Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Vaccine Formulations
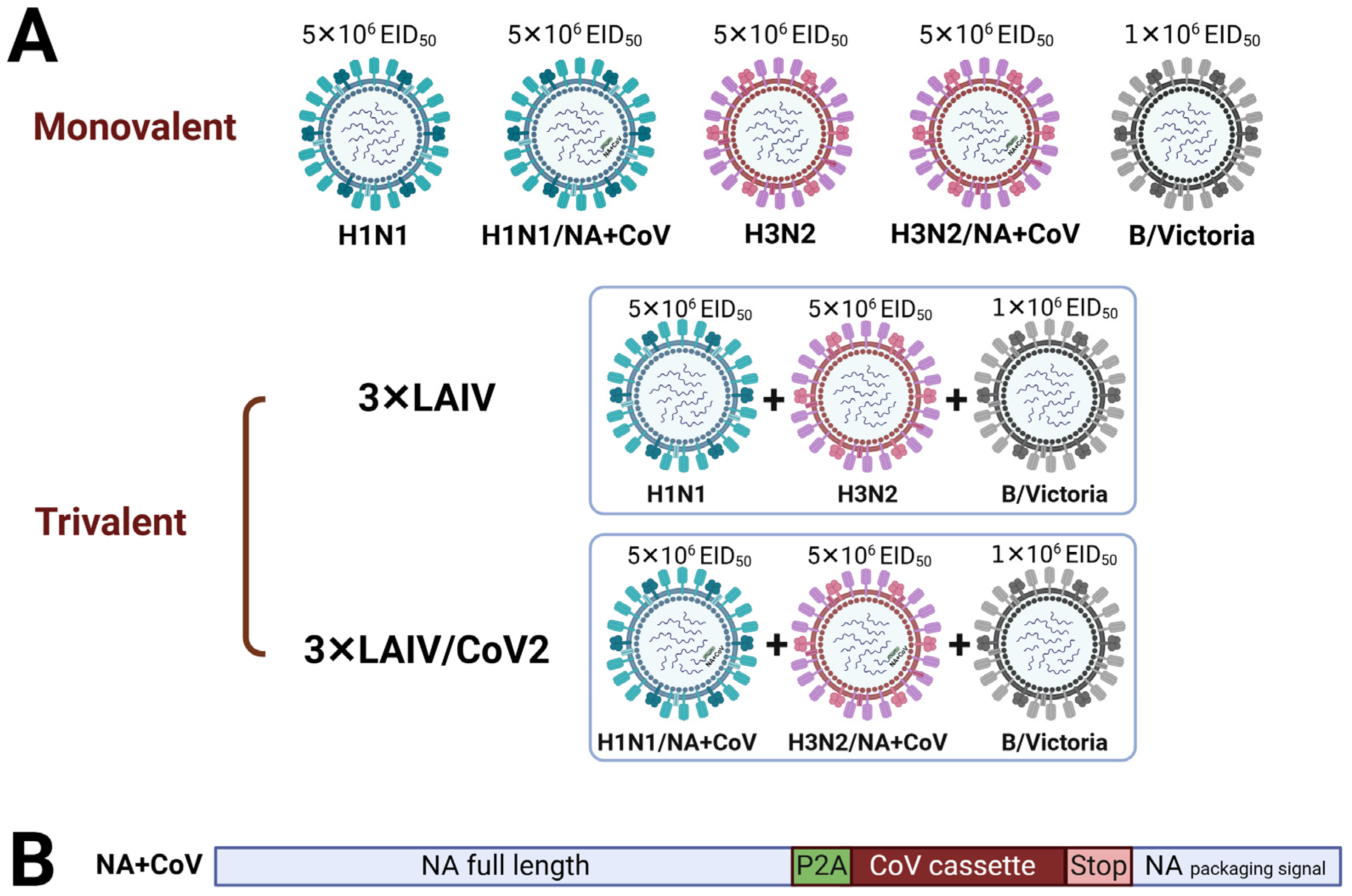
2.2. Titration of Influenza Viruses and SARS-CoV-2
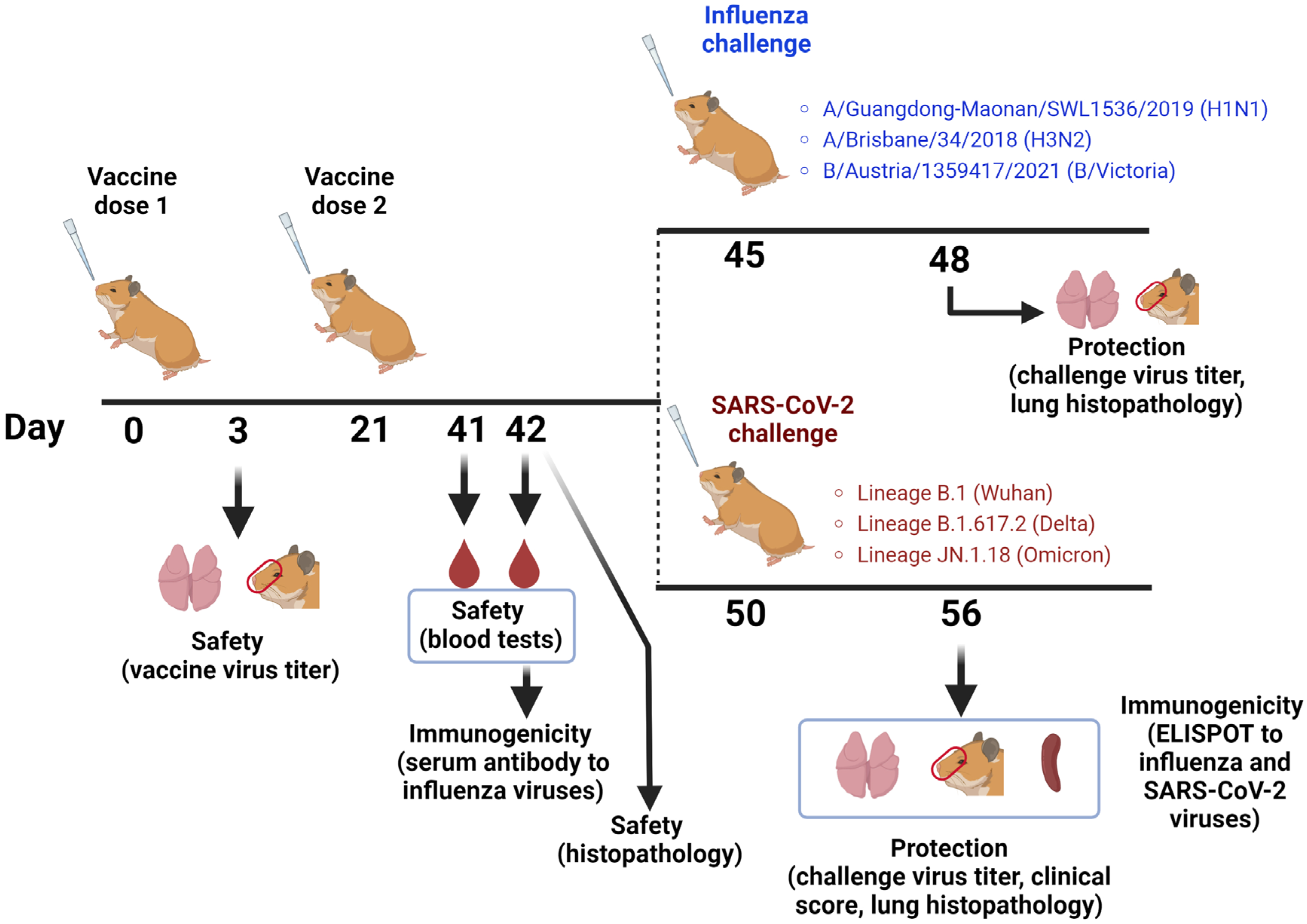
2.3. Hamster Study Design
2.4. Safety Assessment
2.4.1. Animal Health Status
2.4.2. Blood Tests
2.4.3. Histopathology
2.5. Immunogenicity Assessment
2.6. Protection Against Influenza Viruses
2.7. Protection Against SARS-CoV-2 Infection
2.8. Statistical Analysis
3. Results
3.1. Characterization of Vaccine Strains Included in the Trivalent LAIV and LAIV/CoV2 Vaccines
3.2. Safety of the 3×LAIV/CoV2 Vaccine in a Syrian Hamster Model
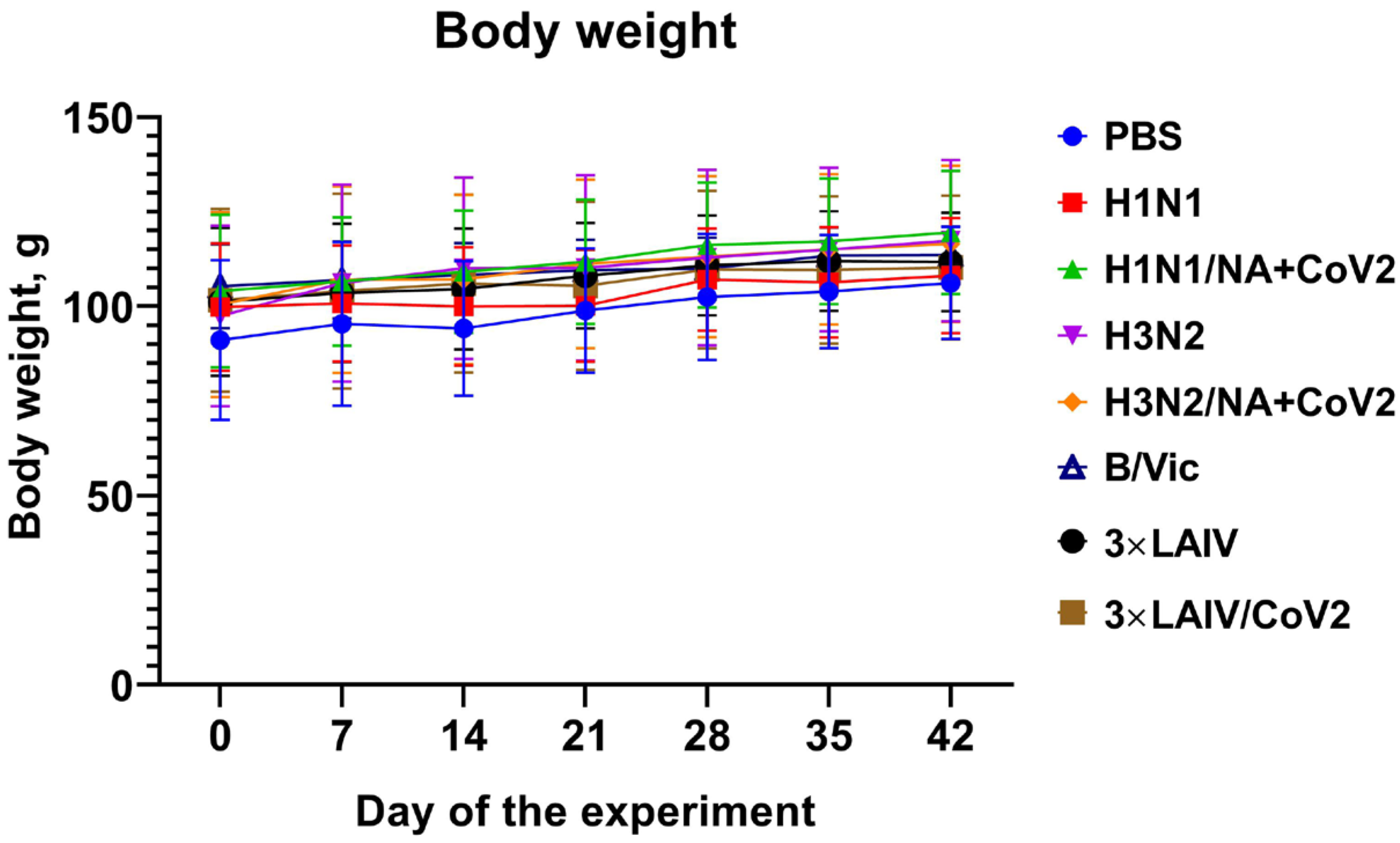
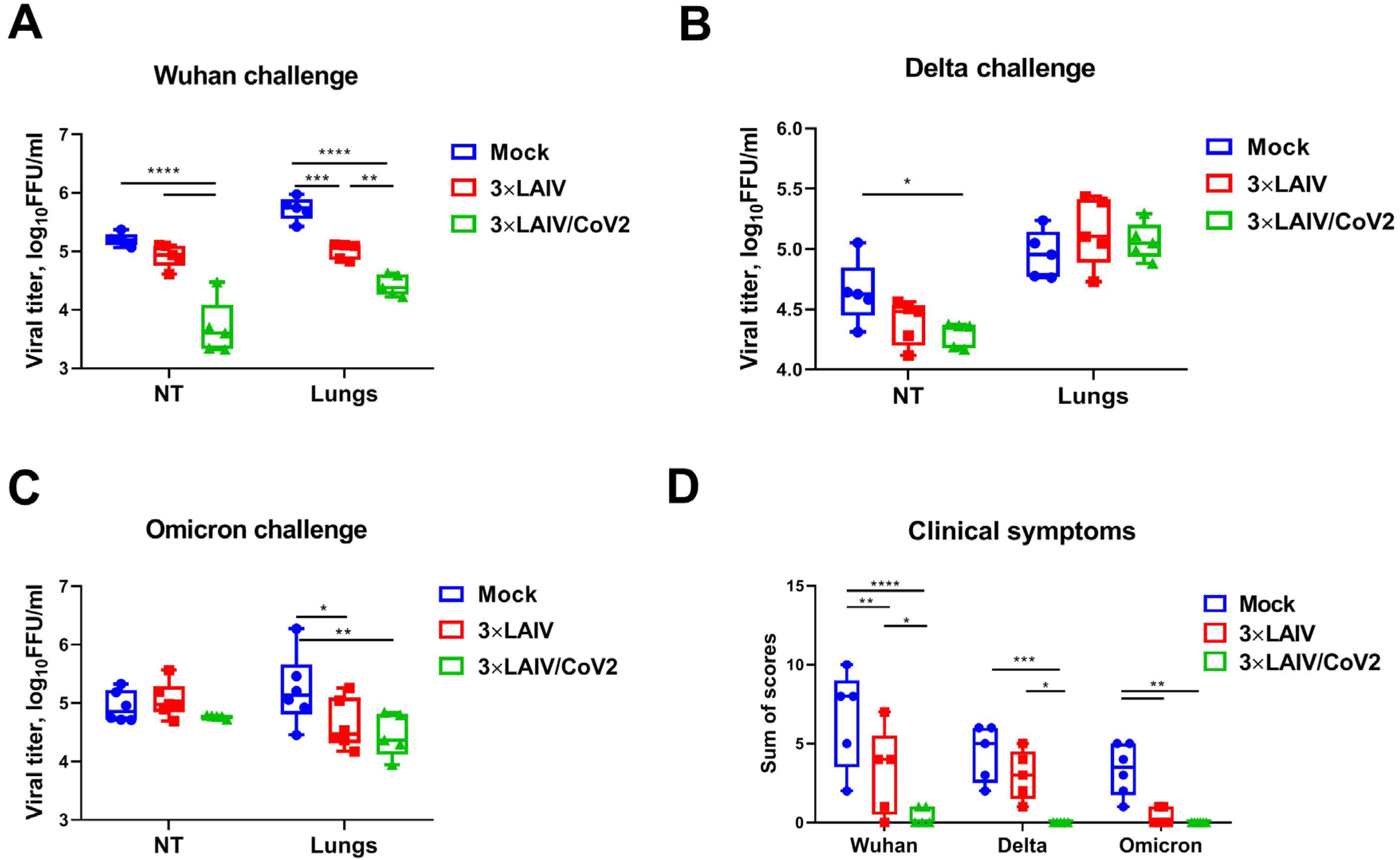
3.2.1. Clinical Observations
3.2.2. Blood Tests
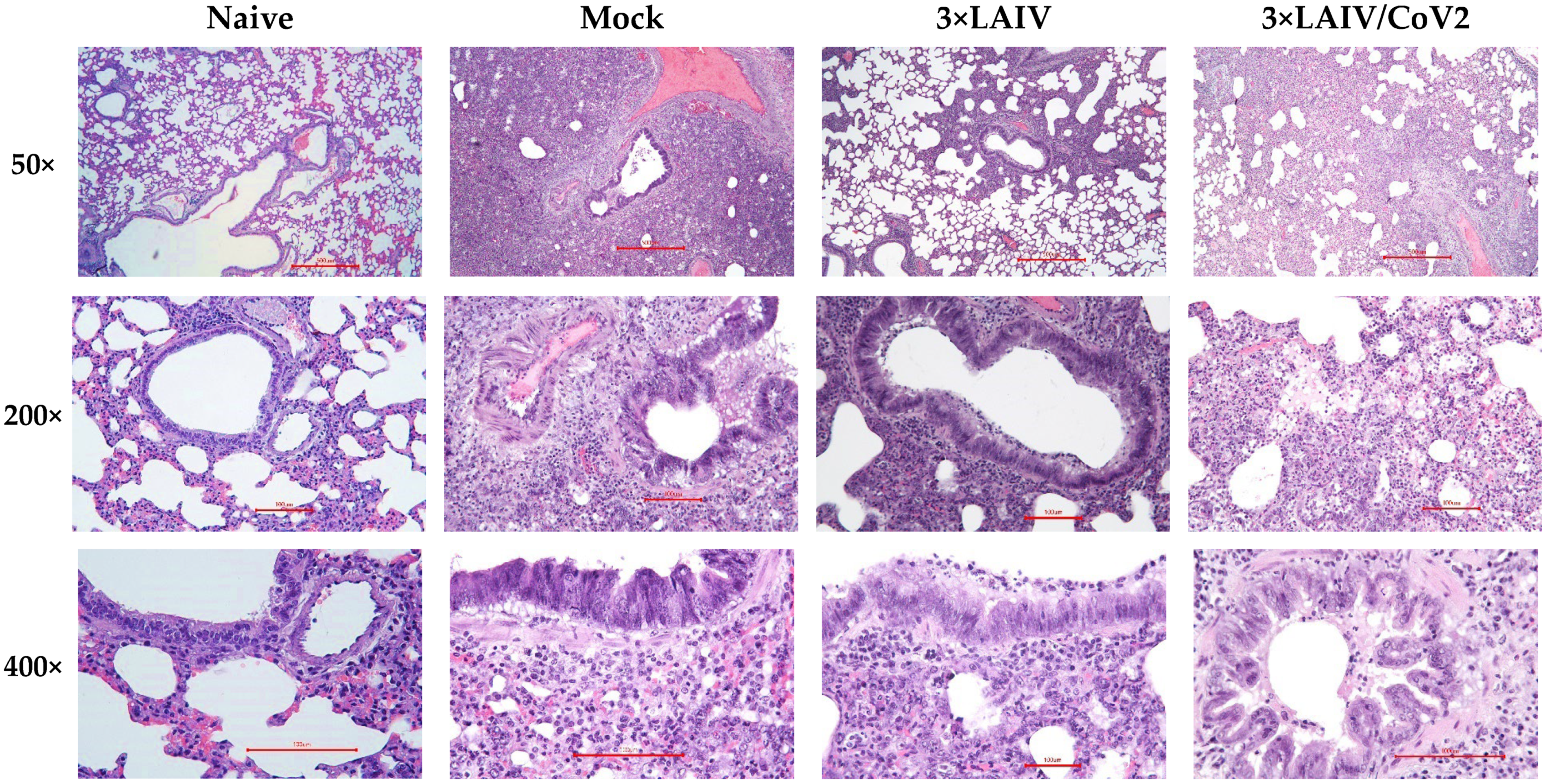
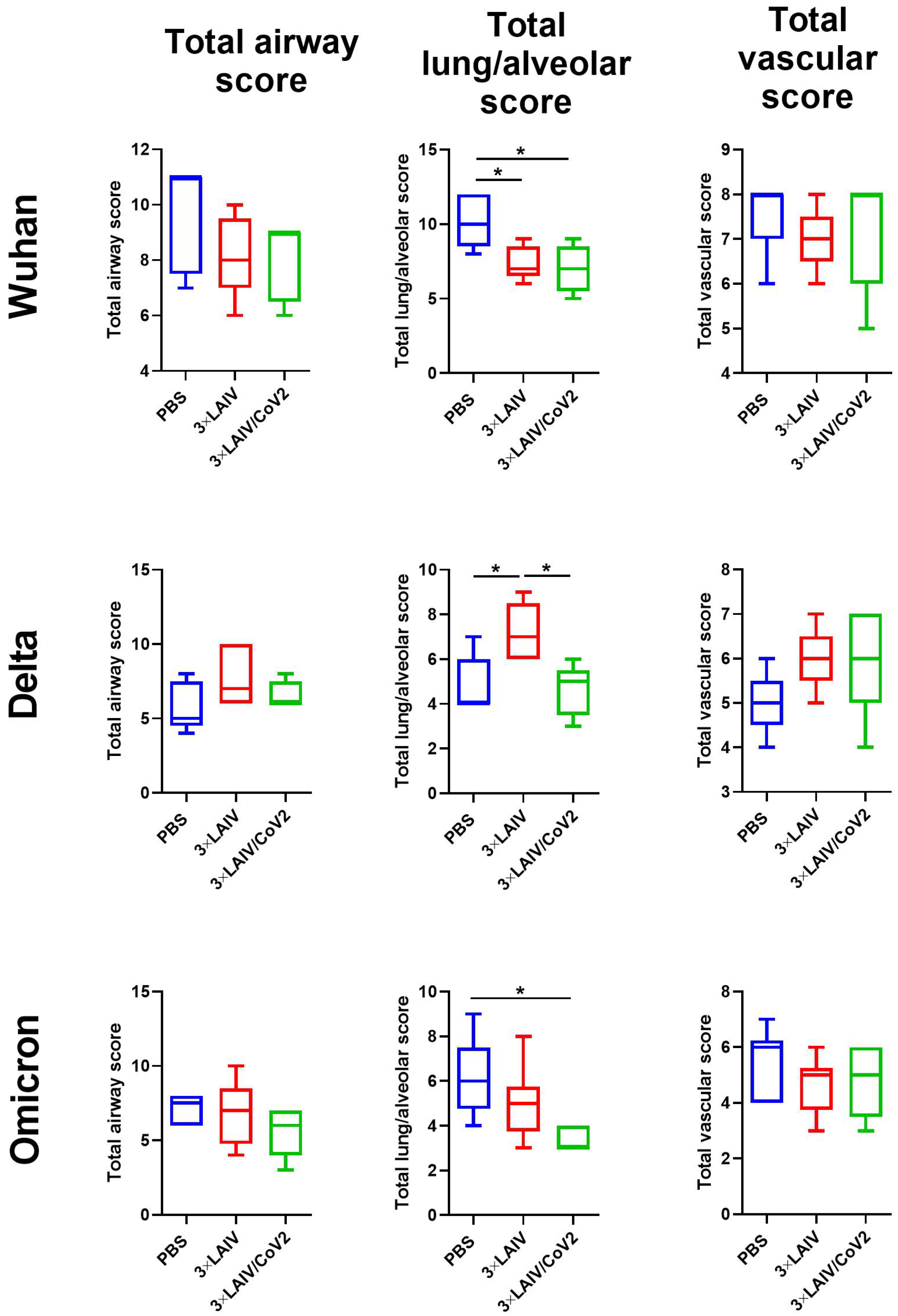
3.2.3. Pathology and Histopathology
3.3. Influenza-Specific Antibody Immune Responses
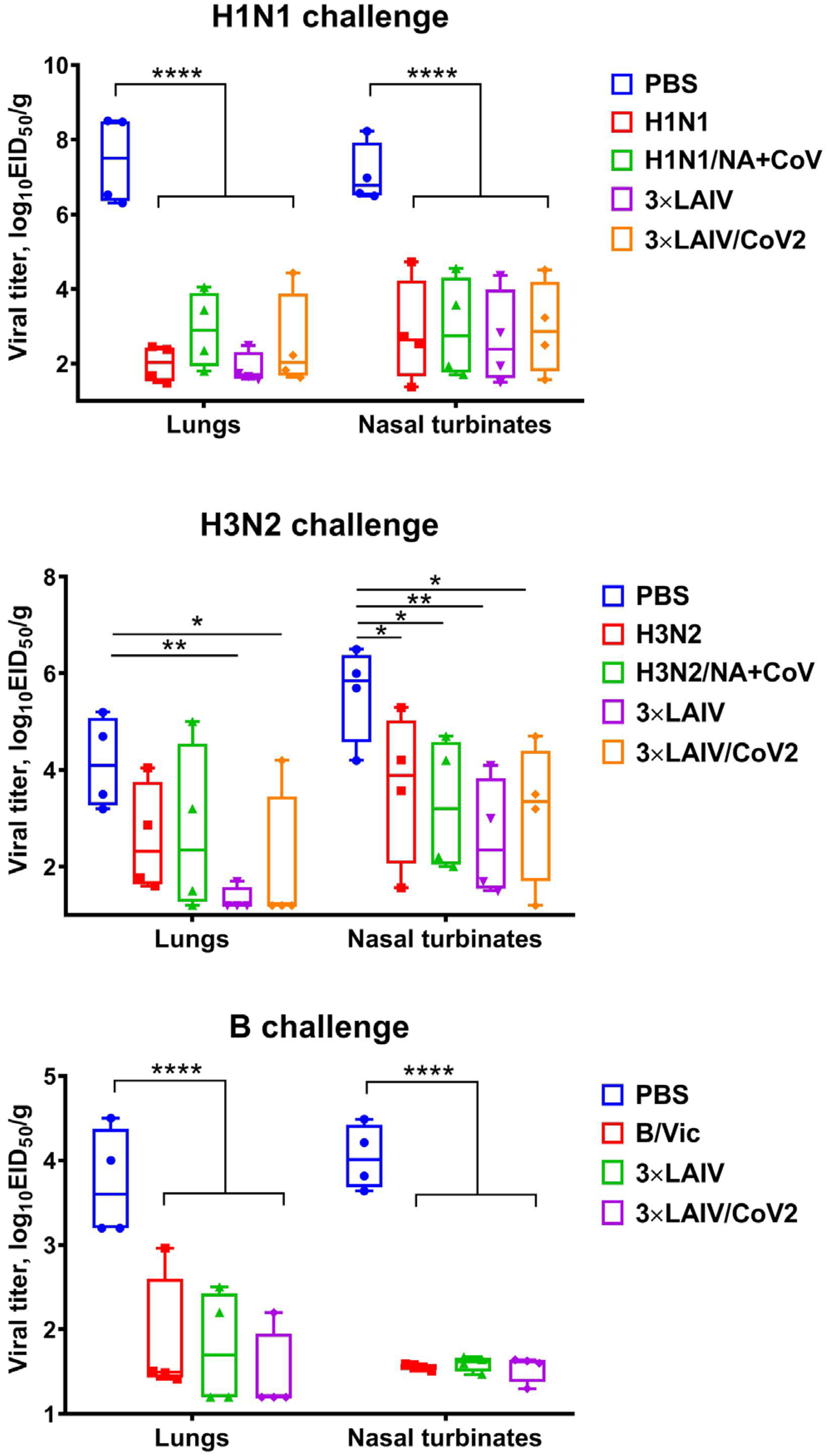
3.4. Protection Against Influenza Challenge
3.5. Protection Against SARS-CoV-2 Infection
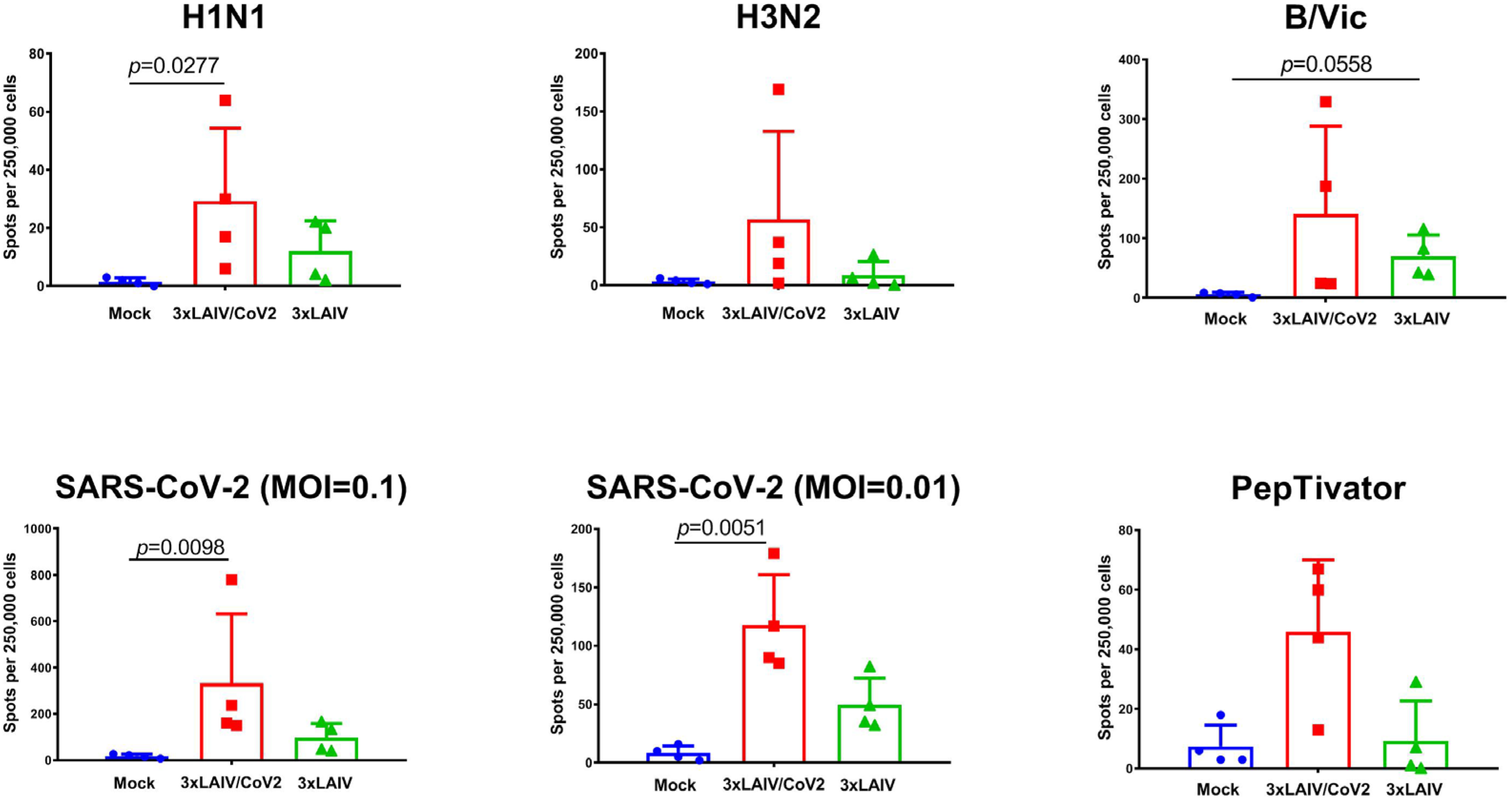
3.6. Cell-Mediated Immune Responses to Influenza and SARS-CoV-2 Antigens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 19 September 2024).
- Caini, S.; Meijer, A.; Nunes, M.C.; Henaff, L.; Zounon, M.; Boudewijns, B.; Del Riccio, M.; Paget, J. Probable Extinction of Influenza B/Yamagata and Its Public Health Implications: A Systematic Literature Review and Assessment of Global Surveillance Databases. Lancet Microbe 2024, 5, 100851. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The Evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Moro, P.L.; Zhang, B.; Ennulat, C.; Harris, M.; McVey, R.; Woody, G.; Marquez, P.; McNeil, M.M.; Su, J.R. Safety of Co-Administration of mRNA COVID-19 and Seasonal Inactivated Influenza Vaccines in the Vaccine Adverse Event Reporting System (VAERS) during July 1, 2021–June 30, 2022. Vaccine 2023, 41, 1859. [Google Scholar] [CrossRef]
- Stepanova, E.; Isakova-Sivak, I.; Rudenko, L. Options for the Development of a Bivalent Vaccine against SARS-CoV-2 and Influenza. Expert. Rev. Vaccines 2022, 21, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Isakova-Sivak, I.; Stepanova, E.; Matyushenko, V.; Niskanen, S.; Mezhenskaya, D.; Bazhenova, E.; Krutikova, E.; Kotomina, T.; Prokopenko, P.; Neterebskii, B.; et al. Development of a T Cell-Based COVID-19 Vaccine Using a Live Attenuated Influenza Vaccine Viral Vector. Vaccines 2022, 10, 1142. [Google Scholar] [CrossRef]
- Stepanova, E.; Isakova-Sivak, I.; Matyushenko, V.; Mezhenskaya, D.; Kudryavtsev, I.; Kostromitina, A.; Chistiakova, A.; Rak, A.; Bazhenova, E.; Prokopenko, P.; et al. Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates. Vaccines 2024, 12, 1099. [Google Scholar] [CrossRef]
- Wong, P.-F.; Isakova-Sivak, I.; Stepanova, E.; Krutikova, E.; Bazhenova, E.; Rekstin, A.; Rudenko, L. Development of Cross-Reactive Live Attenuated Influenza Vaccine Candidates against Both Lineages of Influenza B Virus. Vaccines 2024, 12, 95. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, O.; Wall, J.B.J.; Zheng, M.; Zhou, Y.; Wang, L.; Vaseghi, H.R.; Qian, L.; Liu, J. Systematic Comparison of 2A Peptides for Cloning Multi-Genes in a Polycistronic Vector. Sci. Rep. 2017, 7, 2193. [Google Scholar] [CrossRef]
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance. Available online: http://www.wpro.who.int/emerging_diseases/documents/docs/manualonanimalaidiagnosisandsurveillance.pdf (accessed on 25 August 2018).
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Rak, A.; Matyushenko, V.; Prokopenko, P.; Kostromitina, A.; Polyakov, D.; Sokolov, A.; Rudenko, L.; Isakova-Sivak, I. A Novel Immunofluorescent Test System for SARS-CoV-2 Detection in Infected Cells. PLoS ONE 2024, 19, e0304534. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Official Journal of the European Union. L 276/33. Available online: http://data.europa.eu/eli/dir/2010/63/oj/eng (accessed on 25 August 2018).
- Rak, A.Y.; Donina, S.A.; Isakova-Sivak, I.N.; Rudenko, L.G. Optimization of purification conditions and study of antigenic properties of recombinant nucleocapsid protein of different SARS-CoV-2 strains. Med. Acad. J. 2022, 22, 235–241. [Google Scholar] [CrossRef]
- Lewis, K.D.C.; Ortiz, J.R.; Rahman, M.Z.; Levine, M.Z.; Rudenko, L.; Wright, P.F.; Katz, J.M.; Dally, L.; Rahman, M.; Isakova-Sivak, I.; et al. Immunogenicity and Viral Shedding of Russian-Backbone, Seasonal, Trivalent, Live, Attenuated Influenza Vaccine in a Phase II, Randomized, Placebo-Controlled Trial Among Preschool-Aged Children in Urban Bangladesh. Clin. Infect. Dis. 2019, 69, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Mohn, K.G.-I.; Smith, I.; Sjursen, H.; Cox, R.J. Immune Responses after Live Attenuated Influenza Vaccination. Hum. Vaccines Immunother. 2018, 14, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, N.; Bertzbach, L.D.; Dietert, K.; Abdelgawad, A.; Vladimirova, D.; Kunec, D.; Hoffmann, D.; Beer, M.; Gruber, A.D.; Trimpert, J. Age-Dependent Progression of SARS-CoV-2 Infection in Syrian Hamsters. Viruses 2020, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Lugovskaya, S.A.; Morozova, V.T.; Pochtar, M.E.; Dolgov, V.V. Laboratory Hematology; Department of Clinical and Laboratory Diagnostics; Publisher OOO “Izdatelstvo Triada”: Tver, Russia, 2006; ISBN 5-94789-156-5. (In Russian) [Google Scholar]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef]
- Guo, F.; Yang, X.; Li, X.; Feng, R.; Guan, C.; Wang, Y.; Li, Y.; Sun, C. Nuciferine Prevents Hepatic Steatosis and Injury Induced by a High-Fat Diet in Hamsters. PLoS ONE 2013, 8, e63770. [Google Scholar] [CrossRef]
- van Heek, M.; Austin, T.M.; Farley, C.; Cook, J.A.; Tetzloff, G.G.; Davis, H.R. Ezetimibe, a Potent Cholesterol Absorption Inhibitor, Normalizes Combined Dyslipidemia in Obese Hyperinsulinemic Hamsters. Diabetes 2001, 50, 1330–1335. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, C.; Zhang, X.; Li, N.; Dong, Z.; Sun, G.; Sun, X. Atorvastatin Promotes AMPK Signaling to Protect against High Fat Diet-Induced Non-Alcoholic Fatty Liver in Golden Hamsters. Exp. Ther. Med. 2020, 19, 2133. [Google Scholar] [CrossRef]
- Korb, T.A.; Chernina, V.J.; Blohin, I.A.; Aleshina, O.O.; Vorontsov, A.V.; Morozov, S.P.; Gombolevskiy, V.A. Adrenal Gland Imaging: Normal and Pathological (Literature Review). Probl. Endocrinol. 2021, 67, 26–36. [Google Scholar] [CrossRef]
- Kurganov, I.A.; Bogdanov, D.J.; Emelyanov, S.I.; Matveev, N.L. A Surgeon’s View of Modern Aspects of Adrenal Pathology Diagnostics. Mosc. Surg. J. 2018, 5–13. (In Russian) [Google Scholar] [CrossRef]
- Miedel, E.L.; Hankenson, F.C. Biology and Diseases of Hamsters. In Laboratory Animal Medicine; Academic Press: Cambridge, MA, USA, 2015; pp. 209–245. [Google Scholar] [CrossRef]
- McInnes, E.F.; Ernst, H.; Germann, P.-G. Spontaneous Nonneoplastic Lesions in Control Syrian Hamsters in Three 24-Month Long-Term Carcinogenicity Studies. Toxicol. Pathol. 2015, 43, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Somvanshi, R.; Iyer, P.K.R.; Biswas, J.C.; Koul, G.L. Polycystic Liver Disease in Golden Hamsters. J. Comp. Pathol. 1987, 97, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.V.; Rybakova, A.V.; Guschin, Y.A.; Vaganova, D.S.; Koptyaeva, K.E.; Muzhikyan, A.A.; Makarova, M.; Makarov, V. Pathomorphological diagnostics of lungs in various methods of euthanasia of laboratory animals. Lab. Anim. Sci. Res. 2018, 3, 49–60. (In Russian) [Google Scholar] [CrossRef]
- Gad, S.C.; Gad, S.C. (Eds.) Animal Models in Toxicology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-0-429-09689-1. [Google Scholar]
- Dibben, O.; Crowe, J.; Cooper, S.; Hill, L.; Schewe, K.E.; Bright, H. Defining the Root Cause of Reduced H1N1 Live Attenuated Influenza Vaccine Effectiveness: Low Viral Fitness Leads to Inter-Strain Competition. NPJ Vaccines 2021, 6, 35. [Google Scholar] [CrossRef]
- Parker, L.; Ritter, L.; Wu, W.; Maeso, R.; Bright, H.; Dibben, O. Haemagglutinin Stability Was Not the Primary Cause of the Reduced Effectiveness of Live Attenuated Influenza Vaccine against A/H1N1pdm09 Viruses in the 2013-2014 and 2015-2016 Seasons. Vaccine 2019, 37, 4543–4550. [Google Scholar] [CrossRef]
- Dempsey, R.; Tamburrino, G.; Schewe, K.E.; Crowe, J.; Nuccitelli, A.; Dibben, O. Haemagglutinin Substitutions N125D, D127E, D222G and R223Q Improve Replicative Fitness and Vaccine Effectiveness of an A/H1N1pdm09 Live Attenuated Influenza Vaccine Virus by Enhancing α-2,6 Receptor Binding. PLoS Pathog. 2022, 18, e1010585. [Google Scholar] [CrossRef]
- Hawksworth, A.; Lockhart, R.; Crowe, J.; Maeso, R.; Ritter, L.; Dibben, O.; Bright, H. Replication of Live Attenuated Influenza Vaccine Viruses in Human Nasal Epithelial Cells Is Associated with H1N1 Vaccine Effectiveness. Vaccine 2020, 38, 4209–4218. [Google Scholar] [CrossRef]
- Cobey, S.; Gouma, S.; Parkhouse, K.; Chambers, B.S.; Ertl, H.C.; Schmader, K.E.; Halpin, R.A.; Lin, X.; Stockwell, T.B.; Das, S.R.; et al. Poor Immunogenicity, Not Vaccine Strain Egg Adaptation, May Explain the Low H3N2 Influenza Vaccine Effectiveness in 2012–2013. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, 327. [Google Scholar] [CrossRef]
- Fan, S.; Gu, C.; Kong, H.; Guan, L.; Neumann, G.; Kawaoka, Y. Influenza Viruses Suitable for Studies in Syrian Hamsters. Viruses 2022, 14, 1629. [Google Scholar] [CrossRef]
- Iwatsuki-Horimoto, K.; Nakajima, N.; Ichiko, Y.; Sakai-Tagawa, Y.; Noda, T.; Hasegawa, H.; Kawaoka, Y. Syrian Hamster as an Animal Model for the Study of Human Influenza Virus Infection. J. Virol. 2018, 92, e01693. [Google Scholar] [CrossRef]
- Matyushenko, V.; Kotomina, T.; Kudryavtsev, I.; Mezhenskaya, D.; Prokopenko, P.; Matushkina, A.; Sivak, K.; Muzhikyan, A.; Rudenko, L.; Isakova-Sivak, I. Conserved T-Cell Epitopes of Respiratory Syncytial Virus (RSV) Delivered by Recombinant Live Attenuated Influenza Vaccine Viruses Efficiently Induce RSV-Specific Lung-Localized Memory T Cells and Augment Influenza-Specific Resident Memory T-Cell Responses. Antivir. Res. 2020, 182, 104864. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.J.; Ao, Z.; Olukitibi, T.A.; Lawrynuik, P.; Shieh, C.; Kung, S.K.P.; Fowke, K.R.; Kobasa, D.; Yao, X. Oral Immunization with rVSV Bivalent Vaccine Elicits Protective Immune Responses, Including ADCC, against Both SARS-CoV-2 and Influenza A Viruses. Vaccines 2023, 11, 1404. [Google Scholar] [CrossRef]
- Ao, Z.; Ouyang, M.J.; Olukitibi, T.A.; Warner, B.; Vendramelli, R.; Truong, T.; Meilleur, C.; Zhang, M.; Kung, S.; Fowke, K.R.; et al. A Recombinant VSV-Based Bivalent Vaccine Effectively Protects against Both SARS-CoV-2 and Influenza A Virus Infection. J. Virol. 2022, 96, e01337-22. [Google Scholar] [CrossRef]
- Cao, K.; Wang, X.; Peng, H.; Ding, L.; Wang, X.; Hu, Y.; Dong, L.; Yang, T.; Hong, X.; Xing, M.; et al. A Single Vaccine Protects against SARS-CoV-2 and Influenza Virus in Mice. J. Virol. 2022, 96, e0157821. [Google Scholar] [CrossRef]
- Xing, M.; Hu, G.; Wang, X.; Wang, Y.; He, F.; Dai, W.; Wang, X.; Niu, Y.; Liu, J.; Liu, H.; et al. An Intranasal Combination Vaccine Induces Systemic and Mucosal Immunity against COVID-19 and Influenza. NPJ Vaccines 2024, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Bommireddy, R.; Stone, S.; Bhatnagar, N.; Kumari, P.; Munoz, L.E.; Oh, J.; Kim, K.-H.; Berry, J.T.L.; Jacobsen, K.M.; Jaafar, L.; et al. Influenza Virus-like Particle-Based Hybrid Vaccine Containing RBD Induces Immunity against Influenza and SARS-CoV-2 Viruses. Vaccines 2022, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Kaewborisuth, C.; Wanitchang, A.; Koonpaew, S.; Srisutthisamphan, K.; Saenboonrueng, J.; Im-Erbsin, R.; Inthawong, M.; Sunyakumthorn, P.; Thaweerattanasinp, T.; Tanwattana, N.; et al. Chimeric Virus-like Particle-Based COVID-19 Vaccine Confers Strong Protection against SARS-CoV-2 Viremia in K18-hACE2 Mice. Vaccines 2022, 10, 786. [Google Scholar] [CrossRef]
- Mazunina, E.P.; Gushchin, V.A.; Bykonia, E.N.; Kleymenov, D.A.; Siniavin, A.E.; Kozlova, S.R.; Mukasheva, E.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Usachev, E.V.; et al. Immunogenicity and Efficacy of Combined mRNA Vaccine Against Influenza and SARS-CoV-2 in Mice Animal Models. Vaccines 2024, 12, 1206. [Google Scholar] [CrossRef]
- Loes, A.N.; Gentles, L.E.; Greaney, A.J.; Crawford, K.H.D.; Bloom, J.D. Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice. Viruses 2020, 12, 987. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and Immunogenicity of a Live-Attenuated Influenza Virus Vector-Based Intranasal SARS-CoV-2 Vaccine in Adults: Randomised, Double-Blind, Placebo-Controlled, Phase 1 and 2 Trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Li, Y.; Liu, Q.; Deng, L.; Lu, Y.; Zhang, X.; Li, S.; Ge, J.; Bu, Z.; et al. An Influenza Virus Vector Candidate Vaccine Stably Expressing SARS-CoV-2 Receptor-Binding Domain Produces High and Long-Lasting Neutralizing Antibodies in Mice. Vet. Microbiol. 2022, 271, 109491. [Google Scholar] [CrossRef] [PubMed]
- Chaparian, R.R.; Harding, A.T.; Hamele, C.E.; Riebe, K.; Karlsson, A.; Sempowski, G.D.; Heaton, N.S.; Heaton, B.E. A Virion-Based Combination Vaccine Protects against Influenza and SARS-CoV-2 Disease in Mice. J. Virol. 2022, 96, e00689-22. [Google Scholar] [CrossRef]
- Moser, M.J.; Hill-Batorski, L.; Bowen, R.A.; Matejka, S.M.; Marshall, D.; Kawaoka, Y.; Neumann, G.; Bilsel, P. Intranasal Single-Replication Influenza Vector Induces Cross-Reactive Serum and Mucosal Antibodies against SARS-CoV-2 Variants. Vaccines 2023, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Koonpaew, S.; Kaewborisuth, C.; Srisutthisamphan, K.; Wanitchang, A.; Thaweerattanasinp, T.; Saenboonrueng, J.; Poonsuk, S.; Jengarn, J.; Viriyakitkosol, R.; Kramyu, J.; et al. A Single-Cycle Influenza A Virus-Based SARS-CoV-2 Vaccine Elicits Potent Immune Responses in a Mouse Model. Vaccines 2021, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Rader, N.A.; Lee, K.S.; Loes, A.N.; Miller-Stump, O.A.; Cooper, M.; Wong, T.Y.; Boehm, D.T.; Barbier, M.; Bevere, J.R.; Damron, F.H. Influenza Virus Strains Expressing SARS-CoV-2 Receptor Binding Domain Protein Confer Immunity in K18-hACE2 Mice. Vaccine X 2024, 20, 100543. [Google Scholar] [CrossRef] [PubMed]
- Hill-Batorski, L.; Bowen, R.; Bielefeldt-Ohmann, H.; Moser, M.J.; Matejka, S.M.; Marshall, D.; Kawaoka, Y.; Neumann, G.; Bilsel, P. Mucosal Immunization with Dual Influenza/COVID-19 Single-Replication Virus Vector Protects Hamsters from SARS-CoV-2 Challenge. Vaccine 2024, 42, 2770–2780. [Google Scholar] [CrossRef]
- Wang, D.; Deng, Y.; Zhou, J.; Wang, W.; Huang, B.; Wang, W.; Wei, L.; Ren, J.; Han, R.; Bing, J.; et al. Single-Dose Intranasal Immunisation with Novel Chimeric H1N1 Expressing the Receptor-Binding Domain of SARS-CoV-2 Induces Robust Mucosal Immunity, Tissue-Resident Memory T Cells, and Heterologous Protection in Mice. Vaccines 2023, 11, 1453. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Yuan, L.; Chen, Y.; Wang, X.; Tang, N.; Wei, D.; Ye, X.; Xia, N.; Chen, Y. Intranasal Influenza-Vectored COVID-19 Vaccines Confer Broad Protection against SARS-CoV-2 XBB Variants in Hamsters. PNAS Nexus 2024, 3, 183. [Google Scholar] [CrossRef]
- Li, Y.; Liu, P.; Hao, T.; Liu, S.; Wang, X.; Xie, Y.; Xu, K.; Lei, W.; Zhang, C.; Han, P.; et al. Rational Design of an Influenza-COVID-19 Chimeric Protective Vaccine with HA-Stalk and S-RBD. Emerg. Microbes Infect. 2023, 12, 2231573. [Google Scholar] [CrossRef]
- Devaux, C.A.; Camoin-Jau, L. Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection. Viruses 2023, 15, 1045. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Chen, L.-M.; Matsuoka, Y.; Voeten, J.T.M.; Kiseleva, I.; Heldens, J.G.M.; van den Bosch, H.; Klimov, A.; Rudenko, L.; Cox, N.J.; et al. Genetic Bases of the Temperature-Sensitive Phenotype of a Master Donor Virus Used in Live Attenuated Influenza Vaccines: A/Leningrad/134/17/57 (H2N2). Virology 2011, 412, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, E.A.; Krutikova, E.V.; Kiseleva, I.V.; Rudenko, L.G. The Spatial Location of Single Amino Acid Substitutions in Proteins of Cold-Adapted Influenza B Viruses and Their Impact upon Cold Adaptation. Mol. Genet. Microbiol. Virol. 2018, 33, 169–181. [Google Scholar] [CrossRef]
- Rudenko, L.; Isakova-Sivak, I. Pandemic Preparedness with Live Attenuated Influenza Vaccines Based on A/Leningrad/134/17/57 Master Donor Virus. Expert. Rev. Vaccines 2015, 14, 395–412. [Google Scholar] [CrossRef] [PubMed]
- WHO Coadministration of Seasonal Inactivated Influenza and COVID-19 Vaccines: Interim Guidance, 21 October 2021. Available online: https://iris.who.int/bitstream/handle/10665/346897/WHO-2019-nCoV-SAGE-Vaccines-coadministration-Influenza-2021.1-eng.pdf?sequence=1 (accessed on 16 October 2024).
- Domnich, A.; Grassi, R.; Fallani, E.; Spurio, A.; Bruzzone, B.; Panatto, D.; Marozzi, B.; Cambiaggi, M.; Vasco, A.; Orsi, A.; et al. Changes in Attitudes and Beliefs Concerning Vaccination and Influenza Vaccines between the First and Second COVID-19 Pandemic Waves: A Longitudinal Study. Vaccines 2021, 9, 1016. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Cheong, H.J. Should the COVID-19 Vaccine Be Administered Simultaneously with Other Vaccines? Infect. Chemother. 2021, 53, 589. [Google Scholar] [CrossRef]
- Kwon, S.L.; Kim, S.-Y.; Song, M.; Lee, H.-M.; Ban, S.-H.; Lee, M.-S.; Jeong, H. Assessing the Determinants of Influenza and COVID-19 Vaccine Co-Administration Decisions in the Elderly. Hum. Vaccines Immunother. 2024, 20, 2346966. [Google Scholar] [CrossRef]
- Houle, S.K.D.; Johal, A.; Roumeliotis, P.; Roy, B.; Boivin, W. Co-Administration of Influenza and COVID-19 Vaccines: A Cross-Sectional Survey of Canadian Adults’ Knowledge, Attitudes, and Beliefs. Pharmacy 2024, 12, 70. [Google Scholar] [CrossRef]
- Murdoch, L.; Quan, K.; Baber, J.A.; Ho, A.W.Y.; Zhang, Y.; Xu, X.; Lu, C.; Cooper, D.; Koury, K.; Lockhart, S.P.; et al. Safety and Immunogenicity of the BNT162b2 Vaccine Coadministered with Seasonal Inactivated Influenza Vaccine in Adults. Infect. Dis. Ther. 2023, 12, 2241–2258. [Google Scholar] [CrossRef]
- Pattinson, D.; Jester, P.; Gu, C.; Guan, L.; Armbrust, T.; Petrie, J.G.; King, J.P.; Nguyen, H.Q.; Belongia, E.A.; Halfmann, P.; et al. Ipsilateral and Contralateral Coadministration of Influenza and COVID-19 Vaccines Produce Similar Antibody Responses. eBioMedicine 2024, 103, 105103. [Google Scholar] [CrossRef]
- Toback, S.; Galiza, E.; Cosgrove, C.; Galloway, J.; Goodman, A.L.; Swift, P.A.; Rajaram, S.; Graves-Jones, A.; Edelman, J.; Burns, F.; et al. Safety, Immunogenicity, and Efficacy of a COVID-19 Vaccine (NVX-CoV2373) Co-Administered with Seasonal Influenza Vaccines: An Exploratory Substudy of a Randomised, Observer-Blinded, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2022, 10, 167–179. [Google Scholar] [CrossRef]
- Bai, S.; Zhou, S.; Zhang, J.; Chen, W.; Lv, M.; Wang, J.; Zhang, A.; Wu, J.; Zhao, W. Immunogenicity and Safety of Different Combinations Involving a Third Booster Dose of SARS-CoV-2 Inactivated Vaccine, Inactivated Quadrivalent Influenza Vaccine, and 23-Valent Pneumococcal Polysaccharide Vaccine in Adults Aged ≥60 Years: A Phase 4, Randomized, Open-Label Study. Front. Immunol. 2024, 15, 1437267. [Google Scholar] [CrossRef]
- Izikson, R.; Brune, D.; Bolduc, J.-S.; Bourron, P.; Fournier, M.; Moore, T.M.; Pandey, A.; Perez, L.; Sater, N.; Shrestha, A.; et al. Safety and Immunogenicity of a High-Dose Quadrivalent Influenza Vaccine Administered Concomitantly with a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Adults Aged ≥65 Years: A Phase 2, Randomised, Open-Label Study. Lancet Respir. Med. 2022, 10, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Moderna Announces Positive Phase 3 Data for Combination Vaccine Against Influenza and COVID-19. Available online: https://investors.modernatx.com/news/news-details/2024/Moderna-Announces-Positive-Phase-3-Data-for-Combination-Vaccine-Against-Influenza-and-COVID-19-/default.aspx (accessed on 16 October 2024).
- Pfizer and BioNTech Provide Update on mRNA-Based Combination Vaccine Program Against Influenza and COVID-19 in Individuals 18–64 Years of Age. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-mrna-based-combination (accessed on 16 October 2024).
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class Switch towards Non-Inflammatory, Spike-Specific IgG4 Antibodies after Repeated SARS-CoV-2 mRNA Vaccination. Sci. Immunol. 2022, 8, eade2798. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Rispens, T.; Huijbers, M.G. The Unique Properties of IgG4 and Its Roles in Health and Disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef]
- Kotomina, T.; Isakova-Sivak, I.; Kim, K.-H.; Park, B.R.; Jung, Y.-J.; Lee, Y.; Mezhenskaya, D.; Matyushenko, V.; Kang, S.-M.; Rudenko, L. Generation and Characterization of Universal Live-Attenuated Influenza Vaccine Candidates Containing Multiple M2e Epitopes. Vaccines 2020, 8, 648. [Google Scholar] [CrossRef]
- Wagner, D.K.; Clements, M.L.; Reimer, C.B.; Snyder, M.; Nelson, D.L.; Murphy, B.R. Analysis of Immunoglobulin G Antibody Responses after Administration of Live and Inactivated Influenza A Vaccine Indicates That Nasal Wash Immunoglobulin G Is a Transudate from Serum. J. Clin. Microbiol. 1987, 25, 559–562. [Google Scholar] [CrossRef]
- Stepanova, L.; Naykhin, A.; Kolmskog, C.; Jonson, G.; Barantceva, I.; Bichurina, M.; Kubar, O.; Linde, A. The Humoral Response to Live and Inactivated Influenza Vaccines Administered Alone and in Combination to Young Adults and Elderly. J. Clin. Virol. 2002, 24, 193–201. [Google Scholar] [CrossRef]
- Beyer, W.E.P.; Palache, A.M.; de Jong, J.C.; Osterhaus, A.D.M.E. Cold-Adapted Live Influenza Vaccine versus Inactivated Vaccine: Systemic Vaccine Reactions, Local and Systemic Antibody Response, and Vaccine Efficacy. A Meta-Analysis. Vaccine 2002, 20, 1340–1353. [Google Scholar] [CrossRef]
- Song, Y.; Mehl, F.; Zeichner, S.L. Vaccine Strategies to Elicit Mucosal Immunity. Vaccines 2024, 12, 191. [Google Scholar] [CrossRef]
- Tong, X.; Deng, Y.; Cizmeci, D.; Fontana, L.; Carlock, M.A.; Hanley, H.B.; McNamara, R.P.; Lingwood, D.; Ross, T.M.; Alter, G. Distinct Functional Humoral Immune Responses Are Induced after Live Attenuated and Inactivated Seasonal Influenza Vaccination. J. Immunol. 2024, 212, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, R.S.; Uruchurtu, A.S.S.; Negri, V.A.; Cole, M.E.; Singh, N.; Poshai, N.; Jackson, D.; Hoschler, K.; Baker, T.; Scott, I.C.; et al. Early Mucosal Events Promote Distinct Mucosal and Systemic Antibody Responses to Live Attenuated Influenza Vaccine. Nat. Commun. 2023, 14, 8053. [Google Scholar] [CrossRef]
- Gallichan, W.S.; Rosenthal, K.L. Long-Lived Cytotoxic T Lymphocyte Memory in Mucosal Tissues after Mucosal but Not Systemic Immunization. J. Exp. Med. 1996, 184, 1879–1890. [Google Scholar] [CrossRef]
- Subbarao, K. Live Attenuated Influenza Vaccines for Pandemic Preparedness. J. Pediatr. Infect. Dis. Soc. 2020, 9, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Stacey, H.; Barjesteh, N.; Mapletoft, J.; Miller, M. “Gnothi Seauton”: Leveraging the Host Response to Improve Influenza Virus Vaccine Efficacy. Vaccines 2018, 6, 23. [Google Scholar] [CrossRef]
- Ramirez, S.I.; Faraji, F.; Hills, L.B.; Lopez, P.G.; Goodwin, B.; Stacey, H.D.; Sutton, H.J.; Hastie, K.M.; Saphire, E.O.; Kim, H.J.; et al. Immunological Memory Diversity in the Human Upper Airway. Nature 2024, 632, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-Generated Lung Tissue-Resident Memory T Cells Provide Heterosubtypic Protection to Influenza Infection. JCI Insight 2016, 1, e85832. [Google Scholar] [CrossRef]
- Zheng, M.Z.M.; Wakim, L.M. Tissue Resident Memory T Cells in the Respiratory Tract. Mucosal Immunol. 2022, 15, 379–388. [Google Scholar] [CrossRef]
- Talaat, K.R.; Luke, C.J.; Khurana, S.; Manischewitz, J.; King, L.R.; McMahon, B.A.; Karron, R.A.; Lewis, K.D.C.; Qin, J.; Follmann, D.A.; et al. A Live Attenuated Influenza A(H5N1) Vaccine Induces Long-Term Immunity in the Absence of a Primary Antibody Response. J. Infect. Dis. 2014, 209, 1860–1869. [Google Scholar] [CrossRef]
- Rudenko, L.; Naykhin, A.; Donina, S.; Korenkov, D.; Petukhova, G.; Isakova-Sivak, I.; Losev, I.; Stukova, M.; Erofeeva, M.; Nikiforova, A.; et al. Assessment of Immune Responses to H5N1 Inactivated Influenza Vaccine among Individuals Previously Primed with H5N2 Live Attenuated Influenza Vaccine. Hum. Vaccines Immunother. 2015, 11, 2839–2848. [Google Scholar] [CrossRef]
- Wragg, K.M.; Lee, W.S.; Koutsakos, M.; Tan, H.-X.; Amarasena, T.; Reynaldi, A.; Gare, G.; Konstandopoulos, P.; Field, K.R.; Esterbauer, R.; et al. Establishment and Recall of SARS-CoV-2 Spike Epitope-Specific CD4+ T Cell Memory. Nat. Immunol. 2022, 23, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Sherina, N.; Piralla, A.; Du, L.; Wan, H.; Kumagai-Braesch, M.; Andréll, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; et al. Persistence of SARS-CoV-2-Specific B and T Cell Responses in Convalescent COVID-19 Patients 6–8 Months after the Infection. Med 2021, 2, 281–295.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, P.; Yuan, L.; Zhang, L.; Zhang, L.; Zhao, H.; Chen, C.; Wang, X.; Han, J.; Chen, Y.; et al. A Live Attenuated Virus-Based Intranasal COVID-19 Vaccine Provides Rapid, Prolonged, and Broad Protection against SARS-CoV-2. Sci. Bull. 2022, 67, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Huang, S.; Liu, X.; Chen, Q.; Zhuang, C.; Zhao, H.; Han, J.; Jaen, A.M.; Do, T.H.; Peter, J.G.; et al. Safety and Efficacy of the Intranasal Spray SARS-CoV-2 Vaccine dNS1-RBD: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet. Respir. Med. 2023, 11, 1075. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Lipsitch, M.; Grad, Y.H.; Sette, A.; Crotty, S. Cross-Reactive Memory T Cells and Herd Immunity to SARS-CoV-2. Nat. Rev. Immunol. 2020, 20, 709–713. [Google Scholar] [CrossRef]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Lee, E.; Sandgren, K.; Duette, G.; Stylianou, V.V.; Khanna, R.; Eden, J.-S.; Blyth, E.; Gottlieb, D.; Cunningham, A.L.; Palmer, S. Identification of SARS-CoV-2 Nucleocapsid and Spike T-Cell Epitopes for Assessing T-Cell Immunity. J. Virol. 2021, 95, e02002. [Google Scholar] [CrossRef]
- Clever, S.; Schünemann, L.-M.; Armando, F.; Meyer Zu Natrup, C.; Tuchel, T.; Tscherne, A.; Ciurkiewicz, M.; Baumgärtner, W.; Sutter, G.; Volz, A. Protective MVA-ST Vaccination Robustly Activates T Cells and Antibodies in an Aged-Hamster Model for COVID-19. Vaccines 2024, 12, 52. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Luo, Y.; Ackerson, B.; Tanenbaum, H.C.; Sy, L.S.; Gandhi, A.; Tseng, H.F. Comparison of Vaccine Effectiveness against Influenza Hospitalization of Cell-Based and Egg-Based Influenza Vaccines, 2017–2018. Vaccine 2019, 37, 5807–5811. [Google Scholar] [CrossRef] [PubMed]
- Puig-Barberà, J.; Tamames-Gómez, S.; Plans-Rubio, P.; Eiros-Bouza, J.M. Relative Effectiveness of Cell-Cultured versus Egg-Based Seasonal Influenza Vaccines in Preventing Influenza-Related Outcomes in Subjects 18 Years Old or Older: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 818. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Janjua, N.Z.; Serres, G.D.; Sabaiduc, S.; Eshaghi, A.; Dickinson, J.A.; Fonseca, K.; Winter, A.-L.; Gubbay, J.B.; Krajden, M.; et al. Low 2012–2013 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PLoS ONE 2014, 9, e92153. [Google Scholar] [CrossRef] [PubMed]

| LAIV Virus | Viral Titers in Eggs, lgEID50/mL | Viral Titers in MDCK Cells, lgTCID50/mL | Viral Titers in Hamster Tissues, lgEID50/g | ||||
|---|---|---|---|---|---|---|---|
| 26 °C | 33 °C | 40 °C | NT 1 | Lung | Trachea | ||
| H1N1 | 7.5 | 9.6 | <1.2 | 7.0 | 4.9 ± 0.5 | 3.4 ± 0.5 | 2.0 ± 0.3 |
| H1N1/NA+CoV | 6.8 | 8.5 | <1.2 | 7.0 | 4.1 ± 0.6 | 1.8 ± 0.2 | 2.1 ± 0.1 |
| H3N2 | 6.5 | 9.0 | <1.2 | 8.4 | 3.2 ± 1.9 | 1.8 ± 0.1 | 2.1 ± 0.2 |
| H3N2/NA+CoV | 6.7 | 8.5 | <1.2 | 8.6 | 3.1 ± 0.9 | 2.0 ± 0.4 | 2.2 ± 0.1 |
| B/Victoria | 7.8 | 9.9 | <1.2 | 9.5 | 4.3 ± 1.0 | 2.2 ± 0.4 | 3.6 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, E.; Matyushenko, V.; Mezhenskaya, D.; Bazhenova, E.; Kotomina, T.; Rak, A.; Donina, S.; Chistiakova, A.; Kostromitina, A.; Novitskaya, V.; et al. Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters. Vaccines 2024, 12, 1300. https://doi.org/10.3390/vaccines12121300
Stepanova E, Matyushenko V, Mezhenskaya D, Bazhenova E, Kotomina T, Rak A, Donina S, Chistiakova A, Kostromitina A, Novitskaya V, et al. Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters. Vaccines. 2024; 12(12):1300. https://doi.org/10.3390/vaccines12121300
Chicago/Turabian StyleStepanova, Ekaterina, Victoria Matyushenko, Daria Mezhenskaya, Ekaterina Bazhenova, Tatiana Kotomina, Alexandra Rak, Svetlana Donina, Anna Chistiakova, Arina Kostromitina, Vlada Novitskaya, and et al. 2024. "Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters" Vaccines 12, no. 12: 1300. https://doi.org/10.3390/vaccines12121300
APA StyleStepanova, E., Matyushenko, V., Mezhenskaya, D., Bazhenova, E., Kotomina, T., Rak, A., Donina, S., Chistiakova, A., Kostromitina, A., Novitskaya, V., Prokopenko, P., Rodionova, K., Sivak, K., Kryshen, K., Makarov, V., Rudenko, L., & Isakova-Sivak, I. (2024). Safety, Immunogenicity and Protective Activity of a Modified Trivalent Live Attenuated Influenza Vaccine for Combined Protection Against Seasonal Influenza and COVID-19 in Golden Syrian Hamsters. Vaccines, 12(12), 1300. https://doi.org/10.3390/vaccines12121300






