Respiratory Syncytial Virus: A WAidid Consensus Document on New Preventive Options
Abstract
1. Introduction
2. Virology and Epidemiology of RSV
2.1. Basic Virology and Main Antigens of RSV
2.2. Epidemiology of RSV
2.2.1. Burden of RSV in Infants and Children
2.2.2. Burden of RSV in Adults and Elderly
2.2.3. RSV Hospitalizations in Adults
2.2.4. Admission to ICU and CFR Estimates in Adults
2.2.5. Seasonal Pattern
2.3. The Impact of the COVID-19 Pandemic
2.4. Economic Burden of RSV
3. Preventative Options
3.1. Monoclonal Antibodies
3.2. Vaccines
- (1)
- (2)
- one mRNA formulate: mRNA-1345, from Moderna Inc (mRESVIATM; Moderna Inc., Cambridge, Massachusetts, USA) [233];
- (3)
- two vector-based formulates: adenovirus-based Ad26.RSV.preF from Janssen [234,235,236] that, despite promising results, was temporarily halted in midst of late-stage clinical trials, being only recently relaunched [236], and the poxvirus-vectored vaccine MVA-BN-RSV from Bavarian Nordic A/S (Kvistgård, Denmark), that failed in phase 3, being ultimately discontinued.
3.2.1. Adults
3.2.2. Maternal Vaccination
3.3. Immunization Strategies
3.3.1. Vaccines for Adults
3.3.2. Immunization Strategies for Infants and Small Children
3.3.3. Timing of Immunization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Feng, Z.; Xu, L.; Xie, Z. Receptors for Respiratory Syncytial Virus Infection and Host Factors Regulating the Life Cycle of Respiratory Syncytial Virus. Front. Cell Infect. Microbiol. 2022, 12, 858629. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory Syncytial Virus: Virology, Reverse Genetics, and Pathogenesis of Disease. Curr. Top. Microbiol. Immunol. 2013, 372, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Mousa, J.J. Structural Basis for Respiratory Syncytial Virus and Human Metapneumovirus Neutralization. Curr. Opin. Virol. 2023, 61, 101337. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021, 222, S577–S583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Del Riccio, M.; Spreeuwenberg, P.; Osei-Yeboah, R.; Johannesen, C.K.; Fernandez, L.V.; Teirlinck, A.C.; Wang, X.; Heikkinen, T.; Bangert, M.; Caini, S.; et al. Burden of Respiratory Syncytial Virus in the European Union: Estimation of RSV-Associated Hospitalizations in Children under 5 Years. J. Infect. Dis. 2023, 228, 1528–1538. [Google Scholar] [CrossRef]
- Osei-Yeboah, R.; Spreeuwenberg, P.; Del Riccio, M.; Fischer, T.K.; Egeskov-Cavling, A.M.; Bøås, H.; van Boven, M.; Wang, X.; Lehtonen, T.; Bangert, M.; et al. Estimation of the Number of Respiratory Syncytial Virus–Associated Hospitalizations in Adults in the European Union. J. Infect. Dis. 2023, 228, 1539–1548. [Google Scholar] [CrossRef]
- Gómez, J.A.; Cintra, O.; Berzanskis, A.; Pacheco, S.; Jaswantlal, H.; El Hasnaoui, A.; van Oorschot, D.A.M.; Guzman-Holst, A. Burden of Disease Due to Respiratory Syncytial Virus in Adults in Five Middle-Income Countries. Infect. Dis. Rep. 2024, 16, 750–762. [Google Scholar] [CrossRef]
- Deng, S.; Cong, B.; Edgoose, M.; De Wit, F.; Nair, H.; Li, Y. Risk Factors for Respiratory Syncytial Virus-Associated Acute Lower Respiratory Infection in Children under Five Years: An Updated Systematic Review and Meta–Analysis. Int. J. Infect. Dis. 2024, 146, 107125. [Google Scholar] [CrossRef]
- Staadegaard, L.; Caini, S.; Wangchuk, S.; Thapa, B.; De Almeida, W.A.F.; De Carvalho, F.C.; Njouom, R.; Fasce, R.A.; Bustos, P.; Kyncl, J.; et al. The Global Epidemiology of RSV in Community and Hospitalized Care: Findings from 15 Countries. Open Forum Infect. Dis. 2021, 8, ofab159. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, C.K.; van Wijhe, M.; Tong, S.; Fernández, L.V.; Heikkinen, T.; van Boven, M.; Wang, X.; Bøås, H.; Li, Y.; Campbell, H.; et al. Age-Specific Estimates of Respiratory Syncytial Virus-Associated Hospitalizations in 6 European Countries: A Time Series Analysis. J. Infect. Dis. 2022, 226, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Guiñazú, G.; Dvorkin, J.; Mahmud, S.; Baral, R.; Pecenka, C.; Libster, R.; Clark, A.; Caballero, M.T. Evaluation of the Potential Impact and Cost-Effectiveness of Respiratory Syncytial Virus (RSV) Prevention Strategies for Infants in Argentina. Vaccine 2024, 42, 126234. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Willem, L.; Johannesen, C.K.; Urchueguía-Fornes, A.; Lehtonen, T.; Osei-Yeboah, R.; Salo, H.; Orrico-Sánchez, A.; Diez-Domingo, J.; Jit, M.; et al. Influential Drivers of the Cost-Effectiveness of Respiratory Syncytial Virus Vaccination in European Older Adults: A Multi-Country Analysis. medRxiv 2024. [Google Scholar] [CrossRef]
- Hampp, C.; Kauf, T.L.; Saidi, A.S.; Winterstein, A.G. Cost-Effectiveness of Respiratory Syncytial Virus Prophylaxis in Various Indications. Arch. Pediatr. Adolesc. Med. 2011, 165, 498–505. [Google Scholar] [CrossRef]
- Mac, S.; Sumner, A.; Duchesne-Belanger, S. Cost-Effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019, 143, 20184064. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Bozdemir, S.E.; Yegenoglu, S.; Celebi, S.; McIntosh, E.D.; Unal, S.; Postma, M.J.; Hacimustafaoglu, M. Potential Cost-Effectiveness of RSV Vaccination of Infants and Pregnant Women in Turkey: An Illustration Based on Bursa Data. PLoS ONE 2016, 11, e0163567. [Google Scholar] [CrossRef]
- Laufer, R.S.; Driscoll, A.J.; Baral, R.; Buchwald, A.G.; Campbell, J.D.; Coulibaly, F.; Diallo, F.; Doumbia, M.; Galvani, A.P.; Haidara, F.C.; et al. Cost-Effectiveness of Infant Respiratory Syncytial Virus Preventive Interventions in Mali: A Modeling Study to Inform Policy and Investment Decisions. Vaccine 2021, 39, 5037–5045. [Google Scholar] [CrossRef]
- Cromer, D.; Jan Van Hoek, A.; Newall, A.T.; Pollard, A.J.; Jit, M. Burden of Paediatric Respiratory Syncytial Virus Disease and Potential Effect of Different Immunisation Strategies: A Modelling and Cost-Effectiveness Analysis for England. Lancet Public Health 2017, 2, e367–e374. [Google Scholar] [CrossRef]
- Palmer, L.; Hall, C.B.; Katkin, J.P.; Shi, N.; Masaquel, A.S.; McLaurin, K.K.; Mahadevia, P.J. Healthcare Costs within a Year of Respiratory Syncytial Virus among Medicaid Infants. Pediatr. Pulmonol. 2010, 45, 772–781. [Google Scholar] [CrossRef]
- McLaurin, K.K.; Farr, A.M.; Wade, S.W.; Diakun, D.R.; Stewart, D.L. Respiratory Syncytial Virus Hospitalization Outcomes and Costs of Full-Term and Preterm Infants. J. Perinatol. 2016, 36, 990–996. [Google Scholar] [CrossRef] [PubMed]
- El-Bietar, J.; Nelson, A.; Wallace, G.; Dandoy, C.; Jodele, S.; Myers, K.C.; Teusink, A.; Lane, A.; Davies, S.M.; Danziger-Isakov, L. RSV Infection without Ribavirin Treatment in Pediatric Hematopoietic Stem Cell Transplantation. Bone Marrow Transpl. 2016, 51, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Molinos-Quintana, A.; Pérez-De Soto, C.; Gómez-Rosa, M.; Pérez-Simón, J.A.; Pérez-Hurtado, J.M. Intravenous Ribavirin for Respiratory Syncytial Viral Infections in Pediatric Hematopoietic SCT Recipients. Bone Marrow Transpl. 2013, 48, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Foolad, F.; Aitken, S.L.; Shigle, T.L.; Prayag, A.; Ghantoji, S.; Ariza-Heredia, E.; Chemaly, R.F. Oral versus Aerosolized Ribavirin for the Treatment of Respiratory Syncytial Virus Infections in Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 2019, 68, 1641–1649. [Google Scholar] [CrossRef]
- Viguria, N.; Navascués, A.; Juanbeltz, R.; Echeverría, A.; Ezpeleta, C.; Castilla, J. Effectiveness of Palivizumab in Preventing Respiratory Syncytial Virus Infection in High-Risk Children. Hum. Vaccin. Immunother. 2021, 17, 1867–1872. [Google Scholar] [CrossRef]
- Baraldi, E.; Checcucci Lisi, G.; Costantino, C.; Heinrichs, J.H.; Manzoni, P.; Riccò, M.; Roberts, M.; Vassilouthis, N. RSV Disease in Infants and Young Children: Can We See a Brighter Future? Hum. Vaccin. Immunother. 2022, 18, 2079322. [Google Scholar] [CrossRef]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simões, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef]
- Riccò, M.; Cascio, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Gianluca, P.; Gori, D.; Manzoni, P. Efficacy of Respiratory Syncytial Virus Vaccination to Prevent Lower Respiratory Tract Illness in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines 2024, 12, 500. [Google Scholar] [CrossRef]
- Riccò, M.; Cascio, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Giuri, P.G.; Gori, D.; Manzoni, P. Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024). Vaccines 2024, 12, 640. [Google Scholar] [CrossRef]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef]
- Sullender, W.M. Respiratory Syncytial Virus Genetic and Antigenic Diversity. Clin. Microbiol. Rev. 2000, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, P.; Chin, A.A.; Tan, S.; Jeon, A.H.; Ackerley, C.A.; Siu, K.K.; Lee, J.E.; Hegele, R.G. Identification of Rsv Fusion Protein Interaction Domains on the Virus Receptor, Nucleolin. Viruses 2021, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; McLellan, J.S. Respiratory Syncytial Virus Entry and How to Block It. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef] [PubMed]
- San-Juan-Vergara, H.; Peeples, M.E. Importance of Virus Characteristics in Respiratory Syncytial Virus-Induced Disease. Immunol. Allergy Clin. North. Am. 2019, 39, 321–334. [Google Scholar] [CrossRef]
- Huong, T.N.; Ravi Iyer, L.; Lui, J.; Wang, D.Y.; Tan, B.H.; Sugrue, R.J. The Respiratory Syncytial Virus SH Protein Is Incorporated into Infectious Virus Particles That Form on Virus-Infected Cells. Virology 2023, 580, 28–40. [Google Scholar] [CrossRef]
- Langedijk, A.C.; Harding, E.R.; Konya, B.; Vrancken, B.; Lebbink, R.J.; Evers, A.; Willemsen, J.; Lemey, P.; Bont, L.J. A Systematic Review on Global RSV Genetic Data: Identification of Knowledge Gaps. Rev. Med. Virol. 2021, 32, e2284. [Google Scholar] [CrossRef]
- Anderson, L.J.; Jadhao, S.J.; Paden, C.R.; Tong, S. Functional Features of the Respiratory Syncytial Virus G Protein. Viruses 2021, 13, 1214. [Google Scholar] [CrossRef]
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The Journey to a Respiratory Syncytial Virus Vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef]
- Piedimonte, G.; Perez, M.K. Respiratory Syncytial Virus Infection and Bronchiolitis Practice Gaps. Pediatr. Rev. 2014, 35, 519–530. [Google Scholar] [CrossRef]
- Tramuto, F.; Maida, C.M.; Randazzo, G.; Guzzetta, V.; Santino, A.; Li Muli, R.; Costantino, C.; Graziano, G.; Amodio, E.; Mazzucco, W.; et al. Whole-Genome Sequencing and Genetic Diversity of Human Respiratory Syncytial Virus in Patients with Influenza-like Illness in Sicily (Italy) from 2017 to 2023. Viruses 2024, 16, 851. [Google Scholar] [CrossRef] [PubMed]
- Yunker, M.; Fall, A.; Norton, J.M.; Abdullah, O.; Villafuerte, D.A.; Pekosz, A.; Klein, E.; Mostafa, H.H. Genomic Evolution and Surveillance of Respiratory Syncytial Virus during the 2023–2024 Season. Viruses 2024, 16, 1122. [Google Scholar] [CrossRef] [PubMed]
- Piñana, M.; González-Sánchez, A.; Andrés, C.; Vila, J.; Creus-Costa, A.; Prats-Méndez, I.; Arnedo-Muñoz, M.; Saubi, N.; Esperalba, J.; Rando, A.; et al. Genomic Evolution of Human Respiratory Syncytial Virus during a Decade (2013–2023): Bridging the Path to Monoclonal Antibody Surveillance. J. Infect. 2024, 88, 106153. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Modjarrad, K.; McLellan, J.S. Novel Antigens for RSV Vaccines. Curr. Opin. Immunol. 2015, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Dondi, A.; Scarpini, S.; Rocca, A.; Vandini, S.; Poletti, G.; Lanari, M. Current State and Challenges in Developing Respiratory Syncytial Virus Vaccines. Vaccines 2020, 8, 672. [Google Scholar] [CrossRef]
- Graham, B.S. Vaccine Development for Respiratory Syncytial Virus. Curr. Opin. Virol. 2017, 23, 107–112. [Google Scholar] [CrossRef]
- Tan, J. Clonal Wars: Monoclonal Antibodies Against Infectious Pathogens. DNA Cell Biol. 2022, 41, 34–37. [Google Scholar] [CrossRef]
- Fleming, J.A.; Baral, R.; Higgins, D.; Khan, S.; Kochar, S.; Li, Y.; Ortiz, J.R.; Cherian, T.; Feikin, D.; Jit, M.; et al. Value Profile for Respiratory Syncytial Virus Vaccines and Monoclonal Antibodies. Vaccine 2023, 41, S7–S40. [Google Scholar] [CrossRef]
- Ali, A.; Lopardo, G.; Scarpellini, B.; Stein, R.T.; Ribeiro, D. Systematic Review on Respiratory Syncytial Virus Epidemiology in Adults and the Elderly in Latin America. Int. J. Infect. Dis. 2020, 90, 170–180. [Google Scholar] [CrossRef]
- Heikkinen, T.; Valkonen, H.; Waris, M.; Ruuskanen, O. Transmission of Respiratory Syncytial Virus Infection within Families. Open Forum Infect. Dis. 2015, 2, ofu118. [Google Scholar] [CrossRef]
- Lavergne, V.; Ghannoum, M.; Weiss, K.; Roy, J.; Béliveau, C. Successful Prevention of Respiratory Syncytial Virus Nosocomial Transmission Following an Enhanced Seasonal Infection Control Program. Bone Marrow Transpl. 2011, 46, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ru, X.; Chen, S.; Shao, Q.; Ye, Q. Analysis of the Prevalence and Clinical Features of Respiratory Syncytial Virus Infection in a Pediatric Hospital in Zhejiang Province from 2019 to 2023. J Med Virol 2024, 96, e29758. [Google Scholar] [CrossRef] [PubMed]
- Chuaychoo, B.; Ngamwongwan, S.; Kaewnaphan, B.; Athipanyasilp, N.; Horthongkham, N.; Kantakamalakul, W.; Muangman, N. Clinical Manifestations and Outcomes of Respiratory Syncytial Virus Infection in Adult Hospitalized Patients. J. Clin. Virol. 2019, 117, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Bay, P.; Loegel, C.; Ly, A.; Soulier, A.; N’Debi, M.; Seng, S.; Kassasseya, C.; Rodriguez, C.; Pawlotsky, J.-M.; de Prost, N.; et al. Clinical Phenotypes and Molecular Characteristics of Respiratory Syncytial Virus in Adults: A Monocentric Prospective Study Between 2019 and 2022. J. Infect Dis. 2023, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Provenzano, S.; Corrado, S.; Cerviere, M.P.; Parisi, S.; Marchesi, F.; Bottazzoli, M. Infodemiology of RSV in Italy (2017–2022): An Alternative Option for the Surveillance of Incident Cases in Pediatric Age? Children 2022, 9, 1984. [Google Scholar] [CrossRef]
- Leung, A.K.; Kellner, J.D.; Dele Davies, H.; Calgary, F.; Lansing, E. Respiratory Syncytial Virus Bronchiolitis. J. Natl. Med. Assoc. 2005, 97, 1708. [Google Scholar]
- Peña-López, Y.; Sabater-Riera, J.; Raj, P. Severe Respiratory Syncytial Virus Disease. J. Intensive Med. 2024, 4, 405–416. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Riccò, M.; Corrado, S.; Palmieri, S.; Marchesi, F. Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000–2022). Children 2023, 10, 1169. [Google Scholar] [CrossRef]
- Barr, R.; Green, C.A.; Sande, C.J.; Drysdale, S.B. Respiratory Syncytial Virus: Diagnosis, Prevention and Management. Ther. Adv. Infect. Dis. 2019, 6, 204993611986579. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.; Gilaberte Reyzábal, S.; Salillas, J.; Lago Gómez, M.R.; Rodríguez-Zurita, M.E.; Torralba, M. Respiratory Syncytial Virus Infection among Adults during Influenza Season: A Frequently Overlooked Diagnosis. J. Med. Virol. 2019, 91, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Borg, I.; Rohde, G.; Löseke, S.; Bittscheidt, J.; Schultze-Werninghaus, G.; Stephan, V.; Bufe, A. Evaluation of a Quantitative Real-Time PCR for the Detection of Respiratory Syncytial Virus in Pulmonary Diseases. Eur. Respir. J. 2003, 21, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Do, L.A.H.; van Doorn, H.R.; Bryant, J.E.; Nghiem, M.N.; Nguyen Van, V.C.; Vo, C.K.; Nguyen, M.D.; Tran, T.H.; Farrar, J.; De Jong, M.D. A Sensitive Real-Time PCR for Detection and Subgrouping of Human Respiratory Syncytial Virus. J. Virol. Methods 2012, 179, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Cutrera, R.; d’Angela, D.; Orso, M.; Guadagni, L.; Vittucci, A.C.; Bertoldi, I.; Polistena, B.; Spandonaro, F.; Carrieri, C.; Montuori, E.A.; et al. Trends in Hospitalizations of Children with Respiratory Syncytial Virus Aged Less than 1 Year in Italy, from 2015 to 2019. Ital J Pediatr 2024, 50, 1–7. [Google Scholar] [CrossRef]
- Øymar, K.; Skjerven, H.O.; Mikalsen, I.B. Acute Bronchiolitis in Infants, a Review. Scand. J. Trauma. Resusc. Emerg. Med. 2014, 22, 23. [Google Scholar] [CrossRef]
- Kengne-Nde, C.; Kenmoe, S.; Modiyinji, A.F.; Njouom, R. Prevalence of Respiratory Viruses Using Polymerase Chain Reaction in Children with Wheezing, a Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0243735. [Google Scholar] [CrossRef]
- Ramadan, H.H.; Farr, R.W.; Wetmore, S.J. Adenovirus and Respiratory Syncytial Virus in Chronic Sinusitis Using Polymerase Chain Reaction. Laryngoscope 1997, 107, 923–925. [Google Scholar] [CrossRef]
- Ramirez, J.; Carrico, R.; Wilde, A.; Junkins, A.; Furmanek, S.; Chandler, T.; Schulz, P.; Hubler, R.; Peyrani, P.; Liu, Q.; et al. Diagnosis of Respiratory Syncytial Virus in Adults Substantially Increases When Adding Sputum, Saliva, and Serology Testing to Nasopharyngeal Swab RT–PCR. Infect. Dis. Ther. 2023, 12, 1593–1603. [Google Scholar] [CrossRef]
- Onwuchekwa, C.; Moreo, L.M.; Menon, S.; Machado, B.; Curcio, D.; Kalina, W.; Atwell, J.E.; Gessner, B.D.; Siapka, M.; Agarwal, N.; et al. Underascertainment of Respiratory Syncytial Virus Infection in Adults Due to Diagnostic Testing Limitations: A Systematic Literature Review and Meta-Analysis. J. Infect. Dis. 2023, 228, 173–184. [Google Scholar] [CrossRef]
- Nair, H.; Theodoratou, E.; Rudan, I.; Nokes, D.J.; Ngama HND, M.; Munywoki, P.K.; Dherani, M.; Nair, H.; James Nokes, D.; Gessner, B.D.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Abbas, S.; Raybould, J.E.; Sastry, S.; de la Cruz, O. Respiratory Viruses in Transplant Recipients: More than Just a Cold. Clinical Syndromes and Infection Prevention Principles. Int. J. Infect. Dis. 2017, 62, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, E.; Ciarlitto, C.; Guolo, S.; Brusco, C.; Cerone, G.; Antilici, L.; Schettini, L.; Piscitelli, A.L.; Chiara Vittucci, A.; Cutrera, R.; et al. Respiratory Syncytial Virus Bronchiolitis in Infancy: The Acute Hospitalization Cost. Front. Pediatr. 2021, 8, 594898. [Google Scholar] [CrossRef] [PubMed]
- Rha, B.; Curns, A.T.; Lively, J.Y.; Campbell, A.P.; Englund, J.A.; Boom, J.A.; Azimi, P.H.; Weinberg, G.A.; Staat, M.A.; Selvarangan, R.; et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Young Children: 2015–2016. Pediatrics 2020, 146, e20193611. [Google Scholar] [CrossRef] [PubMed]
- Leader, S.; Kohlhase, K. Respiratory Syncytial Virus-Coded Pediatric Hospitalizations, 1997 to 1999. Pediatr. Infect Dis. J. 2002, 21, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Leader, S.; Kohlhase, K.; Pearlman, M.H.; Williams, J.V.; Engle, W.A. Recent Trends in Severe Respiratory Syncytial Virus (RSV) among US Infants, 1997 to 2000. J. Pediatr. 2003, 143, S127–S132. [Google Scholar] [CrossRef]
- Heemskerk, S.; van Heuvel, L.; Asey, T.; Bangert, M.; Kramer, R.; Paget, J.; van Summeren, J. Disease Burden of RSV Infections and Bronchiolitis in Young Children (< 5 Years) in Primary Care and Emergency Departments: A Systematic Literature Review. Influenza Other Respir. Viruses 2024, 18, e13344. [Google Scholar] [CrossRef]
- Scholz, S.; Dobrindt, K.; Tufts, J.; Adams, S.; Ghaswalla, P.; Ultsch, B.; Gottlieb, J. The Burden of Respiratory Syncytial Virus (RSV) in Germany: A Comprehensive Data Analysis Suggests Underdetection of Hospitalisations and Deaths in Adults 60 Years and Older. Infect. Dis. Ther. 2024, 13, 1759–1770. [Google Scholar] [CrossRef]
- Thomas, E.; Mattila, J.M.; Lehtinen, P.; Vuorinen, T.; Waris, M.; Heikkinen, T. Burden of Respiratory Syncytial Virus Infection during the First Year of Life. J. Infect. Dis. 2021, 223, 811–817. [Google Scholar] [CrossRef]
- Suh, M.; Movva, N.; Jiang, X.; Bylsma, L.C.; Reichert, H.; Fryzek, J.P.; Nelson, C.B. Respiratory Syncytial Virus Is the Leading Cause of United States Infant Hospitalizations, 2009-2019: A Study of the National (Nationwide) Inpatient Sample. J. Infect. Dis. 2022, 226, S154–S163. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Baggett, H.C.; Brooks, W.A.; Feikin, D.R.; Hammitt, L.L.; Higdon, M.M.; Howie, S.R.C.; Deloria Knoll, M.; Kotloff, K.L.; Levine, O.S.; et al. Causes of Severe Pneumonia Requiring Hospital Admission in Children without HIV Infection from Africa and Asia: The PERCH Multi-Country Case-Control Study. Lancet 2019, 394, 757–779. [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Shaw, C.A.; Griffin, P.; Farinola, N.; Railkar, R.A.; Cao, X.; Liu, W.; Sachs, J.R.; Swenson, C.J.; Lee, H.; et al. A Phase 1, Randomized, Placebo-Controlled Study to Evaluate the Safety and Immunogenicity of an MRNA-Based RSV Prefusion F Protein Vaccine in Healthy Younger and Older Adults. Hum. Vaccin. Immunother. 2021, 17, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Cutrera, R.; Wolfler, A.; Picone, S.; Rossi, G.A.; Gualberti, G.; Merolla, R.; Del Vecchio, A.; Villani, A.; Midulla, F.; Dotta, A. Impact of the 2014 American Academy of Pediatrics Recommendation and of the Resulting Limited Financial Coverage by the Italian Medicines Agency for Palivizumab Prophylaxis on the RSV-Associated Hospitalizations in Preterm Infants during the 2016-2017 Epidemic Season: A Systematic Review of Seven Italian Reports. Ital J. Pediatr. 2019, 45, 1–9. [Google Scholar]
- Li, Y.; Reeves, R.M.; Wang, X.; Bassat, Q.; Brooks, W.A.; Cohen, C.; Moore, D.P.; Nunes, M.; Rath, B.; Campbell, H.; et al. Global Patterns in Monthly Activity of Influenza Virus, Respiratory Syncytial Virus, Parainfluenza Virus, and Metapneumovirus: A Systematic Analysis. Lancet Glob. Health 2019, 7, e1031–e1045. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yan, R.; Wu, X.; Zhang, X.; Chen, C.; Jiang, D.; Yang, M.; Cao, K.; Chen, M.; You, Y.; et al. Global Burden and Trends of Respiratory Syncytial Virus Infection across Different Age Groups from 1990 to 2019: A Systematic Analysis of the Global Burden of Disease 2019 Study. Int. J. Infect. Dis. 2023, 135, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Parisi, S.; Corrado, S.; Marchesi, F.; Bottazzoli, M.; Gori, D. Respiratory Syncytial Virus Infections in Recipients of Bone Marrow Transplants: A Systematic Review and Meta-Analysis. Infect. Dis. Rep. 2024, 16, 317–355. [Google Scholar] [CrossRef]
- Wildenbeest, J.G.; Lowe, D.M.; Standing, J.F.; Butler, C.C. Respiratory Syncytial Virus Infections in Adults: A Narrative Review. Lancet Respir. Med. 2024, 12, 822–836. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef]
- Chatzis, O.; Darbre, S.; Pasquier, J.; Meylan, P.; Manuel, O.; Aubert, J.D.; Beck-Popovic, M.; Masouridi-Levrat, S.; Ansari, M.; Kaiser, L.; et al. Burden of Severe RSV Disease among Immunocompromised Children and Adults: A 10 Year Retrospective Study. BMC Infect. Dis. 2018, 18, 111. [Google Scholar] [CrossRef]
- Rozenbaum, M.H.; Begier, E.; Kurosky, S.K.; Whelan, J.; Bem, D.; Pouwels, K.B.; Postma, M.; Bont, L. Incidence of Respiratory Syncytial Virus Infection in Older Adults: Limitations of Current Data. Infect. Dis. Ther. 2023, 12, 1487–1504. [Google Scholar] [CrossRef]
- Kenmoe, S.; Nair, H. The Disease Burden of Respiratory Syncytial Virus in Older Adults. Curr. Opin. Infect. Dis. 2024, 37, 129–136. [Google Scholar] [CrossRef]
- Loubet, P.; Fernandes, J.; de Pouvourville, G.; Sosnowiez, K.; Elong, A.; Guilmet, C.; Omichessan, H.; Bureau, I.; Fagnani, F.; Emery, C.; et al. Respiratory Syncytial Virus-Related Hospital Stays in Adults in France from 2012 to 2021: A National Hospital Database Study. J. Clin. Virol. 2024, 171, 105635. [Google Scholar] [CrossRef] [PubMed]
- Polkowska-Kramek, A.; Begier, E.; Bruyndonckx, R.; Liang, C.; Beese, C.; Brestrich, G.; Tran, T.M.P.; Nuttens, C.; Casas, M.; Bayer, L.J.; et al. Estimated Incidence of Hospitalizations and Deaths Attributable to Respiratory Syncytial Virus Infections Among Adults in Germany Between 2015 and 2019. Infect Dis. Ther. 2024, 13, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Almeida, A.; Christaki, E.; Marques, T.M.; Tosatto, V.; Bianco, G.; Iannaccone, M.; Tsiolakkis, G.; Karagiannis, C.; Maikanti, P.; et al. Severity of RSV Infection in Southern European Elderly Patients during Two Consecutive Winter Seasons (2017–2018). J. Med. Virol. 2021, 93, 5152–5157. [Google Scholar] [CrossRef] [PubMed]
- Nowalk, M.P.; D’Agostino, H.; Dauer, K.; Stiegler, M.; Zimmerman, R.K.; Balasubramani, G.K. Estimating the Burden of Adult Hospitalized RSV Infection Including Special Populations. Vaccine 2022, 40, 4121–4127. [Google Scholar] [CrossRef] [PubMed]
- Narejos Pérez, S.; Ramón Torrell, J.M.; Põder, A.; Leroux-Roels, I.; Pérez-Breva, L.; Steenackers, K.; Vandermeulen, C.; Meisalu, S.; McNally, D.; Bowen, J.S.; et al. Respiratory Syncytial Virus Disease Burden in Community-Dwelling and Long-Term Care Facility Older Adults in Europe and the United States: A Prospective Study. Open Forum Infect. Dis. 2023, 10, ofad111. [Google Scholar] [CrossRef]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory Syncytial Virus Disease Burden in Adults Aged 60 Years and Older in High-Income Countries: A Systematic Literature Review and Meta-Analysis. Influenza Other Respir. Viruses 2022, 17, e13031. [Google Scholar] [CrossRef]
- Permpalung, N.; Mahoney, M.V.; McCoy, C.; Atsawarungruangkit, A.; Gold, H.S.; Levine, J.D.; Wong, M.T.; LaSalvia, M.T.; Alonso, C.D. Clinical Characteristics and Treatment Outcomes among Respiratory Syncytial Virus (RSV)-Infected Hematologic Malignancy and Hematopoietic Stem Cell Transplant Recipients Receiving Palivizumab. Leuk. Lymphoma 2019, 60, 85–91. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.L.; Downs, S.; Jones, S.; Van Niekerk, N.; Simoes, E.A.F.; Nunes, M.C. Burden of Respiratory Syncytial Virus Infection in South African Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Pregnant and Postpartum Women: A Longitudinal Cohort Study. Clin. Infect. Dis. 2018, 66, 1658–1665. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; Zhang, Y.; Zhang, X.; Zheng, M.; Kyaw, M.H. Burden of Respiratory Syncytial Virus Infections in China: Systematic Review and Meta-Analysis. J. Glob. Health 2015, 5, 020417. [Google Scholar] [CrossRef]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality Associated With Influenza and Respiratory Syncytial Virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; King, J.P.; Kieke, B.A.; Pluta, J.; Al-Hilli, A.; Meece, J.K.; Shinde, V. Clinical Features, Severity, and Incidence of RSV Illness during 12 Consecutive Seasons in a Community Cohort of Adults ≥60 Years Old. Open Forum Infect. Dis. 2018, 5, ofy316. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Saiman, L.; Walsh, E.E.; Falsey, A.R.; Jia, H.; Barrett, A.; Alba, L.; Phillips, M.; Finelli, L. Change in Functional Status Associated with Respiratory Syncytial Virus Infection in Hospitalized Older Adults. Influenza Other Respir. Viruses 2022, 16, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Bosco, E.; Van Aalst, R.; McConeghy, K.W.; Silva, J.; Moyo, P.; Eliot, M.N.; Chit, A.; Gravenstein, S.; Zullo, A.R. Estimated Cardiorespiratory Hospitalizations Attributable to Influenza and Respiratory Syncytial Virus among Long-Term Care Facility Residents. JAMA Netw. Open 2021, 4, e2111806. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé (HAS). Stratégie Vaccinale de Prévention Des Infections Par Le VRS Chez l’adulte Âgé de 60 Ans et plus; Haute Autorité de Santé (HAS): Paris, France, 2024. [Google Scholar]
- Widmer, K.; Zhu, Y.; Williams, J.V.; Griffin, M.R.; Edwards, K.M.; Talbot, H.K. Rates of Hospitalizations for Respiratory Syncytial Virus, Human Metapneumovirus, and Influenza Virus in Older Adults. J. Infect. Dis. 2012, 206, 56–62. [Google Scholar] [CrossRef]
- Matias, G.; Taylor, R.; Haguinet, F.; Schuck-Paim, C.; Lustig, R.; Shinde, V. Estimates of Hospitalization Attributable to Influenza and RSV in the US during 1997–2009, by Age and Risk Status. BMC Public Health 2017, 17, 1–14. [Google Scholar] [CrossRef]
- Patel, D.A.; Marcum, Z.A.; Chansakul, A.; Toyip, A.; Nerney, K.; Panozzo, C.A.; St Laurent, S.; Mehta, D.; Ghaswalla, P. Economic Burden of Cardiorespiratory Hospitalizations Associated with Respiratory Syncytial Virus among United States Adults in 2017–2019. Hum. Vaccines Immunother. 2024, 20, 2364493. [Google Scholar] [CrossRef]
- Loubet, P.; Lenzi, N.; Valette, M.; Foulongne, V.; Krivine, A.; Houhou, N.; Lagathu, G.; Rogez, S.; Alain, S.; Duval, X.; et al. Clinical Characteristics and Outcome of Respiratory Syncytial Virus Infection among Adults Hospitalized with Influenza-like Illness in France. Clin. Microbiol. Infect. 2017, 23, 253–259. [Google Scholar] [CrossRef]
- Fleming, D.M.; Taylor, R.J.; Lustig, R.L.; Schuck-Paim, C.; Haguinet, F.; Webb, D.J.; Logie, J.; Matias, G.; Taylor, S. Modelling Estimates of the Burden of Respiratory Syncytial Virus Infection in Adults and the Elderly in the United Kingdom. BMC Infect. Dis. 2015, 15, 443. [Google Scholar] [CrossRef]
- Sharp, A.; Minaji, M.; Panagiotopoulos, N.; Reeves, R.; Charlett, A.; Pebody, R. Estimating the Burden of Adult Hospital Admissions Due to RSV and Other Respiratory Pathogens in England. Influenza Other Respir. Viruses 2022, 16, 125–131. [Google Scholar] [CrossRef]
- Osei-Yeboah, R.; Amankwah, S.; Begier, E.; Adedze, M.; Nyanzu, F.; Appiah, P.; Ansah, J.O.B.; Campbell, H.; Sato, R.; Jodar, L.; et al. Burden of Respiratory Syncytial Virus (RSV) Infection Among Adults in Nursing and Care Homes: A Systematic Review. Influenza Other Respir. Viruses 2024, 18, e70008. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, A.; Pemmerl, S.; Kabesch, M.; Ambrosch, A. Comparative Analysis of RSV-Related Hospitalisations in Children and Adults over a 7 Year-Period before, during and after the COVID-19 Pandemic. J. Clin. Virol. 2023, 166, 105530. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Buda, S.; Schuler, E.; Hirve, S.; Zhang, W.; Haas, W. Risk Factors for Hospitalized Respiratory Syncytial Virus Disease and Its Severe Outcomes. Influenza Other Respir. Viruses 2020, 14, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Niekler, P.; Goettler, D.; Liese, J.G.; Streng, A. Hospitalizations Due to Respiratory Syncytial Virus (RSV) Infections in Germany: A Nationwide Clinical and Direct Cost Data Analysis (2010–2019). Infection 2023, 52, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Recto, C.G.; Fourati, S.; Khellaf, M.; Pawlotsky, J.-M.; De Prost, N.; Diakonoff, H.; Donadio, C.; Pouga, L.; de Tymowski, C.; Kassasseya, C. Respiratory Syncytial Virus vs Influenza Virus Infection: Mortality and Morbidity Comparison Over 7 Epidemic Seasons in an Elderly Population. J. Infect. Dis. 2024, 230, 1130–1138. [Google Scholar] [CrossRef]
- Celante, H.; Oubaya, N.; Fourati, S.; Beaune, S.; Khellaf, M.; Casalino, E.; Ricard, J.D.; Vieillard-Baron, A.; Heming, N.; Mekontso Dessap, A.; et al. Prognosis of Hospitalised Adult Patients with Respiratory Syncytial Virus Infection: A Multicentre Retrospective Cohort Study. Clin. Microbiol. Infect. 2023, 29, 943.e1–943.e8. [Google Scholar] [CrossRef]
- Luo, W.; Liu, Q.; Zhou, Y.; Ran, Y.; Liu, Z.; Hou, W.; Pei, S.; Lai, S. Spatiotemporal Variations of “Triple-Demic” Outbreaks of Respiratory Infections in the United States in the Post-COVID-19 Era. BMC Public Health 2023, 23, 2452. [Google Scholar] [CrossRef]
- Patel, T.A.; Jain, B.; Raifman, J. Revamping Public Health Systems: Lessons Learned From the Tripledemic. Am. J. Prev. Med. 2023, 66, 185–188. [Google Scholar] [CrossRef]
- Mazela, J.; Jackowska, T.; Czech, M.; Helwich, E.; Martyn, O.; Aleksiejuk, P.; Smaga, A.; Glazewska, J.; Wysocki, J. Epidemiology of Respiratory Syncytial Virus Hospitalizations in Poland: An Analysis from 2015 to 2023 Covering the Entire Polish Population of Children Aged under Five Years. Viruses 2024, 16, 704. [Google Scholar] [CrossRef]
- Scarpaci, M.; Bracaloni, S.; Esposito, E.; De Angelis, L.; Baglivo, F.; Casini, B.; Panatto, D.; Ogliastro, M.; Loconsole, D.; Chironna, M.; et al. RSV Disease Burden in Primary Care in Italy: A Multi-Region Pediatric Study, Winter Season 2022–2023. Influenza Other Respir. Viruses 2024, 18, e13282. [Google Scholar] [CrossRef]
- Rose, E.B.; Wheatley, A.; Langley, G.; Gerber, S.; Haynes, A. Morbidity and Mortality Weekly Report Respiratory Syncytial Virus Seasonality-United States, 2014–2017. Morb. Mortal. Wkly. Rep. 2018, 67, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Janet, S.; Broad, J.; Snape, M.D. Respiratory Syncytial Virus Seasonality and Its Implications on Prevention Strategies. Hum. Vaccin. Immunother. 2018, 14, 234–244. [Google Scholar] [CrossRef]
- Morley, C.; Grimwood, K.; Maloney, S.; Ware, R.S. Meteorological Factors and Respiratory Syncytial Virus Seasonality in Subtropical Australia. Epidemiol. Infect. 2018, 146, 757–762. [Google Scholar] [CrossRef]
- Pica, N.; Bouvier, N.M. Environmental Factors Affecting the Transmission of Respiratory Viruses. Curr. Opin. Virol. 2012, 2, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory Syncytial Virus Infection in Adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef]
- Regassa, B.T.; Gebrewold, L.A.; Mekuria, W.T.; Kassa, N.A. Molecular Epidemiology of Respiratory Syncytial Virus in Children with Acute Respiratory Illnesses in Africa: A Systematic Review and Meta-Analysis. J. Glob. Health 2023, 13, 04001. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Y.; Chmaisse, A.; Boutros, C.; Chamseddine, S.; Fayad, D.; Zaraket, H.; Dbaibo, G. The Burden of Respiratory Syncytial Virus (RSV) Infection in the Middle East and North Africa (MENA) Region across Age Groups: A Systematic Review. Vaccine 2021, 39, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F. Respiratory Syncytial Virus, Influenza and SARS-CoV-2 in Homeless People from Urban Shelters: A Systematic Review and Meta-Analysis (2023). Epidemiologia 2024, 5, 41–79. [Google Scholar] [CrossRef]
- Paynter, S. Humidity and Respiratory Virus Transmission in Tropical and Temperate Settings. Epidemiol. Infect. 2015, 143, 1110–1118. [Google Scholar] [CrossRef]
- Kenmoe, S.; Bigna, J.J.; Well, E.A.; Simo, F.B.N.; Penlap, V.B.; Vabret, A.; Njouom, R. Prevalence of Human Respiratory Syncytial Virus Infection in People with Acute Respiratory Tract Infections in Africa: A Systematic Review and Meta-Analysis. Influenza Other Respir. Viruses 2018, 12, 793–803. [Google Scholar] [CrossRef]
- Yassine, H.M.; Sohail, M.U.; Younes, N.; Nasrallah, G.K. Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region. Microorganisms 2020, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Pebody, R.; Moyes, J.; Hirve, S.; Campbell, H.; Jackson, S.; Moen, A.; Nair, H.; Simões, E.A.F.; Smith, P.G.; Wairagkar, N.; et al. Approaches to Use the WHO Respiratory Syncytial Virus Surveillance Platform to Estimate Disease Burden. Influenza Other Respir. Viruses 2020, 14, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; de Conto, F.; Buttrini, M.; Piccolo, G.; Montecchini, S.; Maccari, C.; Martinelli, M.; di Maio, A.; Ferraglia, F.; Pinardi, F.; et al. Human Respiratory Viruses, Including SARS-CoV-2, Circulating in the Winter Season 2019–2020 in Parma, Northern Italy. Int. J. Infect. Dis. 2021, 102, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Sherman, A.C.; Babiker, A.; Sieben, A.J.; Pyden, A.; Steinberg, J.; Kraft, C.S.; Koelle, K.; Kanjilal, S. The Effect of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Mitigation Strategies on Seasonal Respiratory Viruses: A Tale of 2 Large Metropolitan Centers in the United States. Clin. Infect. Dis. 2021, 72, E154–E157. [Google Scholar] [CrossRef]
- Kuitunen, I.; Artama, M.; Mäkelä, L.; Backman, K.; Heiskanen-Kosma, T.; Renko, M. Effect of Social Distancing Due to the COVID-19 Pandemic on the Incidence of Viral Respiratory Tract Infections in Children in Finland during Early 2020. Pediatr. Infect. Dis. J. 2020, 39, E423–E427. [Google Scholar] [CrossRef]
- van Brusselen, D.; de Troeyer, K.; ter Haar, E.; vander Auwera, A.; Poschet, K.; van Nuijs, S.; Bael, A.; Stobbelaar, K.; Verhulst, S.; van Herendael, B.; et al. Bronchiolitis in COVID-19 Times: A Nearly Absent Disease? Eur. J. Pediatr. 2021, 180, 1969–1973. [Google Scholar] [CrossRef]
- Britton, P.N.; Hu, N.; Saravanos, G.; Shrapnel, J.; Davis, J.; Snelling, T.; Dalby-Payne, J.; Kesson, A.M.; Wood, N.; Macartney, K.; et al. COVID-19 Public Health Measures and Respiratory Syncytial Virus. Lancet Child. Adolesc. Health 2020, 4, e42–e43. [Google Scholar] [CrossRef]
- Ippolito, G.; la Vecchia, A.; Umbrello, G.; di Pietro, G.; Bono, P.; Scalia, S.; Pinzani, R.; Tagliabue, C.; Bosis, S.; Agostoni, C.; et al. Disappearance of Seasonal Respiratory Viruses in Children Under Two Years Old During COVID-19 Pandemic: A Monocentric Retrospective Study in Milan, Italy. Front. Pediatr. 2021, 9, 721005. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC) Surveillance Atlas of Infectious Diseases. Available online: https://atlas.ecdc.europa.eu/public/index.aspx (accessed on 6 November 2024).
- Wang, H.; Zheng, Y.; de Jonge, M.I.; Wang, R.; Verhagen, L.M.; Chen, Y.; Li, L.; Xu, Z.; Wang, W. Lockdown Measures during the COVID-19 Pandemic Strongly Impacted the Circulation of Respiratory Pathogens in Southern China. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Maglione, M.; Pascarella, A.; Botti, C.; Ricci, G.; Morelli, F.; Camelia, F.; Micillo, A.; Calì, C.; Savoia, F.; Tipo, V.; et al. Changing Epidemiology of Acute Viral Respiratory Infections in Hospitalized Children: The Post-Lockdown Effect. Children 2022, 9, 1242. [Google Scholar] [CrossRef]
- Caini, S.; Meijer, A.; Nunes, M.C.; Henaff, L.; Zounon, M.; Boudewijns, B.; Del Riccio, M.; Paget, J. Probable Extinction of Influenza B/Yamagata and Its Public Health Implications: A Systematic Literature Review and Assessment of Global Surveillance Databases. Lancet Microbe. 2024, 5, 100851. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.A.; Phuong, L.K.; Peplinski, J.; Lim, S.M.; Lee, W.H.; Farhat, A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; et al. Examining the Interseasonal Resurgence of Respiratory Syncytial Virus in Western Australia. Arch. Dis. Child. 2021, 7, e1.2–e7. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.A.; Yeoh, D.K.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Moore, H.C.; Blyth, C.C. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019-Related Public Health Measures. Clin. Infect. Dis. 2021, 73, E2829–E2830. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Aminisani, N.; Huang, S.; O’Donnell, J.; Trenholme, A.; Broderick, D.; Paynter, J.; Castelino, L.; Grant, C.; McIntyre, P. Comparison of the Burden and Temporal Pattern of Hospitalisations Associated With Respiratory Syncytial Virus (RSV) Before and After COVID-19 in New Zealand. Influenza Other Respir. Viruses 2024, 18, e13346. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, R.; Liu, E.; Deng, Y. Timing Patterns of Initial Respiratory Syncytial Virus Infection and Factors Influencing Disease Severity in Hospitalized Infants with Different Health Status. J. Med. Virol. 2024, 96, e29719. [Google Scholar] [CrossRef]
- Miyama, T.; Kakimoto, K.; Yamanaka, Y.; Nishida, Y.; Iritani, N.; Motomura, K. Irregular Seasonality of Respiratory Syncytial Virus Infection Persists in 2023 in Osaka, Japan. IJID Reg. 2024, 13, 100442. [Google Scholar] [CrossRef]
- Lastrucci, V.; Pacifici, M.; Puglia, M.; Alderotti, G.; Berti, E.; Del Riccio, M.; Bonaccorsi, G.; Moriondo, M.; Resti, M.; Peroni, D.; et al. Seasonality and Severity of Respiratory Syncytial Virus during the COVID-19 Pandemic: A Dynamic Cohort Study. Int. J. Infect. Dis. 2024, 148, 107231. [Google Scholar] [CrossRef]
- Li, M.; Cong, B.; Wei, X.; Wang, Y.; Kang, L.; Gong, C.; Huang, Q.; Wang, X.; Li, Y.; Huang, F. Characterising the Changes in RSV Epidemiology in Beijing, China during 2015–2023: Results from a Prospective, Multi-Centre, Hospital-Based Surveillance and Serology Study. Lancet Reg. Health West Pac. 2024, 45, 101050. [Google Scholar] [CrossRef]
- Camporesi, A.; Morello, R.; Pierucci, U.M.; Proli, F.; Lazzareschi, I.; Bersani, G.; Valentini, P.; Roland, D.; Buonsenso, D. 2021/22 and 2022/23 Post-Pandemic Bronchiolitis Seasons in Two Major Italian Cities: A Prospective Study. Children 2023, 10, 1081. [Google Scholar] [CrossRef]
- Piedra, P.A.; Hause, A.M.; Aideyan, L. Respiratory Syncytial Virus (RSV): Neutralizing Antibody, a Correlate of Immune Protection. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1442, pp. 77–91. [Google Scholar]
- Walsh, E.E.; Wang, L.; Falsey, A.R.; Qiu, X.; Corbett, A.; Holden-Wiltse, J.; Mariani, T.J.; Topham, D.J.; Caserta, M.T. Virus-Specific Antibody, Viral Load, and Disease Severity in Respiratory Syncytial Virus Infection. J. Infect. Dis. 2018, 218, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Scarselli, E.; Lelii, M.; Scala, A.; Vitelli, A.; Capone, S.; Fornili, M.; Biganzoli, E.; Orenti, A.; Nicosia, A.; et al. Antibody Response to Respiratory Syncytial Virus Infection in Children <18 Months Old. Hum. Vaccin. Immunother. 2016, 12, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Kara, M.; Sutcu, M.; Mese, S.; Demircili, M.E.; Sivrikoz, T.S.; Torun, S.H.; Agacfidan, A.; Coban, A.; Unuvar, E.; et al. Evaluation of Respiratory Syncytial Virus IgG Antibody Dynamics in Mother-Infant Pairs Cohort. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Steinhoff, M.C.; Magaret, A.; Zaman, K.; Roy, E.; Langdon, G.; Formica, M.A.; Walsh, E.E.; Englund, J.A. Respiratory Syncytial Virus Transplacental Antibody Transfer and Kinetics in Mother-Infant Pairs in Bangladesh. J. Infect. Dis. 2014, 210, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Berbers, G.; Mollema, L.; Van Der Klis, F.; Den Hartog, G.; Schepp, R. Antibody Responses to Respiratory Syncytial Virus: A Cross-Sectional Serosurveillance Study in the Dutch Population Focusing on Infants Younger Than 2 Years. J. Infect. Dis. 2021, 224, 269–278. [Google Scholar] [CrossRef]
- Hatter, L.; Eathorne, A.; Hills, T.; Bruce, P.; Beasley, R. Respiratory Syncytial Virus: Paying the Immunity Debt with Interest. Lancet Child Adolesc. Health 2021, 5, e44–e45. [Google Scholar] [CrossRef]
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N.; Ujiie, M. Resurgence of Respiratory Syncytial Virus Infections during Covid-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970. [Google Scholar] [CrossRef]
- Azzari, C.; Baraldi, E.; Bonanni, P.; Bozzola, E.; Coscia, A.; Lanari, M.; Manzoni, P.; Mazzone, T.; Sandri, F.; Checcucci Lisi, G.; et al. Epidemiology and Prevention of Respiratory Syncytial Virus Infections in Children in Italy. Ital. J. Pediatr. 2021, 47, 198. [Google Scholar] [CrossRef]
- Tramuto, F.; Maida, C.M.; Di Naro, D.; Randazzo, G.; Vitale, F.; Restivo, V.; Costantino, C.; Amodio, E.; Casuccio, A.; Graziano, G.; et al. Respiratory Syncytial Virus: New Challenges for Molecular Epidemiology Surveillance and Vaccination Strategy in Patients with ILI/SARI. Vaccines 2021, 9, 1334. [Google Scholar] [CrossRef]
- Lodi, L.; Catamerò, F.; Voarino, M.; Barbati, F.; Moriondo, M.; Nieddu, F.; Sarli, W.M.; Citera, F.; Astorino, V.; Pelosi, C.; et al. Epidemiology of Respiratory Syncytial Virus in Hospitalized Children over a 9-Year Period and Preventive Strategy Impact. Front. Pharmacol. 2024, 15, 1381107. [Google Scholar] [CrossRef]
- Wick, M.; Kliemt, R.; Poshtiban, A.; Kossack, N.; Diller, G.P.; Soudani, S.; Bangert, M.; Kramer, R.; Damm, O. Respiratory Syncytial Virus Immunization Patterns in Germany, 2015–2020. Hum. Vaccin. Immunother. 2024, 20, 2380110. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, J.; Brannman, L.; Wade, S.W.; Gonzales, T.; Kong, A.M. Healthcare Resource Utilization and Costs in the 12 Months Following Hospitalization for Respiratory Syncytial Virus or Unspecified Bronchiolitis among Infants. J. Med. Econ. 2020, 23, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Thampi, N.; Knight, B.D.; Thavorn, K.; Webster, R.J.; Lanctot, K.; Hawken, S.; McNally, J.D. Health Care Costs of Hospitalization of Young Children for Respiratory Syncytial Virus Infections: A Population-Based Matched Cohort Study. CMAJ Open 2021, 9, E948–E956. [Google Scholar] [CrossRef] [PubMed]
- Carrico, J.; Hicks, K.A.; Wilson, E.; Panozzo, C.A.; Ghaswalla, P. The Annual Economic Burden of Respiratory Syncytial Virus in Adults in the United States. J. Infect. Dis. 2023, 230, e342–e352. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.T.; Nduaguba, S.O.; Wang, Y.; Diaby, V.; Finelli, L.; Choi, Y.; Winterstein, A.G. Economic Burden of Medically Attended Respiratory Syncytial Virus Infections Among Privately Insured Children Under 5 Years of Age in the USA. Influenza Other Respir. Viruses 2024, 18, e13347. [Google Scholar] [CrossRef]
- Ackerson, B.; An, J.; Sy, L.S.; Solano, Z.; Slezak, J.; Tseng, H.F. Cost of Hospitalization Associated with Respiratory Syncytial Virus Infection versus Influenza Infection in Hospitalized Older Adults. J. Infect. Dis. 2020, 222, 962–966. [Google Scholar] [CrossRef]
- Averin, A.; Atwood, M.; Sato, R.; Yacisin, K.; Begier, E.; Shea, K.; Curcio, D.; Houde, L.; Weycker, D. Attributable Cost of Adult Respiratory Syncytial Virus Illness Beyond the Acute Phase. Open Forum Infect. Dis. 2024, 11, ofae097. [Google Scholar] [CrossRef]
- Mac, S.; Shi, S.; Millson, B.; Tehrani, A.; Eberg, M.; Myageri, V.; Langley, J.M.; Simpson, S. Burden of Illness Associated with Respiratory Syncytial Virus (RSV)-Related Hospitalizations among Adults in Ontario, Canada: A Retrospective Population-Based Study. Vaccine 2023, 41, 5141–5149. [Google Scholar] [CrossRef]
- Rafferty, E.; Paulden, M.; Buchan, S.A.; Robinson, J.L.; Bettinger, J.A.; Kumar, M.; Svenson, L.W.; MacDonald, S.E. Evaluating the Individual Healthcare Costs and Burden of Disease Associated with RSV Across Age Groups. Pharmacoeconomics 2022, 40, 633–645. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Zaniboni, A.; Ranzieri, S. Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021). Pediatr. Rep. 2022, 14, 147–165. [Google Scholar] [CrossRef]
- Riccò, M.; Corrado, S.; Cerviere, M.P.; Ranzieri, S.; Marchesi, F. Respiratory Syncytial Virus Prevention through Monoclonal Antibodies: A Cross-Sectional Study on Knowledge, Attitudes, and Practices of Italian Pediatricians. Pediatr. Rep. 2023, 15, 154–174. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Frogel, M.P.; Stewart, D.L.; Hoopes, M.; Fernandes, A.W.; Mahadevia, P.J. A Systematic Review of Compliance with Palivizumab Administration for RSV Immunoprophylaxis. J. Manag. Care Pharm. 2010, 16, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Olchanski, N.; Hansen, R.N.; Pope, E.; D’Cruz, B.; Fergie, J.; Goldstein, M.; Krilov, L.R.; McLaurin, K.K.; Nabrit-Stephens, B.; Oster, G.; et al. Palivizumab Prophylaxis for Respiratory Syncytial Virus: Examining the Evidence around Value. Open Forum Infect. Dis. 2018, 5, ofy031. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Luna, M.; Manzoni, P.; Paes, B.; Baraldi, E.; Cossey, V.; Kugelman, A.; Chawla, R.; Dotta, A.; Rodríguez Fernández, R.; Resch, B.; et al. Expert Consensus on Palivizumab Use for Respiratory Syncytial Virus in Developed Countries. Paediatr. Respir. Rev. 2020, 33, 35–44. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Committee on Infectious Diseases and Bronchiolitis Guidelines Committee Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics 2014, 134, e620–e638. [Google Scholar] [CrossRef]
- Meissner, H.C.; Long, S.S. Committee on Infectious Diseases and Committee on Fetus and Newborn Revised Indications for the Use of Palivizumab and Respiratory Syncytial Virus Immune Globulin Intravenous for the Prevention of Respiratory Syncytial Virus Infections. Pediatrics 2003, 112, 1447–1452. [Google Scholar] [CrossRef]
- Yu, T.; Padula, W.V.; Yieh, L.; Gong, C.L. Cost-Effectiveness of Nirsevimab and Palivizumab for Respiratory Syncytial Virus Prophylaxis in Preterm Infants 29–34 6/7 Weeks’ Gestation in the United States. Pediatr. Neonatol. 2023, 65, 152–158. [Google Scholar] [CrossRef]
- Weiner, J.H. Respiratory Syncytial Virus Infection and Palivizumab: Are Families Receiving Accurate Information? Am. J. Perinatol. 2010, 27, 219–223. [Google Scholar] [CrossRef]
- Barbati, F.; Moriondo, M.; Pisano, L.; Calistri, E.; Lodi, L.; Ricci, S.; Giovannini, M.; Canessa, C.; Indolfi, G.; Azzari, C. Epidemiology of Respiratory Syncytial Virus-Related Hospitalization over a 5-Year Period in Italy: Evaluation of Seasonality and Age Distribution before Vaccine Introduction. Vaccines 2020, 8, 15. [Google Scholar] [CrossRef]
- Bozzola, E. Respiratory Syncytial Virus Resurgence in Italy: The Need to Protect All Neonates and Young Infants. Int. J. Environ. Res. Public Health 2022, 19, 380. [Google Scholar] [CrossRef]
- Dovizio, M.; Veronesi, C.; Bartolini, F.; Cavaliere, A.; Grego, S.; Pagliaro, R.; Procacci, C.; Ubertazzo, L.; Bertizzolo, L.; Muzii, B.; et al. Clinical and Economic Burden of Respiratory Syncytial Virus in Children Aged 0–5 Years in Italy. Ital. J. Pediatr. 2024, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lai, H.; Na, F.; Li, S.; Qiu, X.; Tian, J.; Zhang, Z.; Ge, L. Monoclonal Antibody for the Prevention of Respiratory Syncytial Virus in Infants and Children: A Systematic Review and Network Meta-Analysis. JAMA Netw. Open 2023, 6, E230023. [Google Scholar] [CrossRef] [PubMed]
- Cingo, O. Motavizumab. MAbs 2009, 1, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.A.F.; Forleo-Neto, E.; Geba, G.P.; Kamal, M.; Yang, F.; Cicirello, H.; Houghton, M.R.; Rideman, R.; Zhao, Q.; Benvin, S.L.; et al. Suptavumab for the Prevention of Medically Attended Respiratory Syncytial Virus Infection in Preterm Infants. Clin. Infect. Dis. 2021, 73, E4400–E4408. [Google Scholar] [CrossRef]
- Pecenka, C.; Sparrow, E.; Feikin, D.R.; Srikantiah, P.; Darko, D.M.; Karikari-Boateng, E.; Baral, R.; Vizzotti, C.; Rearte, A.; Jalang’o, R.; et al. Respiratory Syncytial Virus Vaccination and Immunoprophylaxis: Realising the Potential for Protection of Young Children. Lancet 2024, 404, 1157–1170. [Google Scholar] [CrossRef]
- Keam, S.J. Nirsevimab: First Approval. Drugs 2023, 83, 181–187. [Google Scholar] [CrossRef]
- Talha, M.; Ali, M.H. Latest FDA-Approved Drug Nirsevimab-Alip (Beyfortus): A Gamechanger for Treatment of Respiratory Syncytial Virus. J. Med. Virol. 2023, 95, e29169. [Google Scholar] [CrossRef]
- Moline, H.L.; Tannis, A.; Toepfer, A.P.; Williams, J.V.; Boom, J.A.; Englund, J.A.; Halasa, N.B.; Mary; Staat, A.; Weinberg, G.A.; et al. Morbidity and Mortality Weekly Report Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus-Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season-New Vaccine Surveillance Network. Morb. Mortal. Wkly. Rev. 2024, 73, 209–214. [Google Scholar] [CrossRef]
- Consolati, A.; Farinelli, M.; Serravalle, P.; Rollandin, C.; Apprato, L.; Esposito, S.; Bongiorno, S. Safety and Efficacy of Nirsevimab in a Universal Prevention Program of Respiratory Syncytial Virus Bronchiolitis in Newborns and Infants in the First Year of Life in the Valle d’Aosta Region, Italy, in the 2023–2024 Epidemic Season. Vaccines 2024, 12, 549. [Google Scholar] [CrossRef]
- López-Lacort, M.; Muñoz-Quiles, C.; Mira-Iglesias, A.; Xavier López-Labrador, F.; Mengual-Chuliá, B.; Fernández-García, C.; Carballido-Fernández, M.; Pineda-Caplliure, A.; Mollar-Maseres, J.; Shalabi Benavent, M.; et al. Early Estimates of Nirsevimab Immunoprophylaxis Effectiveness against Hospital Admission for Respiratory Syncytial Virus Lower Respiratory Tract Infections in Infants. Euro Surveill. 2024, 29, 2400046. [Google Scholar] [CrossRef]
- Ernst, C.; Bejko, D.; Gaasch, L.; Hannelas, E.; Kahn, I.; Pierron, C.; Del Lero, N.; Schalbar, C.; Do Carmo, E.; Kohnen, M.; et al. Impact of Nirsevimab Prophylaxis on Paediatric Respiratory Syncytial Virus (RSV)-Related Hospitalisations during the Initial 2023/24 Season in Luxembourg. Eurosurveillance 2024, 29, 2400033. [Google Scholar] [CrossRef]
- Ezpeleta, G.; Navascués, A.; Viguria, N.; Herranz-Aguirre, M.; Juan Belloc, S.E.; Gimeno Ballester, J.; Muruzábal, J.C.; García-Cenoz, M.; Trobajo-Sanmartín, C.; Echeverria, A.; et al. Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines 2024, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Ares-Gómez, S.; Mallah, N.; Santiago-Pérez, M.-I.; Pardo-Seco, J.; Pérez-Martínez, O.; Otero-Barrós, M.-T.; Suárez-Gaiche, N.; Kramer, R.; Jin, J.; Platero-Alonso, L.; et al. Effectiveness and Impact of Universal Prophylaxis with Nirsevimab in Infants against Hospitalisation for Respiratory Syncytial Virus in Galicia, Spain: Initial Results of a Population-Based Longitudinal Study. Lancet Infect. Dis. 2024, 24, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Cantais, A.; Annino, N.; Thuiller, C.; Tripodi, L.; Cesana, P.; Seigle-Ferrand, E.; Zekre, F.; Pillet, S.; Pozzetto, B. First RSV Epidemic with Nirsevimab. Older Children than Previous Epidemics, Even When Hospitalized. J. Med. Virol. 2024, 96, e29483. [Google Scholar] [CrossRef] [PubMed]
- Martinón-Torre, F.; Mirás-Carballa, S.; Durán-Parrond, C. Early Lessons from the Implementation of Universal Respiratory Syncytial Virus Prophylaxis in Infants with Long-Acting Monoclonal Antibodies, Galicia, Spain, September and October 2023. Eurosurveillance 2023, 28, 2300606. [Google Scholar] [CrossRef]
- Barbas Del Buey, J.F.; Íñigo Martínez, J.; Gutiérrez Rodríguez, M.Á.; Alonso García, M.; Sánchez-Gómez, A.; Lasheras Carbajo, M.D.; Jiménez Bueno, S.; Esteban Vasallo, M.D.; López Zambrano, M.A.; Calvo Rey, C.; et al. The Effectiveness of Nirsevimab in Reducing the Burden of Disease Due to Respiratory Syncytial Virus (RSV) Infection over Time in the Madrid Region (Spain): A Prospective Population-Based Cohort Study. Front. Public Health 2024, 12, 1441786. [Google Scholar] [CrossRef]
- Assad, Z.; Romain, A.-S.; Aupiais, C.; Shum, M.; Schrimpf, C.; Lorrot, M.; Corvol, H.; Prevost, B.; Ferrandiz, C.; Giolito, A.; et al. Nirsevimab and Hospitalization for RSV Bronchiolitis. N. Engl. J. Med. 2024, 391, 144–154. [Google Scholar] [CrossRef]
- Francisco, L.; Cruz-Cañete, M.; Pérez, C.; Couceiro, J.A.; Otheo, E.; Launes, C.; Rodrigo, C.; Jiménez, A.B.; Llorente, M.; Montesdeoca, A.; et al. Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease in Children: Statement of the Spanish Society of Paediatric Infectious Disease (SEIP). An. Pediatr. 2023, 99, 257–263. [Google Scholar] [CrossRef]
- Rki. Epidemiologisches Bulletin STIKO: Prophylaxe von RSV-Erkrankungen Mit Nirsevimab Bei Neugeborenen Und Säuglingen; Robert Koch Institute: Berlin, Germany, 2024; Available online: https://www.rki.de/SharedDocs/FAQ/Impfen/RSV-Prophylaxe/FAQ_Liste_gesamt.html (accessed on 10 October 2024).
- Merk/MSD Merck’s Clesrovimab (MK-1654), an Investigational Respiratory Syncytial Virus (RSV) Preventative Monoclonal Antibody, Significantly Reduced Incidence of RSV Disease and Hospitalization in Healthy Preterm and Full-Term Infants—Merck.Com. Available online: https://www.merck.com/news/mercks-clesrovimab-mk-1654-an-investigational-respiratory-syncytial-virus-rsv-preventative-monoclonal-antibody-significantly-reduced-incidence-of-rsv-disease-and-hospitalization-in-heal/ (accessed on 5 November 2024).
- Phuah, J.Y.; Maas, B.M.; Tang, A.; Zhang, Y.; Caro, L.; Railkar, R.A.; Swanson, M.D.; Cao, Y.; Li, H.; Roadcap, B.; et al. Quantification of Clesrovimab, an Investigational, Half-Life Extended, Anti-Respiratory Syncytial Virus Protein F Human Monoclonal Antibody in the Nasal Epithelial Lining Fluid of Healthy Adults. Biomed. Pharmacother. 2023, 169, 115851. [Google Scholar] [CrossRef]
- Scotta, M.C.; Stein, R.T. Current Strategies and Perspectives for Active and Passive Immunization against Respiratory Syncytial Virus in Childhood. J. Pediatr. 2023, 99, S4–S11. [Google Scholar] [CrossRef]
- Cicconi, P.; Jones, C.; Sarkar, E.; Silva-Reyes, L.; Klenerman, P.; de Lara, C.; Hutchings, C.; Moris, P.; Janssens, M.; Fissette, L.A.; et al. First-in-Human Randomized Study to Assess the Safety and Immunogenicity of an Investigational Respiratory Syncytial Virus (RSV) Vaccine Based on Chimpanzee-Adenovirus-155 Viral Vector-Expressing RSV Fusion, Nucleocapsid, and Antitermination Viral Proteins in Healthy Adults. Clin. Infect. Dis. 2020, 70, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Essink, B.; Harper, C.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; Guo, R.; Panozzo, C.A.; Wilson, E.; et al. Safety and Immunogenicity of an MRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase 1 Clinical Trial in Healthy Older Adults. J. Infect. Dis. 2024, 230, e647–e656. [Google Scholar] [CrossRef] [PubMed]
- Spearman, P.; Jin, H.; Knopp, K.; Xiao, P.; Gingerich, M.C.; Kidd, J.; Singh, K.; Tellier, M.; Radziewicz, H.; Wu, S.; et al. Intranasal Parainfluenza Virus Type 5 (PIV5)-Vectored RSV Vaccine Is Safe and Immunogenic in Healthy Adults in a Phase 1 Clinical Study. Sci. Adv. 2023, 9, eadj7611. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G.; Papi, A.; Athan, E.; Feldman, R.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; et al. Efficacy and Safety of Respiratory Syncytial Virus (RSV) Prefusion F Protein Vaccine (RSVPreF3 OA) in Older Adults Over 2 RSV Seasons. Clin. Infect. Dis. 2024, 78, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Kabir, G.; Schultz, S.; Silbernagl, G.; Schmidt, D.; Jenkins, V.A.; Weidenthaler, H.; Stroukova, D.; Martin, B.K.; De Moerlooze, L. Reduced Respiratory Syncytial Virus Load, Symptoms, and Infections: A Human Challenge Trial of MVA-BN-RSV Vaccine. J. Infect. Dis. 2023, 228, 999–1011. [Google Scholar] [CrossRef]
- Chang, L.A.; Phung, E.; Crank, M.C.; Morabito, K.M.; Villafana, T.; Dubovsky, F.; Falloon, J.; Esser, M.T.; Lin, B.C.; Chen, G.L.; et al. A Prefusion-Stabilized RSV F Subunit Vaccine Elicits B Cell Responses with Greater Breadth and Potency than a Postfusion F Vaccine. J. Infect. Dis. 2022, 228, 999–1011. [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an MRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Cox, F.; Saeland, E.; Thoma, A.; van den Hoogen, W.; Tettero, L.; Drijver, J.; Vaneman, C.; van Polanen, Y.; Ritschel, T.; Bastian, A.R.; et al. RSV A2-Based Prefusion F Vaccine Candidates Induce RSV A and RSV B Cross Binding and Neutralizing Antibodies and Provide Protection against RSV A and RSV B Challenge in Preclinical Models. Vaccines 2023, 11, 672. [Google Scholar] [CrossRef]
- Nussbaum, J.; Cao, X.; Railkar, R.A.; Sachs, J.R.; Spellman, D.S.; Luk, J.; Shaw, C.A.; Cejas, P.J.; Citron, M.P.; Al-Ibrahim, M.; et al. Evaluation of a Stabilized RSV Pre-Fusion F MRNA Vaccine: Preclinical Studies and Phase 1 Clinical Testing in Healthy Adults. Vaccine 2023, 41, 6488–6501. [Google Scholar] [CrossRef]
- Samy, N.; Reichhardt, D.; Schmidt, D.; Chen, L.M.; Silbernagl, G.; Vidojkovic, S.; Meyer, T.P.; Jordan, E.; Adams, T.; Weidenthaler, H.; et al. Safety and Immunogenicity of Novel Modified Vaccinia Ankara-Vectored RSV Vaccine: A Randomized Phase I Clinical Trial. Vaccine 2020, 38, 2608–2619. [Google Scholar] [CrossRef]
- Stuart, A.S.V.; Virta, M.; Williams, K.; Seppa, I.; Hartvickson, R.; Greenland, M.; Omoruyi, E.; Bastian, A.R.; Haazen, W.; Salisch, N.; et al. Phase 1/2a Safety and Immunogenicity of an Adenovirus 26 Vector Respiratory Syncytial Virus (RSV) Vaccine Encoding Prefusion F in Adults 18–50 Years and RSV-Seropositive Children 12–24 Months. J. Infect. Dis. 2023, 227, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Díez-Domingo, J.; Sáez-Llorens, X.; Rodriguez-Weber, M.A.; Epalza, C.; Chatterjee, A.; Chiu, C.H.; Lin, C.Y.; Berry, A.A.; Martinón-Torres, F.; Baquero-Artigao, F.; et al. Safety and Immunogenicity of a ChAd155-Vectored Respiratory Syncytial Virus (RSV) Vaccine in Healthy RSV-Seropositive Children 12–23 Months of Age. J. Infect. Dis. 2023, 227, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Green, C.A.; Scarselli, E.; Voysey, M.; Capone, S.; Vitelli, A.; Nicosia, A.; Cortese, R.; Thompson, A.J.; Sande, C.S.; Lara, C.D.; et al. Safety and Immunogenicity of Novel Respiratory Syncytial Virus (RSV) Vaccines Based on the RSV Viral Proteins F, N and M2-1 Encoded by Simian Adenovirus (PanAd3-RSV) and MVA (MVA-RSV); Protocol for an Open-Label, Dose-Escalation, Single-Centre, Phase 1 Clinical Trial in Healthy Adults. BMJ Open 2015, 5, e008748. [Google Scholar] [PubMed]
- Malkin, E.; Yogev, R.; Abughali, N.; Sliman, J.; Wang, C.K.; Zuo, F.; Yang, C.F.; Eickhoff, M.; Esser, M.T.; Tang, R.S.; et al. Safety and Immunogenicity of a Live Attenuated RSV Vaccine in Healthy RSV-Seronegative Children 5 to 24 Months of Age. PLoS ONE 2013, 8, e77104. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.; Falsey, A.; Patton, M.; Stacey, H.; Eiras, D.P.; Jiang, Q.; Woodside, J.; Mikati, T.; Kalinina, E.; Cooper, D.; et al. Efficacy of a Bivalent RSVpreF Vaccine in Older Adults Beyond a First RSV Season. In Proceedings of the 8th ReSViNET Conference, Mumbay, India, 13–16 February 2024; Respiratory Syncytial Virus Foundation: Mumbay, India, 13 February 2024; pp. 99–100. [Google Scholar]
- Karron, R.A.; Luongo, C.; Woods, S.; Oliva, J.; Collins, P.L.; Buchholz, U.J.; Council-Dibitetto, C.; Gatto, M.; Ghasri, T.; Gormley, A.; et al. Evaluation of the Live-Attenuated Intranasal Respiratory Syncytial Virus (RSV) Vaccine RSV/6120/ΔNS2/1030s in RSV-Seronegative Young Children. J. Infect. Dis. 2024, 229, 346–354. [Google Scholar] [CrossRef]
- Cunningham, C.K.; Karron, R.A.; Muresan, P.; Kelly, M.S.; McFarland, E.J.; Perlowski, C.; Libous, J.; Oliva, J.; Jean-Philippe, P.; Moye, J.; et al. Evaluation of Recombinant Live-Attenuated Respiratory Syncytial Virus (RSV) Vaccines RSV/ΔNS2/Δ1313/I1314L and RSV/276 in RSV-Seronegative Children. J. Infect. Dis. 2022, 226, 2069–2078. [Google Scholar] [CrossRef]
- Athan, E.; Baber, J.; Quan, K.; Scott, R.J.; Jaques, A.; Jiang, Q.; Li, W.; Cooper, D.; Cutler, M.W.; Kalinina, E.V.; et al. Safety and Immunogenicity of Bivalent RSVpreF Vaccine Coadministered with Seasonal Inactivated Influenza Vaccine in Older Adults. Clin. Infect. Dis. 2024, 78, 1360–1368. [Google Scholar] [CrossRef]
- Karron, R.A.; Atwell, J.E.; McFarland, E.J.; Cunningham, C.K.; Muresan, P.; Perlowski, C.; Libous, J.; Spector, S.A.; Yogev, R.; Aziz, M.; et al. Live-Attenuated Vaccines Prevent Respiratory Syncytial Virus-Associated Illness in Young Children. Am. J. Respir. Crit. Care Med. 2021, 203, 594–603. [Google Scholar] [CrossRef]
- Verdijk, P.; van der Plas, J.L.; van Brummelen, E.M.J.; Jeeninga, R.E.; de Haan, C.A.M.; Roestenberg, M.; Burggraaf, J.; Kamerling, I.M.C. First-in-Human Administration of a Live-Attenuated RSV Vaccine Lacking the G-Protein Assessing Safety, Tolerability, Shedding and Immunogenicity: A Randomized Controlled Trial. Vaccine 2020, 38, 6088–6095. [Google Scholar] [CrossRef]
- Karron, R.A.; Luongo, C.; Mateo, J.S.; Wanionek, K.; Collins, P.L.; Buchholz, U.J. Safety and Immunogenicity of the Respiratory Syncytial Virus Vaccine RSV/ΔNS2/Δ1313/I1314L in RSVSeronegative Children. J. Infect. Dis. 2020, 222, 82–91. [Google Scholar] [CrossRef]
- Abarca, K.; Rey-Jurado, E.; Muñoz-Durango, N.; Vázquez, Y.; Soto, J.A.; Gálvez, N.M.S.; Valdés-Ferrada, J.; Iturriaga, C.; Urzúa, M.; Borzutzky, A.; et al. Safety and Immunogenicity Evaluation of Recombinant BCG Vaccine against Respiratory Syncytial Virus in a Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial. EClinicalMedicine 2020, 27, 100517. [Google Scholar] [CrossRef] [PubMed]
- McFarland, E.J.; Karron, R.A.; Muresan, P.; Cunningham, C.K.; Libous, J.; Perlowski, C.; Thumar, B.; Gnanashanmugam, D.; Moye, J.; Schappell, E.; et al. Live Respiratory Syncytial Virus Attenuated by M2-2 Deletion and Stabilized Temperature Sensitivity Mutation 1030s Is a Promising Vaccine Candidate in Children. J. Infect. Dis. 2020, 221, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Eto, T.; Okubo, Y.; Momose, A.; Tamura, H.; Zheng, R.; Callendret, B.; Bastian, A.R.; Comeaux, C.A. A randomized, double-blind, placebo-controlled, Phase 1 Study to evaluate the safety, Reactogenicity, and Immunogenicity of Single Vaccination of Ad26.RSV.preF-Based Regimen in Japanese Adults aged 60 years. Influenza Other Respir. Viruses 2024, 18, e13336. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Jones, J.M.; Roper, L.E.; Prill, M.M.; Ortega-Sanchez, I.R.; Moulia, D.L.; Wallace, M.; Godfrey, M.; Broder, K.R.; Tepper, N.K.; et al. Morbidity and Mortality Weekly Report Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus-Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices-United States. Morb. Mortal. Wkly. Rep. 2023, 72, 1115–1122. [Google Scholar]
- Melgar, M.; Britton, A.; Roper, L.E.; Keipp Talbot, H.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices-United States, 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 793–801. [Google Scholar] [CrossRef]
- Mullard, A. FDA Approves MRNA-Based RSV Vaccine. Nat. Rev. Drug Discov. 2024, 23, 487. [Google Scholar] [CrossRef]
- Falsey, A.R.; Williams, K.; Gymnopoulou, E.; Bart, S.; Ervin, J.; Bastian, A.R.; Menten, J.; De Paepe, E.; Vandenberghe, S.; Chan, E.K.H.; et al. Efficacy and Safety of an Ad26.RSV.PreF–RSV PreF Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 609–620. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Comeaux, C.A.; Bart, S.; Bastian, A.R.; Klyashtornyy, V.; De Paepe, E.; Omoruyi, E.; Van Der Fits, L.; Van Heesbeen, R.; Heijnen, E.; Callendret, B.; et al. Safety, Immunogenicity, and Regimen Selection of Ad26.RSV.PreF-Based Vaccine Combinations: A Randomized, Double-Blind, Placebo-Controlled, Phase 1/2a Study. J. Infect. Dis. 2024, 229, 19–29. [Google Scholar] [CrossRef]
- Melgar, M.; Britton, A.; Roper, L.E.; Talbot, H.K.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Am. J. Transplant. 2023, 23, 1631–1640. [Google Scholar] [CrossRef]
- United States Food and Drugs Administration (FDA). AREXVY (Product Information) 2024. Available online: https://www.fda.gov/vaccines-blood-biologics/arexvy (accessed on 2 November 2024).
- United States Food and Drugs Administration (FDA). MRESVIA (Product Information) 2024. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/mresvia (accessed on 2 November 2024).
- United States Food and Drug Administration (FDA). ABRYSVO (Product Information) 2024. Available online: https://www.fda.gov/vaccines-blood-biologics/abrysvo (accessed on 2 November 2024).
- Feldman, R.G.; Antonelli-Incalzi, R.; Steenackers, K.; Lee, D.G.; Papi, A.; Ison, M.G.; Fissette, L.; David, M.P.; Marechal, C.; Van Der Wielen, M.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine Is Efficacious in Older Adults With Underlying Medical Conditions. Clin. Infect. Dis. 2024, 78, 202–209. [Google Scholar] [CrossRef]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Doreski, P.A.; Marc, G.P.; Jimenez, G.; Priddy, F.; Lin, N.; Le Cam, N.; Slobod, K.; Sonia, K.; et al. Efficacy and Safety of MRNA-1345, an RSV Vaccine, in Older Adults: Results through ≥6 Months of Follow-up and Evaluation of Correlate of Protection Against RSV. In Proceedings of the 8th ReSViNET Conference; Respiratory Syncytial Virus Society, Mumbay, India, 15 February 2024; pp. 87–88. [Google Scholar]
- Rki Epidemiologisches Bulletin STIKO. Beschluss zur Empfehlung der STIKO für eine Standardimpfung gegen Erkrankungen durch Respiratorische Synzytial-Viren (RSV) für Personen ≥75 Jahre sowie zur Indikationsimpfung von Personen im Alter von 60 bis 74 Jahren mit Risikofaktoren. RKI Epidemiologisches Bulletin 2024, 32, 3–28. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2024/Ausgaben/32_24.pdf?__blob=publicationFile (accessed on 2 November 2024).
- Falsey, A.R.; Walsh, E.E.; Scott, D.A.; Gurtman, A.; Zareba, A.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Jiang, Q.; et al. Phase 1/2 Randomized Study of the Immunogenicity, Safety, and Tolerability of a Respiratory Syncytial Virus Prefusion F Vaccine in Adults with Concomitant Inactivated Influenza Vaccine. In Proceedings of the Journal of Infectious Diseases; Oxford University Press: Oxford, UK, 2022; Volume 225, pp. 2056–2066. [Google Scholar]
- Walsh, E.E.; Falsey, A.R.; Scott, D.A.; Gurtman, A.; Zareba, A.M.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Radley, D.; et al. A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine. J. Infect. Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Chin, J.; Pollard, J.; Zerr, D.M.; Englund, J.A. Clinical Outcomes in Outpatient Respiratory Syncytial Virus Infection in Immunocompromised Children. Influenza Other Respir. Viruses 2016, 10, 205–210. [Google Scholar] [CrossRef]
- Yoon, J.G.; Noh, J.Y.; Choi, W.S.; Park, J.J.; Suh, Y.B.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Clinical Characteristics and Disease Burden of Respiratory Syncytial Virus Infection among Hospitalized Adults. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Reeves, R.M.; van Wijhe, M.; Lehtonen, T.; Stona, L.; Teirlinck, A.C.; Vazquez Fernandez, L.; Li, Y.; Osei-Yeboah, R.; Fischer, T.K.; Heikkinen, T.; et al. A Systematic Review of European Clinical Practice Guidelines for Respiratory Syncytial Virus Prophylaxis. J. Infect. Dis. 2022, 226, S110–S116. [Google Scholar] [CrossRef]
- Billard, M.; van de Ven, P.M.; Baraldi, B.; Kragten-Tabatabaie, L.; Bont, L.J.; Wildenbeest, J.G. International Changes in Respiratory Syncytial Virus (RSV) Epidemiology during the COVID-19 Pandemic: Association with School Closures. Influenza Other Respir. Viruses 2022, 16, 926–936. [Google Scholar] [CrossRef]
- Juhn, Y.J.; Wi, C.-I.; Takahashi, P.Y.; Ryu, E.; King, K.S.; Hickman, J.A.; Yao, J.D.; Binnicker, M.J.; Natoli, T.L.; Evans, T.K.; et al. Incidence of Respiratory Syncytial Virus Infection in Older Adults Before and During the COVID-19 Pandemic. JAMA Netw. Open 2023, 6, e2250634. [Google Scholar] [CrossRef]
- Cong, B.; Koç, U.; Bandeira, T.; Bassat, Q.; Bont, L.; Chakhunashvili, G.; Cohen, C.; Desnoyers, C.; Hammitt, L.L.; Heikkinen, T.; et al. Changes in the Global Hospitalisation Burden of Respiratory Syncytial Virus in Young Children during the COVID-19 Pandemic: A Systematic Analysis. Lancet Infect. Dis. 2023, 24, 361–374. [Google Scholar] [CrossRef]
- Yonts, A.B.; Gaviria-Agudelo, C.; Kimberlin, D.W.; Paulsen, G.C.; O’Leary, S.T. June 2024 ACIP Meeting Update: Influenza, COVID-19, RSV and Other Vaccines. Pediatrics 2024, 154, e2024068310. [Google Scholar] [CrossRef] [PubMed]
- Britton, A.; Roper, L.E.; Kotton, C.N.; David; Hutton, W.; Fleming-Dutra, K.E.; Godfrey, M.; Ortega-Sanchez, I.R.; Broder, K.R. Use of Respiratory Syncytial Virus Vaccines in Adults Aged ≥60 Years: Updated Recommendations of the Advisory Committee on Immunization Practices-United States, 2024. Morb. Mortal. Wkly. Rep. 2024, 73, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Surie, D.; Self, W.H.; Zhu, Y.; Yuengling, K.A.; Johnson, C.A.; Grijalva, C.G.; Dawood, F.S.; Gaglani, M.; Ghamande, S.; McNeal, T.; et al. RSV Vaccine Effectiveness Against Hospitalization Among US Adults 60 Years and Older. JAMA 2024, 332, 1105. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of Effectiveness of Vaccines against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Pérez Marc, G.; Falsey, A.R.; Jiang, Q.; Eiras, D.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Zareba, A.M.; et al. RENOIR Trial—RSVpreF Vaccine Efficacy over Two Seasons. N. Engl. J. Med. 2024, 391, 1459–1460. [Google Scholar] [CrossRef]
- Gerber, S. Meeting of the Advisory Committee on Immunization Practices (ACIP)—AREXVY. In Proceedings of the MEETING OF THE ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES (ACIP) Respiratory Syncytial Virus (RSV) Vaccine, Adults, Atlanta, GA, USA, 23–24 October 2024; Advisory Commitee on Immunization Practices (ACIP): Atlanta, GA, USA, 2024. [Google Scholar]
- Britton, A.; Melgar, M. Evidence to Recommendations Framework (EtR): RSV Vaccination in Adults Aged 50-59 years, 60-74 years, and 75 years and older. Available online: https://www.cdc.gov/acip/downloads/slides-2024-06-26-28/11-RSV-Adult-Melgar-Roper-Britton-508.pdf (accessed on 2 November 2024).
- Pfizer Inc. Pfizer Announces Positive Top-Line Data for Full Season Two Efficacy of ABRYSVO® for RSV in Older Adults. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two (accessed on 28 March 2024).
- Das, R. RSV Vaccine (MRESVIA, MRNA-1345) Concomitant Administration Overview Advisory Committee on Immunization Practices. In Proceedings of the Meeting of the Advisory Committee on Immunization Practices (ACIP) Respiratory Syncytial Virus (RSV) Vaccine, Adults, Atlanta, GA, USA, 23–24 October 2024; Advisory Committee on Immunization Practices (ACIP): Atlanta, GA, USA, 2024. [Google Scholar]
- Buynak, R.; Cannon, K.; DeAtkine, D.; Kirby, J.; Usdan, L.; Bhavsar, A.; Gérard, C.; Kuznetsova, A.; Jayadev, A.; Amare, H.; et al. Randomized, Open-Label Phase 3 Study Evaluating Immunogenicity, Safety, and Reactogenicity of RSVPreF3 OA Coadministered with FLU-QIV-HD in Adults Aged ≥ 65. Infect. Dis. Ther. 2024, 13, 1789–1805. [Google Scholar] [CrossRef]
- Chandler, R.; Montenegro, N.; Llorach, C.; Aguirre, L.N.; Germain, S.; Kuriyakose, S.O.; Lambert, A.; Descamps, D.; Olivier, A.; Hulstrøm, V. Immunogenicity, Reactogenicity, and Safety of AS01E-Adjuvanted Respiratory Syncytial Virus (RSV) Prefusion F Protein-Based Candidate Vaccine (RSVPreF3 OA) When Co-Administered With a Seasonal Quadrivalent Influenza Vaccine in Older Adults: Results of a Phase 3, Open-Label, Randomized Controlled Trial. Clin. Infect. Dis. 2024, ciad786. [Google Scholar] [CrossRef]
- Melgar, M.; Britton, A. RSV Vaccination in Adults: Work Group Interpretations National Center for Immunization and Respiratory Diseases. In Proceedings of the Meeting of the Advisory Committee On Immunization Practices (ACIP) Respiratory Syncytial Virus (RSV) Vaccine, Adults, Atlanta, GA, USA, 23–24 October 2024; Advisory Committee on Immunization Practices (ACIP): Atlanta, GA, USA, 2024. [Google Scholar]
- Hause, A.M.; Moro, P.L.; Baggs, J.; Zhang, B.; Marquez, P.; Melgar, M.; Britton, A.; Stroud, E.; Myers, T.R.; Rakickas, J.; et al. Early Safety Findings Among Persons Aged ≥ 60 Years Who Received a Respiratory Syncytial Virus Vaccine—United States, 3 May 2023–14 April 2024. Morb. Mortal. Wkly. Rev. 2024, 30, 489–494. [Google Scholar] [CrossRef]
- Lloyd, P.; Phd, S.; Statistician, H. Evaluation of Guillain-Barré Syndrome (GBS) Following Respiratory Syncytial Virus (RSV) Vaccination Among Adults 65 Years and Older. In Proceedings of the Meeting of the Advisory Committee on Immunization Practices (ACIP) Respiratory Syncytial Virus (RSV) Vaccine, Adults, Atlanta, GA, USA, 23–24 October 2024; Advisory Committee on Immunization Practices (ACIP): Atlanta, GA, USA, 2024. [Google Scholar]
- Cohen, M.K.; Ikeda, R.M.; Kent, C.K.; Gottardy, A.J.; Leahy, M.A.; Spriggs, S.R.; Velarde, A.; Yang, T.; Doan, Q.M.; King, P.H.; et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices-United States, 2023-24 Influenza Season. Morb. Mortal. Wkly. Rev. 2023, 72, 1–28. [Google Scholar]
- Janusz, C.B.; Anderson, T.C.; Leidner, A.J.; Lee, G.M.; Dooling, K.; Prosser, L.A. Projected Risks and Health Benefits of Vaccination against Herpes Zoster and Related Complications in US Adults. Hum. Vaccin. Immunother. 2022, 18, 2060668. [Google Scholar] [CrossRef]
- Anderson, T.C.; Masters, N.B.; Guo, A.; Shepersky, L.; Leidner, A.J.; Lee, G.M.; Kotton, C.N.; Dooling, K.L. Use of Recombinant Zoster Vaccine in Immunocompromised Adults Aged ≥19 Years: Recommendations of the Advisory Committee on Immunization Practices-United States, 2022. Morb. Mortal. Wkly. Rev. 2022, 71, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Vellozzi, C.; Iqbal, S.; Broder, K. Guillain-Barré Syndrome, Influenza, and Influenza Vaccination: The Epidemiologic Evidence. Clin. Infect. Dis. 2014, 58, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Vidal Valero, M. “A Good Day”: FDA Approves World’s First RSV Vaccine. Nature 2023, 617, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.A.; Al-Ansari, K.; Nasrallah, G.K.; Elrayess, M.A.; Al-Thani, A.A.; Derrien-Colemyn, A.; Ruckwardt, T.J.; Graham, B.S.; Yassine, H.M. Level of Maternal Respiratory Syncytial Virus (RSV) F Antibodies in Hospitalized Children and Correlates of Protection. Int. J. Infect. Dis. 2021, 109, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Tielsch, J.; Katz, J.; Magaret, A.S.; Khatry, S.; LeClerq, S.C.; Shrestha, L.; Kuypers, J.; Steinhoff, M.C.; Englund, J.A. Transplacental Transfer of Maternal Respiratory Syncytial Virus (RSV) Antibody and Protection against RSV Disease in Infants in Rural Nepal. J. Clin. Virol. 2017, 95, 90–95. [Google Scholar] [CrossRef]
- Kachikis, A.B.; Rumfelt, K.; Pike, M.; Sosa, M.; Stolarczuk, J.E.; Cho, H.; Eckert, L.O.; Martin, E.T.; Englund, J.A. Transfer of Respiratory Syncytial Virus Prefusion F Protein Antibody in Low Birthweight Infants. Open Forum Infect. Dis. 2024, 11, ofae314. [Google Scholar] [CrossRef]
- Willemsen, J.E.; Borghans, J.A.M.; Bont, L.J.; Drylewicz, J. Disagreement FDA and EMA on RSV Maternal Vaccination: Possible Consequence for Global Mortality. Pediatr. Infect. Dis. J. 2024, 43, E1–E2. [Google Scholar] [CrossRef]
- Dieussaert, I.; Hyung Kim, J.; Luik, S.; Seidl, C.; Pu, W.; Stegmann, J.-U.; Swamy, G.K.; Webster, P.; Dormitzer, P.R. RSV Prefusion F Protein–Based Maternal Vaccine—Preterm Birth and Other Outcomes. N. Engl. J. Med. 2024, 390, 1009–1021. [Google Scholar] [CrossRef]
- Regan, A.K. Perinatal Outcomes after RSV Vaccination during Pregnancy—Addressing Emerging Concerns. JAMA Netw. Open 2024, 7, e2419229. [Google Scholar] [CrossRef]
- Son, M.; Riley, L.E.; Staniczenko, A.P.; Cron, J.; Yen, S.; Thomas, C.; Sholle, E.; Osborne, L.M.; Lipkind, H.S. Nonadjuvanted Bivalent Respiratory Syncytial Virus Vaccination and Perinatal Outcomes. JAMA Netw. Open 2024, 7, e2419268. [Google Scholar] [CrossRef]
- Otsuki, T.; Akada, S.; Anami, A.; Kosaka, K.; Munjal, I.; Baber, J.; Shoji, Y.; Aizawa, M.; Swanson, K.A.; Gurtman, A. Efficacy and Safety of Bivalent RSVpreF Maternal Vaccination to Prevent RSV Illness in Japanese Infants: Subset Analysis from the Pivotal Randomized Phase 3 MATISSE Trial. Vaccine 2024, 42, 126041. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Gouvernement du Canada Respiratory Syncytial Virus (RSV) Vaccines; Canadian Immunization Guide: Ottawa, ON, Canada, 2024.
- Haute Authorité de Santé (HAS). Vaccination Maternelle Contre Le VRS: Une Nouvelle Possibilité Pour Protéger Le Nouveau-Né; Communiqué de Presse: Paris, Frace, 2024. [Google Scholar]
- Haute Autorité de Santé (HAS). Recommandation Vaccinale Contre Les Infections à VRS Chez Les Femmes Enceintes; Haute Autorité de Santé (HAS): Paris, France, 2024. [Google Scholar]
- Joint Committee on Vaccination and Immunisation (JCVI). Respiratory Syncytial Virus (RSV) Immunisation Programme for Infants and Older Adults; JCVI Full Statement: London, UK, 2023. Available online: https://www.gov.uk/government/publications/rsv-immunisation-programme-jcvi-advice-7-june-2023/respiratory-syncytial-virus-rsv-immunisation-programme-for-infants-and-older-adults-jcvi-full-statement-11-september-2023 (accessed on 2 November 2024).
- Superior Health Council. Preventive Strategies Against Rsv Disease in Children. SHC № 9760, 6 December 2023. Available online: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/20231222_shc-9760_advice_rsv_children_vweb.pdf (accessed on 2 November 2024).
- Jasset Ba, O.J.; Andrea Lopez Zapana, P.; Bahadir, Z.; Shook, L.; Dennis, M.B.; Gilbert, E.B.; Ariel LIU, Z.B.; Yinger Bs, R.V.; Bald, C.B.; Bradford Bs, C.G.; et al. Longer Interval between Maternal RSV Vaccination and Birth Increases Placental Transfer Efficiency. medRxiv 2024. [Google Scholar] [CrossRef]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Phijffer, E.W.; de Bruin, O.; Ahmadizar, F.; Bont, L.J.; Van der Maas, N.A.; Sturkenboom, M.C.; Wildenbeest, J.G.; Bloemenkamp, K.W. Respiratory Syncytial Virus Vaccination during Pregnancy for Improving Infant Outcomes. Cochrane Database Syst. Rev. 2024, 2024, CD015134. [Google Scholar] [CrossRef]
- Moghadas, S.M.; Shoukat, A.; Bawden, C.E.; Langley, J.M.; Singer, B.H.; Fitzpatrick, M.C.; Galvani, A.P. Cost-Effectiveness of Prefusion F Protein-Based Vaccines Against Respiratory Syncytial Virus Disease for Older Adults in the United States. Clin. Infect. Dis. 2023, 78, 1328–1335. [Google Scholar] [CrossRef]
- Hutton, D.W.; Prosser, L.A.; Rose, A.M.; Mercon, K.; Leidner, A.J.; Havers, F.P.; Prill, M.M.; Roper, L.E.; Pike, J.; Britton, A.; et al. Cost-Effectiveness of Vaccinating Adults Aged 60 Years and Older against Respiratory Syncytial Virus. Vaccine 2024, 42, 126294. [Google Scholar] [CrossRef]
- Crawford, R.; Bailey, S.; Cornelissen, T. CADTH Health Technology Review Cost-Effectiveness of Respiratory Syncytial Virus Vaccines for Adults Cost-Effectiveness of Respiratory Syncytial Virus Vaccines for Adults. Can. Agency Drugs Technol. Health 2024, 4, 1–18. Available online: https://www.ncbi.nlm.nih.gov/books/NBK602524/pdf/Bookshelf_NBK602524.pdf (accessed on 2 November 2024).
- Superior Health Council. Vaccination Against RSV (Adults)—SHC N. 9725; SHC: Brussels, Belgium, 2023. [Google Scholar]
- Folkhälsomyndigheten. Vaccination Mot RS-Virus; Folkhälsomyndigheten: Stockholm, Sweden, 2024. [Google Scholar]
- Bundesministerium Soziales Gesundheit Pflege und Konsumentenschutz. Impfplan Österreich 2023/2024; Bundesministerium Soziales Gesundheit Pflege und Konsumentenschutz: Wien, Austria, 2024. [Google Scholar]
- National Immunisation Advisory Committee. Recommendations for Passive Immunisation and Vaccination Against Respiratory Syncytial Virus in Infants, Children and Older Adults; National Immunisation Advisory Committee: Dublin, Ireland, 2023. [Google Scholar]
- Folkehelseinstitutet (FHI). RSV-Vaksine-Håndbok for Helsepersonell RSV-Infeksjon; Folkehelseinstitutet (FHI): Oslo, Norway, 2024. [Google Scholar]
- National Advisory Committee on Immunization (NACI). Advisory Committyee Statement (ACS): Statement on the Prevention of Respiratory Syncytial Virus(RSV) Disease in Older Adults; Editions Universitaires E: Ottawa, ON, Canada, 2024. [Google Scholar]
- Joint Committee on Vaccination and Immunisation (JCVI). RSV Immunisation Programme: JCVI Advice, 7 June 2023 (updated 11 September 2023). London, UK, 2023. Available online: https://www.gov.uk/government/publications/rsv-immunisation-programme-jcvi-advice-7-june-2023 (accessed on 2 November 2024).
- Respiratory Syncytial Virus (RSV). The Australian Immunisation Handbook. Available online: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/respiratory-syncytial-virus-rsv (accessed on 2 November 2024).
- Gunatilaka, A.; Giles, M.L. Maternal RSV Vaccine Development. Where to from Here? Hum. Vaccin. Immunother. 2021, 17, 4542–4548. [Google Scholar] [CrossRef]
- Nourbakhsh, S.; Shoukat, A.; Zhang, K.; Poliquin, G.; Halperin, D.; Sheffield, H.; Halperin, S.A.; Langley, J.M.; Moghadas, S.M. Effectiveness and Cost-Effectiveness of RSV Infant and Maternal Immunization Programs: A Case Study of Nunavik, Canada. EClinicalMedicine 2021, 41, 101141. [Google Scholar] [CrossRef]
- Li, X.; Bilcke, J.; Vázquez Fernández, L.; Bont, L.; Willem, L.; Wisløff, T.; Jit, M.; Beutels, P.; Beutels, P.; Bont, L.; et al. Cost-Effectiveness of Respiratory Syncytial Virus Disease Prevention Strategies: Maternal Vaccine Versus Seasonal or Year-Round Monoclonal Antibody Program in Norwegian Children. J. Infect. Dis. 2022, 226, S95–S101. [Google Scholar] [CrossRef]
- Health Council of the Netherlands. Immunisation against RSV in the First Year of Life—Executive Summary; Health Council of the Netherlands: The Hague, The Netherlands, 2024; Available online: https://www.healthcouncil.nl/documents/advisory-reports/2024/02/14/immunisation-against-rsv-in-the-first-year-of-life (accessed on 2 November 2024).
- American Academy of Pediatrics (AAP). AAP Recommendations for the Prevention of RSV Disease in Infants and Children; 2024; Available online: https://www.aap.org/en/patient-care/respiratory-syncytial-virus-rsv-prevention/?srsltid=AfmBOopCbPVgy-sSXA11JMWteVSnhyJJ6-DYrEKeSAtU--gdurNp3rFH (accessed on 2 November 2024).
- United States Centers for Disease Control and Prevention RSV Immunization Guidance for Infants and Young Children. Available online: https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/infants-young-children.html#:~:text=What%20to%20know,in%20most%20of%20the%20U.S (accessed on 2 November 2024).
- United States Centers for Disease Control and Prevention RSV Vaccine Guidance for Pregnant People. Available online: https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/pregnant-people.html#:~:text=To (accessed on 2 November 2024).
- United States Centers for Disease Control and Prevention Respiratory Syncytial Virus Infection (RSV)—Vaccines for Older Adults. Available online: https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/older-adults.html (accessed on 2 November 2024).
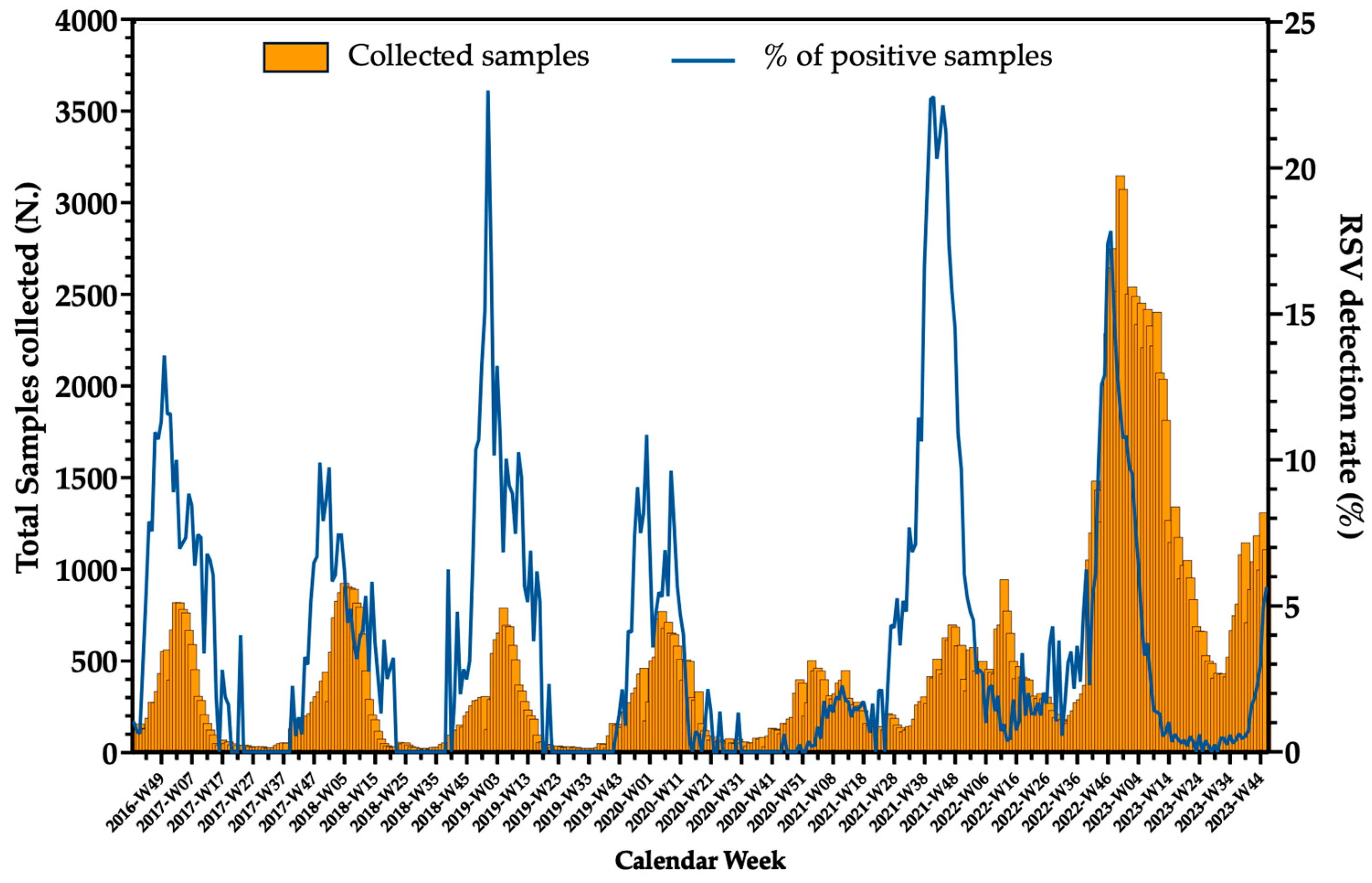

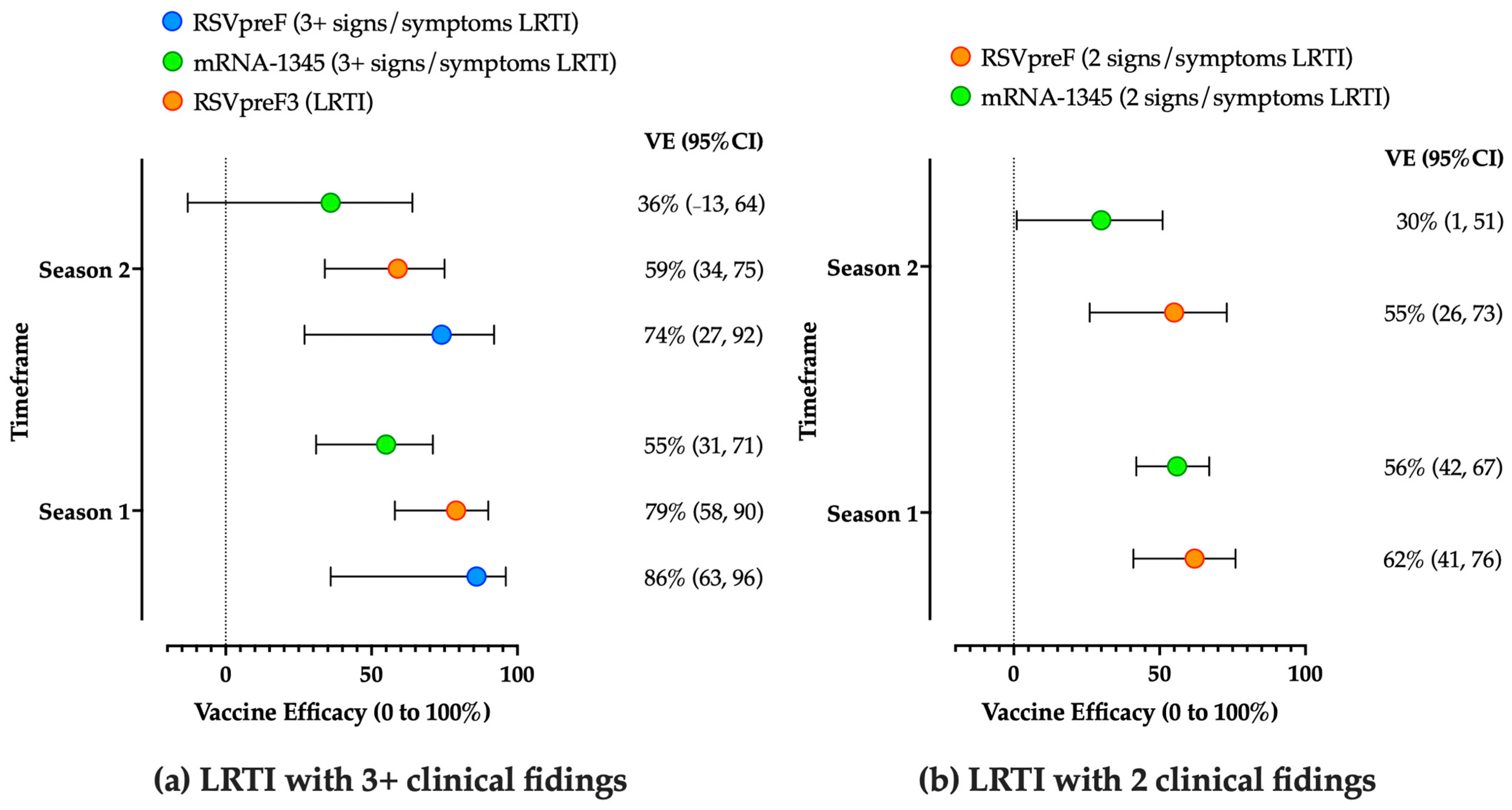


| Vaccine | Characteristics | Age Groups | Reference |
|---|---|---|---|
| Arexvy RSVPreF3 | Monovalent (RSV A) stabilized prefusion F protein (120 µg) Adjuvant: AS01 Single dose | 49–59 years (chronic medical conditions) 60–74 years (chronic medical conditions) ≥75 years (all) | [210,241,242] |
| Abrysvo RSVpreF | Bivalent (RSV A and B) stabilized prefusion F protein (60 µg + 60 µg); Adjuvant: none Single dose | 18–59 years (chronic medical conditions) 60–74 years (chronic medical conditions) ≥75 years (all) Maternal use (all age groups) | [87,221] |
| mRESVIA mRNA-1345 | Monovalent (RSV A) Single mRNA sequence encoding for a stabilized prefusion F protein (50 µg) Adjuvant: none Monovalent | 60–74 years (chronic medical conditions) ≥75 years (all) | [213,243] |
| Vaccine | Primary Outcome | Efficacy (Point Estimate, 95%CI) | |
|---|---|---|---|
| First RSV Season (0 to 12 Months) | Second RSV Season (13 to 24 Months) | ||
| RSVpreF | LRTI with two signs/symptoms | 62% (41, 76) | 55% (26, 73) |
| LRTI with ≥three signs/symptoms | 86% (63, 96) | 74% (27, 92) | |
| RSVpreF3, single dose | LRTI a | 79% (58, 90) | 59% (34, 75) |
| RSVpreF3, two doses | LRTI a | - | 58% (34, 75) |
| mRNA-1345 | LRTI with two signs/symptoms | 56% (42, 67) | 30% (1, 51) b |
| LRTI with ≥three signs/symptoms | 55% (31, 71) | 36% (−13, 64) b | |
| USA [253,254] | Canada [296] | UK [297] | Germany [244] | France [105] | Austria [293] | Belgium [291] | Sweden [292] | Ireland [294] | Norway [295] | Australia [298] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication by age | |||||||||||
| 18–59 years | AUTH. | NO | NO | NO | NO | COND. | NO | NO | NO | NO | NO |
| 60–64 years | COND. | COND. | NO | COND. | COND. | ALL | COND. | COND. | NO | COND. | COND. |
| 65–74 years | COND. | COND. | NO | COND. | COND. | ALL | COND. | COND. | ALL | COND. | COND. |
| ≥75 years | ALL | ALL | ALL | ALL | ALL | ALL | ALL | ALL | ALL | COND. | ALL |
| High-risk groups comorbidities | |||||||||||
| Chronic cardiovascular disease | X | X | X | X | X | X | X | X | |||
| Chronic lung or respiratory disease | X | X | X | X | X | X | X | X | |||
| Chronic renal disease | X | X | X | X | X | ||||||
| End-stage renal disease | X | X | X | X | X | ||||||
| Uncomplicated diabetes mellitus | X | X | X | X | |||||||
| Complicated diabetes mellitus | X | X | X | X | X | X | |||||
| Any neurologic or neuromuscular conditions | X | ||||||||||
| Neurologic or neuromuscular conditions causing impaired airway clearance or respiratory muscle weakness | X | X | X | X | |||||||
| Chronic liver disease | X | X | X | ||||||||
| Neoplasia | X | ||||||||||
| Chronic hematologic conditions | X | X | X | X | |||||||
| Obesity (BMI ≥ 30 kg/m2) | X | ||||||||||
| Severe obesity (BMI ≥ 40 kg/m2) | X | X | X | X | |||||||
| Any immune compromise | X | X | X | X | X | X | |||||
| Severe immune compromise | X | X | X | X | X | X | |||||
| Residence in a nursing home | X | X | X | X | X | X | |||||
| Chronic medical conditions or risk factors that a health care provider determines would increase the risk for severe disease due to viral respiratory infection | X | X |
| Maternal Vaccination | Monoclonal Antibodies | |
|---|---|---|
| Advantages | Not requiring shots to the newborns. | Can be delivered any time up to 2 years of age. |
| Effective protection guaranteed from birth up to 180 days. | Effective protection expected up to 150 days after the delivery. | |
| Potential resistance to preF mutations solicited by nirsevimab. | Likely well-accepted by parents from countries with high pediatric vaccination rates. | |
| Disadvantages | Transplacental transfer of maternal antibodies depends on the production of IgG-class neutralizing antibodies: it could be insufficient due to maternal conditions (e.g., any immunodeficiency status), or premature delivery. | Requiring shots to the newborns. |
| Doubts on its efficacy in pre-term infants (even if delivered in optimal maternal:foetus transfer windows). | High cost of conventional mAb (serial shots during the winter season) | |
| Possibly affected by general vaccine hesitancy for maternal vaccination (likely heterogeneous acceptance in various countries). | Potential emergence of mutated strains due to selective pressure of the monoclonal antibody. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Abu-Raya, B.; Icardi, G.; Spoulou, V.; Greenberg, D.; Pecurariu, O.F.; Hung, I.F.-N.; Osterhaus, A.; Sambri, V.; Esposito, S. Respiratory Syncytial Virus: A WAidid Consensus Document on New Preventive Options. Vaccines 2024, 12, 1317. https://doi.org/10.3390/vaccines12121317
Riccò M, Abu-Raya B, Icardi G, Spoulou V, Greenberg D, Pecurariu OF, Hung IF-N, Osterhaus A, Sambri V, Esposito S. Respiratory Syncytial Virus: A WAidid Consensus Document on New Preventive Options. Vaccines. 2024; 12(12):1317. https://doi.org/10.3390/vaccines12121317
Chicago/Turabian StyleRiccò, Matteo, Bahaa Abu-Raya, Giancarlo Icardi, Vana Spoulou, David Greenberg, Oana Falup Pecurariu, Ivan Fan-Ngai Hung, Albert Osterhaus, Vittorio Sambri, and Susanna Esposito. 2024. "Respiratory Syncytial Virus: A WAidid Consensus Document on New Preventive Options" Vaccines 12, no. 12: 1317. https://doi.org/10.3390/vaccines12121317
APA StyleRiccò, M., Abu-Raya, B., Icardi, G., Spoulou, V., Greenberg, D., Pecurariu, O. F., Hung, I. F.-N., Osterhaus, A., Sambri, V., & Esposito, S. (2024). Respiratory Syncytial Virus: A WAidid Consensus Document on New Preventive Options. Vaccines, 12(12), 1317. https://doi.org/10.3390/vaccines12121317










