Live Attenuated aTJ Vaccine Effectively Protects Pigeons Against Homologous PPMV-1 Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Virus, Cells, and Plasmids
2.3. Construction of Infectious cDNA Clones
2.4. Construction of the Helper Plasmids
2.5. Rescue and Pathogenicity of Viruses
2.6. Virus Titration and Growth Curve
2.7. Immunization and Challenge
2.8. HI Assay
2.9. Virus Isolation
2.10. Statistical Analysis
3. Results
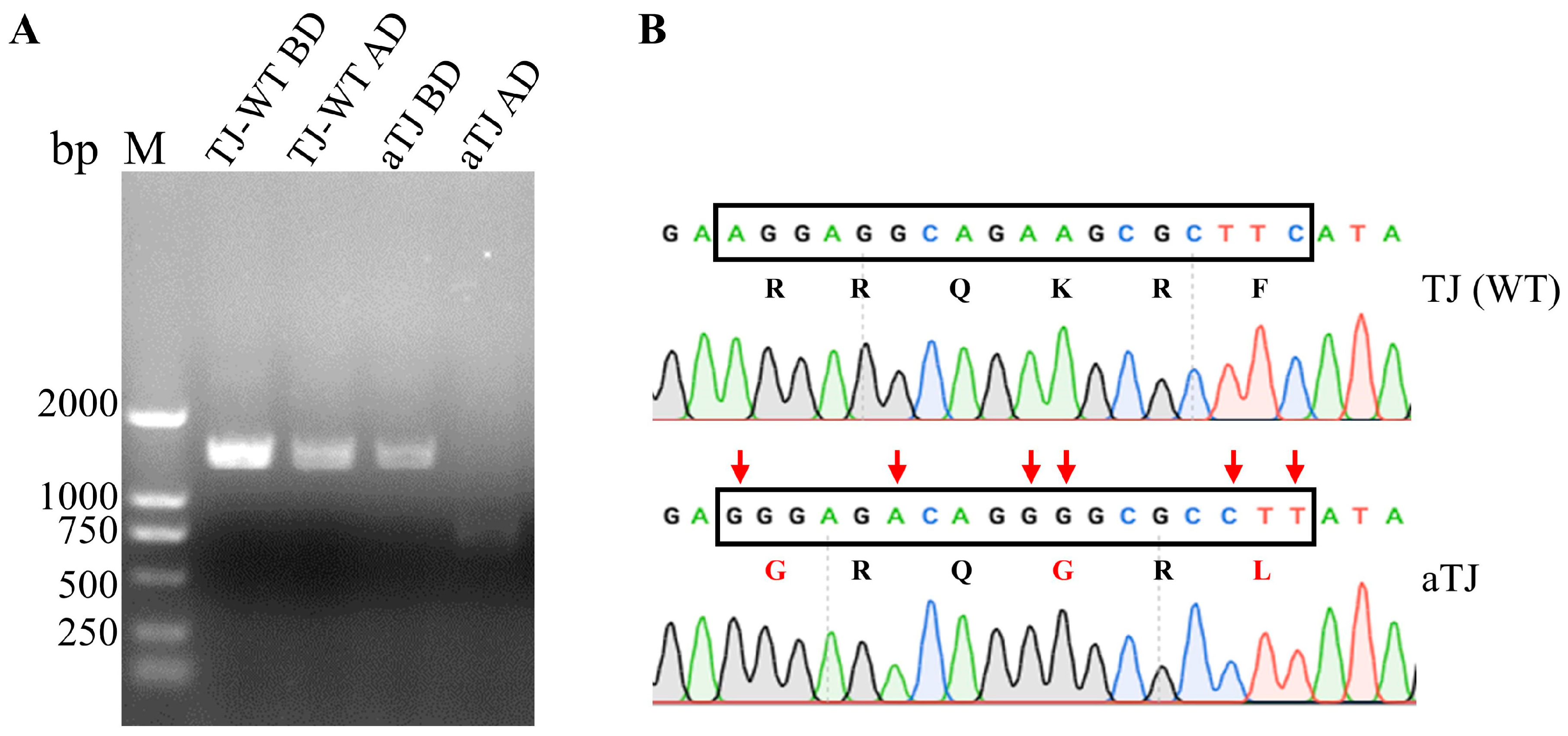
3.1. Generation of Recombinant aTJ Virus
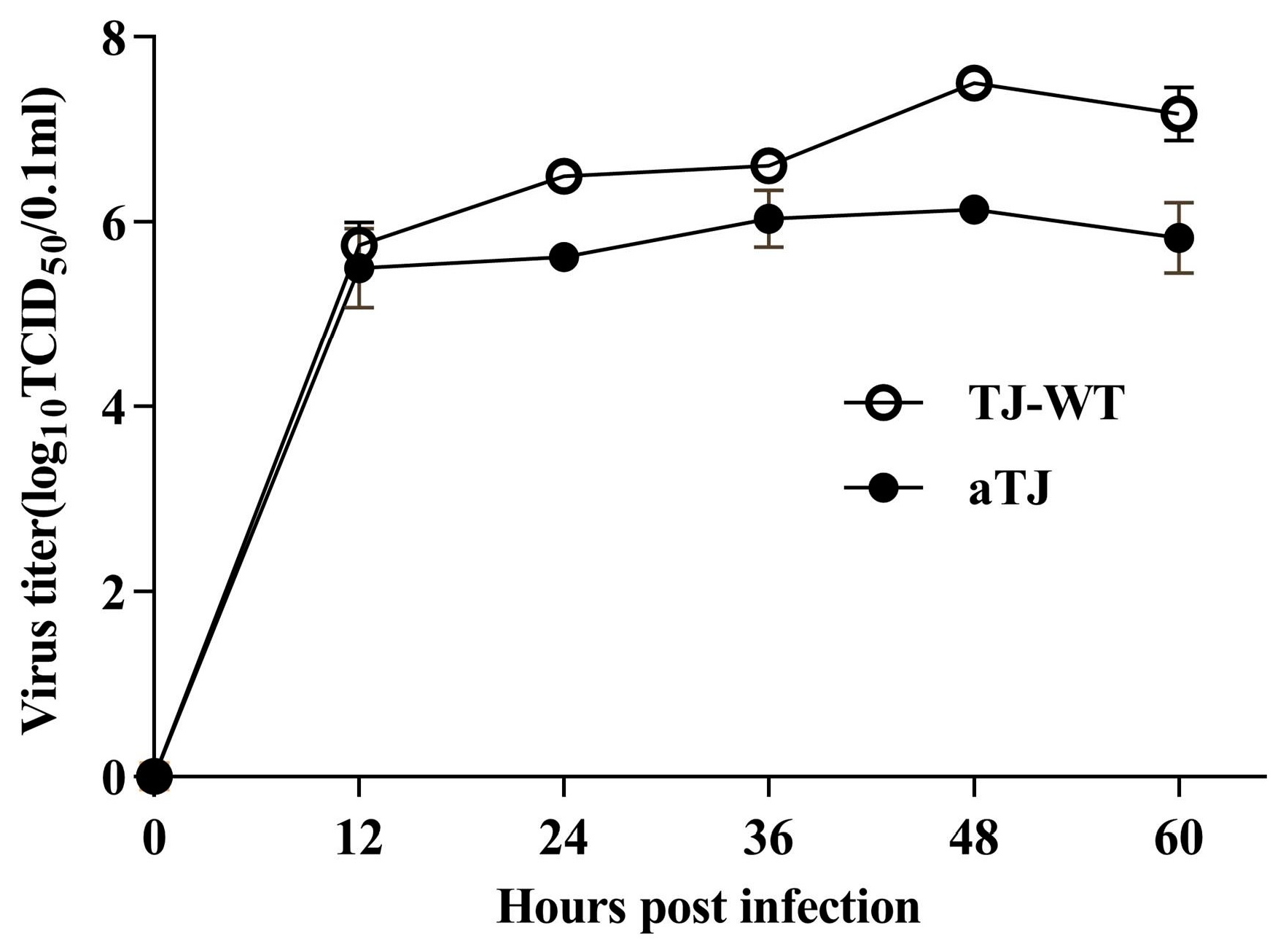
3.2. Biological Characterization of aTJ
| Strains | lgTCID50 1 (0.1 mL) | lgEID50 2 (0.1 mL) | MDT 3 (h) | ICPI 4 |
|---|---|---|---|---|
| aTJ | −8.63 | −9.00 | 146 | 0.20 |
| TJ-WT | −8.17 | −8.38 | 62 | 1.19 |
3.3. Antibody Response
3.4. Clinical Signs in Vaccinated Pigeon After Challenge
3.5. Reduction in Virulent NDV Shedding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayo, M.A. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 2002, 147, 1655–1663. [Google Scholar] [CrossRef]
- Dimitrov, K.M.; Abolnik, C.; Afonso, C.L.; Albina, E.; Bahl, J.; Berg, M.; Briand, F.X.; Brown, I.H.; Choi, K.S.; Chvala, I.; et al. Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect. Genet. Evol. 2019, 74, 103917. [Google Scholar] [CrossRef]
- Millar, N.S.; Chambers, P.; Emmerson, P.T. Nucleotide sequence of the fusion and haemagglutinin-neuraminidase glycoprotein genes of Newcastle disease virus, strain Ulster: Molecular basis for variations in pathogenicity between strains. J. Gen. Virol. 1988, 69 Pt 3, 613–620. [Google Scholar] [CrossRef]
- Aldous, E.W.; Fuller, C.M.; Mynn, J.K.; Alexander, D.J. A molecular epidemiological investigation of isolates of the variant avian paramyxovirus type 1 virus (PPMV-1) responsible for the 1978 to present panzootic in pigeons. Avian Pathol 2004, 33, 258–269. [Google Scholar] [CrossRef]
- Kaleta, E.F.; Alexander, D.J.; Russell, P.H. The first isolation of the avian PMV-1 virus responsible for the current panzootic in pigeons ? Avian Pathol. 1985, 14, 553–557. [Google Scholar] [CrossRef]
- Xie, P.; Chen, L.; Zhang, Y.; Lin, Q.; Ding, C.; Liao, M.; Xu, C.; Xiang, B.; Ren, T. Evolutionary Dynamics and Age-Dependent Pathogenesis of Sub-Genotype VI.2.1.1.2.2 PPMV-1 in Pigeons. Viruses 2020, 12, 433. [Google Scholar] [CrossRef]
- Ren, S.; Wang, C.; Zhang, X.; Zhao, L.; Wang, X.; Yao, W.; Han, Q.; Wang, Y.; Fan, M.; Gao, X.; et al. Phylogenetic and pathogenic characterization of a pigeon paramyxovirus type 1 isolate reveals cross-species transmission and potential outbreak risks in the northwest region of China. Arch. Virol. 2017, 162, 2755–2767. [Google Scholar] [CrossRef]
- Śmietanka, K.; Olszewska, M.; Domańska-Blicharz, K.; Bocian, A.L.; Minta, Z. Experimental infection of different species of birds with pigeon paramyxovirus type 1 virus—Evaluation of clinical outcomes, viral shedding, and distribution in tissues. Avian Dis. 2014, 58, 523–530. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Han, Z.; Shao, Y.; Chen, J.; Zhao, S.; Kong, X.; Liu, S. Phylogenetic analysis and comparison of eight strains of pigeon paramyxovirus type 1 (PPMV-1) isolated in China between 2010 and 2012. Arch. Virol. 2013, 158, 1121–1131. [Google Scholar] [CrossRef]
- He, Y.; Lu, B.; Dimitrov, K.M.; Liang, J.; Chen, Z.; Zhao, W.; Qin, Y.; Duan, Q.; Zhou, Y.; Liu, L.; et al. Complete Genome Sequencing, Molecular Epidemiological, and Pathogenicity Analysis of Pigeon Paramyxoviruses Type 1 Isolated in Guangxi, China during 2012–2018. Viruses 2020, 12, 366. [Google Scholar] [CrossRef]
- Dortmans, J.C.; Venema-Kemper, S.; Peeters, B.P.; Koch, G. Field vaccinated chickens with low antibody titres show equally insufficient protection against matching and non-matching genotypes of virulent Newcastle disease virus. Vet. Microbiol. 2014, 172, 100–107. [Google Scholar] [CrossRef]
- Zeng, T.; Xie, L.; Xie, Z.; Hua, J.; Huang, J.; Xie, Z.; Zhang, Y.; Zhang, M.; Luo, S.; Li, M.; et al. Analysis of Newcastle disease virus prevalence in wild birds reveals interhost transmission of genotype VI strains. Microbiol. Spectr. 2024, e0081624, Epub ahead of print. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Li, Y.; Liu, J.; Wang, W.; Bai, J.; Yang, Z.; Liu, H.; Xiao, S. A pigeon paramyxovirus type 1 isolated from racing pigeon as an inactivated vaccine candidate provides effective protection. Poult. Sci. 2022, 101, 102097. [Google Scholar] [CrossRef]
- Abolnik, C. A current review of avian influenza in pigeons and doves (Columbidae). Vet. Microbiol. 2014, 170, 181–196. [Google Scholar] [CrossRef]
- Liu, M.; Qu, Y.; Wang, F.; Liu, S.; Sun, H. Genotypic and pathotypic characterization of Newcastle disease virus isolated from racing pigeons in China. Poult. Sci. 2015, 94, 1476–1482. [Google Scholar] [CrossRef]
- Dortmans, J.C.; Rottier, P.J.; Koch, G.; Peeters, B.P. Passaging of a Newcastle disease virus pigeon variant in chickens results in selection of viruses with mutations in the polymerase complex enhancing virus replication and virulence. J. Gen. Virol. 2011, 92, 336–345. [Google Scholar] [CrossRef]
- Kommers, G.D.; King, D.J.; Seal, B.S.; Brown, C.C. Virulence of six heterogeneous-origin Newcastle disease virus isolates before and after sequential passages in domestic chickens. Avian Pathol. 2003, 32, 81–93. [Google Scholar] [CrossRef]
- Cui, S.; Xiong, H.; Feng, Z.; Chu, Y.; Que, C.; Qin, J.; Pan, Y.; Yu, K.; Jia, L.; Yao, X.; et al. Severe pigeon paramyxovirus 1 infection in a human case with probable post-COVID-19 condition. Emerg. Microbes Infect. 2023, 12, 2251600. [Google Scholar] [CrossRef]
- Miller, P.J.; King, D.J.; Afonso, C.L.; Suarez, D.L. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine 2007, 25, 7238–7246. [Google Scholar] [CrossRef]
- Hu, S.; Ma, H.; Wu, Y.; Liu, W.; Wang, X.; Liu, Y.; Liu, X. A vaccine candidate of attenuated genotype VII Newcastle disease virus generated by reverse genetics. Vaccine 2009, 27, 904–910. [Google Scholar] [CrossRef]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.; Miller, P.J. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef]
- Hamouda, E.E.; Eid, A.A.M.; Gouda, H.F.; Dessouki, A.A.; El-Deeb, A.H.; Daines, R.; Iqbal, M.; ElBakrey, R.M. Assessment of PPMV-1 Genotype VI Virulence in Pigeons and Chickens and Protective Effectiveness of Paramyxovirus Vaccines in Pigeons. Viruses 2024, 16, 1585. [Google Scholar] [CrossRef]
- Dimitrov, K.M.; Ramey, A.M.; Qiu, X.; Bahl, J.; Afonso, C.L. Temporal, geographic, and host distribution of avian paramyxovirus 1 (Newcastle disease virus). Infect. Genet. Evol. 2016, 39, 22–34. [Google Scholar] [CrossRef]
- Wakamatsu, N.; King, D.J.; Seal, B.S.; Peeters, B.P.; Brown, C.C. The effect on pathogenesis of Newcastle disease virus LaSota strain from a mutation of the fusion cleavage site to a virulent sequence. Avian Dis. 2006, 50, 483–488. [Google Scholar] [CrossRef]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef]
- Dortmans, J.C.; Koch, G.; Rottier, P.J.; Peeters, B.P. Virulence of Newcastle disease virus: What is known so far? Vet. Res. 2011, 42, 122. [Google Scholar] [CrossRef]
- Selim, K.M.; Selim, A.; Arafa, A.; Hussein, H.A.; Elsanousi, A.A. Molecular characterization of full fusion protein (F) of Newcastle disease virus genotype VIId isolated from Egypt during 2012–2016. Vet World 2018, 11, 930–938. [Google Scholar] [CrossRef]
- Peeters, B.P.; de Leeuw, O.S.; Koch, G.; Gielkens, A.L. Rescue of Newcastle disease virus from cloned cDNA: Evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 1999, 73, 5001–5009. [Google Scholar] [CrossRef]
- Xiao, S.; Nayak, B.; Samuel, A.; Paldurai, A.; Kanabagattebasavarajappa, M.; Prajitno, T.Y.; Bharoto, E.E.; Collins, P.L.; Samal, S.K. Generation by reverse genetics of an effective, stable, live-attenuated newcastle disease virus vaccine based on a currently circulating, highly virulent Indonesian strain. PLoS ONE 2012, 7, e52751. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, D.; Liu, B.; Liang, L.; Liang, R.; Tang, X.; Qiu, X.; Ding, C.; Ding, J.; Hou, S. Isolation, identification and pathogenicity analysis of pigeon paramyxovirus-1 TJ20 strain. Acta Vet. Zootech. Sin. 2024, 55, 4051–4060. [Google Scholar]
- Peng, T.; Qiu, X.; Tan, L.; Yu, S.; Yang, B.; Dai, J.; Liu, X.; Sun, Y.; Song, C.; Liu, W.; et al. Ubiquitination on Lysine 247 of Newcastle Disease Virus Matrix Protein Enhances Viral Replication and Virulence by Driving Nuclear-Cytoplasmic Trafficking. J. Virol. 2022, 96, e0162921. [Google Scholar] [CrossRef]
- Zhan, T.; He, D.; Lu, X.; Liao, T.; Wang, W.; Chen, Q.; Liu, X.; Gu, M.; Wang, X.; Hu, S.; et al. Biological Characterization and Evolutionary Dynamics of Pigeon Paramyxovirus Type 1 in China. Front. Vet. Sci. 2021, 8, 721102. [Google Scholar] [CrossRef]
- WOAH. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, World Organisation for Animal Health, Twelfth Edition 2023 Chapter 3.3.14. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.03.14_NEWCASTLE_DIS.pdf (accessed on 9 October 2024).
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the Calculation of TCID(50) for Quantitation of Virus Infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Chen, H.; Tian, J.; Zhao, X.; Jia, Y.; Xiao, S.; Wang, X.; Liu, H.; Yang, Z. Comparative biology of two genetically closely related Newcastle disease virus strains with strongly contrasting pathogenicity. Vet. Microbiol. 2021, 253, 108977. [Google Scholar] [CrossRef]
- Paldurai, A.; Kim, S.H.; Nayak, B.; Xiao, S.; Shive, H.; Collins, P.L.; Samal, S.K. Evaluation of the contributions of individual viral genes to newcastle disease virus virulence and pathogenesis. J. Virol. 2014, 88, 8579–8596. [Google Scholar] [CrossRef]
- Tian, Y.; Xue, R.; Yang, W.; Li, Y.; Xue, J.; Zhang, G. Characterization of ten paramyxovirus type 1 viruses isolated from pigeons in China during 1996-2019. Vet Microbiol. 2020, 244, 108661. [Google Scholar] [CrossRef]
- Zhan, T.; Lu, X.; He, D.; Gao, X.; Chen, Y.; Hu, Z.; Wang, X.; Hu, S.; Liu, X. Phylogenetic analysis and pathogenicity assessment of pigeon paramyxovirus type 1 circulating in China during 2007–2019. Transbound. Emerg. Dis. 2022, 69, 2076–2088. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhao, J.; Xue, J.; Yang, Y.L.; Zhang, G.Z. Antigenic variation of LaSota and genotype VII Newcastle disease virus (NDV) and their efficacy against challenge with velogenic NDV. Vaccine 2017, 35, 27–32. [Google Scholar] [CrossRef]
- Liu, M.M.; Cheng, J.L.; Yu, X.H.; Qin, Z.M.; Tian, F.L.; Zhang, G.Z. Generation by reverse genetics of an effective attenuated Newcastle disease virus vaccine based on a prevalent highly virulent Chinese strain. Biotechnol. Lett. 2015, 37, 1287–1296. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Xu, Y.; Han, Z.; Shao, Y.; Kong, X.; Liu, S. A comparative study of pigeons and chickens experimentally infected with PPMV-1 to determine antigenic relationships between PPMV-1 and NDV strains. Vet. Microbiol. 2014, 168, 88–97. [Google Scholar] [CrossRef]
- Kapczynski, D.R.; Afonso, C.L.; Miller, P.J. Immune responses of poultry to Newcastle disease virus. Dev. Comp. Immunol. 2013, 41, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Q.; Tian, M.X.; Wang, Y.P.; Zhao, Y.; Zou, N.L.; Zhao, F.F.; Cao, S.J.; Wen, X.T.; Liu, P.; Huang, Y. The different expression of immune-related cytokine genes in response to velogenic and lentogenic Newcastle disease viruses infection in chicken peripheral blood. Mol. Biol. Rep. 2012, 39, 3611–3618. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Saxena, V.K.; Ara, A.; Singh, K.B.; Sundaresan, N.R.; Saxena, M.; Rasool, T.J. Immune response to Newcastle disease virus in chicken lines divergently selected for cutaneous hypersensitivity. Int. J. Immunogenet. 2007, 34, 445–455. [Google Scholar] [CrossRef]
- Degen, W.G.; Daal, N.; Rothwell, L.; Kaiser, P.; Schijns, V.E. Th1/Th2 polarization by viral and helminth infection in birds. Vet. Microbiol. 2005, 105, 163–167. [Google Scholar] [CrossRef]
- Liu, H.; Tian, J.; Lu, K.; Li, Y.; Guan, Z.; Cao, X.; Li, X.; Chang, Z.; Wang, X.; Sa, X.; et al. Chicken ISG12(2) attenuates Newcastle disease virus and enhances the efficiency of Newcastle disease vaccine via activating immune pathways. Transbound. Emerg. Dis. 2022, 69, 2634–2648. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Xu, L.; Hu, Y.; Zhang, B.; Liu, J. Immunologic synergism with IL-2 and effects of cCHMIs on mRNA expression of IL-2 and IFN-gamma in chicken peripheral T lymphocyte. Vaccine 2006, 24, 7109–7114. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, L.A.; de Leeuw, O.S.; Tacken, M.G.; Klos, H.C.; de Vries, R.P.; de Boer-Luijtze, E.A.; van Zoelen-Bos, D.J.; Rigter, A.; Rottier, P.J.; Moormann, R.J.; et al. Protective efficacy of Newcastle disease virus expressing soluble trimeric hemagglutinin against highly pathogenic H5N1 influenza in chickens and mice. PLoS ONE 2012, 7, e44447. [Google Scholar] [CrossRef]
- Miller, P.J.; Afonso, C.L.; El Attrache, J.; Dorsey, K.M.; Courtney, S.C.; Guo, Z.; Kapczynski, D.R. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Dev. Comp. Immunol. 2013, 41, 505–513. [Google Scholar] [CrossRef]
- Dortmans, J.C.; Peeters, B.P.; Koch, G. Newcastle disease virus outbreaks: Vaccine mismatch or inadequate application? Vet. Microbiol. 2012, 160, 17–22. [Google Scholar] [CrossRef]

| Primer Names | Primer Sequence (5′→3′) |
|---|---|
| C1-F | CGACTCACTATAGGACCAAACAGAGAATCCGTGAGTTA |
| C1-R | CCgGTaCCTTGTAGGACGATC |
| S2-F | GTTAGCATTCCCGATCGTCCTACAAGGtACcGGTG |
| S2-R | AGGAGGTGGAGATGCCATGCCGACCCACGCGTTATaAGgCGCccCTGTCTCCc |
| S3-F | GAgGGAGaCAGggGCGCCTTATAG |
| S3-R | GTGGAGATGCCATGCCGACCCACGCGTGGAGCTCGCCATTTCCTACCCG |
| C4-F | ACGGGTAGGAAATGGCGAG |
| C4-R | AGGAGGTGGAGATGCCATGCCGACCCACGCGTCTGTTCCGGGCATAGTCTG |
| C5-F | CTCGCTGACGCTAGCAG |
| C5-R | GAGGAGGTGGAGATGCCATGCCGACCCACCAAACAAAGATTTGGTGAATG |
| TVT-F | GGGTCGGCATGGCATCTCCAC |
| TVT-R | TGGTCCTATAGTGAGTCGTATTAATTTCGCGGG |
| 6NP-F | CACTATAGGCTAGCCTCGAGGCCACCATGTCGTCCGTCTTTG |
| 6NP-R | CTCTAGAGGTACCACGCGTTCAGTACCCCCAGTCGGTG |
| 6P-F | CACTATAGGCTAGCCTCGAGGCCACCATGGCAACTTTTACTGATGCTG |
| 6P-R | TCTAGAGGTACCACGCGTTCAACCATTCAGTGCAAGGC |
| 6L1-F | CACTATAGGCTAGCCTCGAGGCCACCATGGCGAGCTCCGGTCCTGAAAG |
| 6L1-R | GCTAGCGTCAGCGAGCACATAGC |
| 6L2-F | GTCTGCTATGTGCTCGCTG |
| 6L2-R | CTCTAGAGGTACCACGCGTTTAGGAGTCATTGTTACTGTAATATCCCTTTG |
| pCI-V-F | ACGCGTGGTACCTCTAGAGTC |
| pCI-V-R | CTCGAGGCTAGCCTATAGTGAGTC |
| PPMV1-3F 1 | TCAAAGCAGACATCCTCCA |
| PPMV1-3R 1 | AAATGTMACTTTCTTTCCCCTCT |
| PPMV1-4F 2 | ATGTCACTATTGAYGTGGAGGTA |
| PPMV1-4R 2 | GGACAAGTGCTGAGGCAAAYC |
| Groups | Number | Virus Shedding | Morbidity | Mortality | |
|---|---|---|---|---|---|
| 5 dpc | 10 dpc | ||||
| aTJ | 10 | 3/10 | 1/10 | 1/10 | 0/10 |
| PBS | 10 | 10/10 | 5/5 | 10/10 *** | 10/10 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liu, D.; Liu, B.; Liang, R.; Liang, L.; Tang, X.; Hou, S.; Ding, C.; Qiu, X.; Ding, J. Live Attenuated aTJ Vaccine Effectively Protects Pigeons Against Homologous PPMV-1 Challenge. Vaccines 2024, 12, 1304. https://doi.org/10.3390/vaccines12121304
Zhang S, Liu D, Liu B, Liang R, Liang L, Tang X, Hou S, Ding C, Qiu X, Ding J. Live Attenuated aTJ Vaccine Effectively Protects Pigeons Against Homologous PPMV-1 Challenge. Vaccines. 2024; 12(12):1304. https://doi.org/10.3390/vaccines12121304
Chicago/Turabian StyleZhang, Shan, Dahu Liu, Baojing Liu, Ruinying Liang, Lin Liang, Xinming Tang, Shaohua Hou, Chan Ding, Xusheng Qiu, and Jiabo Ding. 2024. "Live Attenuated aTJ Vaccine Effectively Protects Pigeons Against Homologous PPMV-1 Challenge" Vaccines 12, no. 12: 1304. https://doi.org/10.3390/vaccines12121304
APA StyleZhang, S., Liu, D., Liu, B., Liang, R., Liang, L., Tang, X., Hou, S., Ding, C., Qiu, X., & Ding, J. (2024). Live Attenuated aTJ Vaccine Effectively Protects Pigeons Against Homologous PPMV-1 Challenge. Vaccines, 12(12), 1304. https://doi.org/10.3390/vaccines12121304







