonstrating protection in pigs against pH1N1 and in chickens against H6N1 | [101,102] |
| Lassa virus | Glycoprotein (GP) | Experimental vaccine eliciting high antibody titres in mice | [88] |
| Rift Valley fever virus | Gn and Gc Glycoproteins | Experimental vaccine demonstrating complete protection in sheep | [103,104] |
| SARS-CoV-2 | RBD | Experimental vaccine demonstrating protection in NHPs | [105,106] |
| Zika virus | E80 and EDIII proteins | Experimental vaccine demonstrating protection in mice | [89] |
| Virus | Vaccine | Cell Line | Antigen | Manufacturer | Reference |
|---|---|---|---|---|---|
| Classical swine fever | Porvac® | HEK 293 | E2-CD154 | The Centre for Genetic Engineering and Biotechnology (CIGB) (Havana, Cuba) | [139] |
| Dengue virus | Dengvaxia® | Vero | preM and E | Sanofi Pasteur (Val de Reuil France) | [13] |
| Influenza A (H1N1) 2009 | Celvapan® | Vero | Whole virion | Baxter (Orth an der Donau Austria) | [140,141] |
| Influenza A (trivalent) | Flucelvax® | MDCK | HA and NA | Novartis Vaccines and Diagnostics (Basel, Switzerland) | [12,142] |
| Influenza A (trivalent) | Preflucel® | Vero | HA | Baxter (Orth an der Donau Austria) | [11] |
| Influenza H5N1 | Celvapan® | Vero | Whole virion | Baxter (Orth an der Donau Austria) | [140,141] |
| Respiratory syncytial virus | AREXEVY® | CHO | RSVPreF | GlaxoSmithKline (London, UK) | [143,144,145,146,147] |
| Respiratory syncytial virus | ABRYSVO® | CHO | RSVPreF | Pfizer (New York, NY, USA) | [148,149,150,151] |
| Virus | Antigen | Cell Line | Application | Reference |
|---|---|---|---|---|
| Classical swine fever virus | E2 Protein | CHO | Experimental mucosal vaccine demonstrating protection in pigs. | [152] |
| Hendra virus | sGHeV | HeLa | Experimental vaccine demonstrating protective efficacy in African green monkeys and cats. | [153,154,155] |
| Hendra virus | sGHeV | 293F | Experimental vaccine demonstrating protective efficacy in ferrets and horses. | [156,157,158] |
| SARS-CoV-2 | RBD-nanoparticle | HEK-293 | Experimental vaccine eliciting strong neutralizing antibody responses and rapid viral clearance in nasal washes. | [159] |
| SARS-CoV-2 | Pan-HLA-DR mAb fused to RBD | HEK-293 | Experimental vaccine demonstrating robust protection, strong neutralizing antibody responses and viral clearance in ferret nasal washes. | [160] |
| Yeast Species | Virus | Antigen | Reference |
|---|---|---|---|
| H. polymorpha | Papillomavirus | L1 protein | [188] |
| Hepatitis B virus | VrHB-IB | [189] | |
| Porcine circovirus type 2 | PCV2b capsid protein | [190] | |
| P. pastoris | Dengue virus | Envelope glycoproteins | [191,192,193,194,195] |
| Hand, foot and mouth disease (HFMD) | P1 and 3CD proteins | [196] | |
| Hepatitis B virus | HBsAg | [197] | |
| Hepatitis C virus | HCV Core protein | [198] | |
| Papillomavirus | HPV 16L1, 18L1 | [199,200] | |
| Influenza virus | Hemagglutinin protein | [201,202,203] | |
| Classical swine fever virus | E2 glycoprotein | [204,205] | |
| Chikungunya virus | Structural protein VLPs | [206] | |
| SARS-CoV-2 | RBD monomers and dimers | [207] | |
| S. cerevisiae | Papillomavirus | VLPs of hrHPV16 and 18, and lrHPV6 and 11—Gardasil® | [187] |
| HPV16 | [208] | ||
| Hepatitis B virus | Hepatitis B surface antigen (HBsAg)—Recombivax® | [209,210] | |
| HBV X, S and C antigens | [211,212] | ||
| Surface protein GS-4774 | [213] | ||
| Enterovirus 71 (hand, foot and mouth disease) | EV71 Structural antigens | [214,215] | |
| Parvovirus B19 | VP | [216,217] | |
| Dengue virus | Dengue envelope domain III | [218] | |
| Human immunodeficiency virus-1 | Envelope glycoprotein | [219] |
| Plant | Virus | Antigen | Application | Reference |
|---|---|---|---|---|
| Nicotiana benthamiana | Influenza | H1 and H5 VLPs | Experimental vaccine eliciting strong humoral and long-term cell-mediated responses in adults. | [270] |
| Influenza | H7N9 VLP | Experimental vaccine demonstrating strong antibody response in mice and ferrets. | [271] | |
| Influenza | HA subunit | Experimental vaccine demonstrating immunogenicity in mice, rabbits and ferrets. | [272] | |
| Influenza | H6 VLP | Experimental vaccine demonstrating reduced viral shedding in chickens. | [273] | |
| Infectious bronchitis virus | S protein VLP | Experimental vaccine demonstrating immunogenicity in chickens. | [274] | |
| SARS-CoV-2 | RBD | Experimental vaccine shown to induce neutralizing antibodies in mice and NHPs. | [275] |
| Cellular Expression | Cell-Free Expression | References | |
|---|---|---|---|
| Time | 1–2 weeks | 24–72 h | [308,344] |
| Protein Toxicity | Often a major issue | High tolerance for toxic proteins | [289,359] |
| Membrane Proteins | Overexpression can cause cytotoxicity and cell death | Variety of different sizes can be produced depending on lysate source | [331,332,360] |
| Protein Yield | High yields (mg/mL) | Yield varies depending on the protein from µg/mL to mg/mL | [288,304] |
| Post-translational Modifications | All possible depending on the system. | Mainly in eukaryotic CF systems with translationally active microsomes. Limited in prokaryotic and eukaryotic lysates without endogenous microsomes. O-linked glycosylation does not occur. | [302,361] |
| Scalability | Minimum volume of 5 mL to several litres. | Range from 5 µL (chip-based, Eppendorf tube) to 100 L (fermenter, commercial bioreactor) | [362,363] |
| Flexibility and Manipulation | Closed system, difficult to manipulate | Open system, easy to manipulate reaction conditions, no cell membrane constraints. | [364] |
| Point-of-care protein production | Difficult due to time-consuming process, infrastructural facilities and cold storage. | Lyophilization allows for removal of cold-chain and on-site production without major facilities. | [298,365] |
| Applications | Cells need to be lysed for membrane protein applications. | Simple as protein can be purified and reconstituted immediately post-synthesis. | [288,364] |
| Acceptance | Reliable, current standard for protein production approved by regulatory authorities. | Currently limited to research at laboratory level with few, limited commercial applications | [289,304,344] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sookhoo, J.R.V.; Schiffman, Z.; Ambagala, A.; Kobasa, D.; Pardee, K.; Babiuk, S. Protein Expression Platforms and the Challenges of Viral Antigen Production. Vaccines 2024, 12, 1344. https://doi.org/10.3390/vaccines12121344
Sookhoo JRV, Schiffman Z, Ambagala A, Kobasa D, Pardee K, Babiuk S. Protein Expression Platforms and the Challenges of Viral Antigen Production. Vaccines. 2024; 12(12):1344. https://doi.org/10.3390/vaccines12121344
Chicago/Turabian StyleSookhoo, Jamie R. V., Zachary Schiffman, Aruna Ambagala, Darwyn Kobasa, Keith Pardee, and Shawn Babiuk. 2024. "Protein Expression Platforms and the Challenges of Viral Antigen Production" Vaccines 12, no. 12: 1344. https://doi.org/10.3390/vaccines12121344
APA StyleSookhoo, J. R. V., Schiffman, Z., Ambagala, A., Kobasa, D., Pardee, K., & Babiuk, S. (2024). Protein Expression Platforms and the Challenges of Viral Antigen Production. Vaccines, 12(12), 1344. https://doi.org/10.3390/vaccines12121344







