Evaluation of a Vaccine Candidate Designed for Broad-Spectrum Protection against Type A Foot-and-Mouth Disease in Asia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Site-Directed Mutagenesis and Subcloning
2.3. Virus Recovery and Cell Culture
2.4. Virus Replication and Purification
2.5. Preparation of the Experimental Vaccines
2.6. Vaccination and Viral Challenge in Mice
2.7. Vaccination and Viral Challenge in Pigs
2.8. The Immunogenicity of the Candidate Vaccine in Pigs
2.9. Detection of Antibodies against Structural Proteins
2.10. Virus-Neutralization Test (VNT)
2.11. Virus Detection in Vaccinated and Challenged Pigs
2.12. Statistical Analysis
2.13. Ethics Statement
3. Results
3.1. Construction of Chimeric Viruses
3.2. The Apo22 and Apo22-B Vaccines Protected Mice against Challenge with Type A Viruses
3.3. The Apo22-B Vaccine Completely Protected Pigs against Challenge with Type A Viruses
3.4. Comparison of Virus-Neutralizing Antibody Titers in Apo22-B-Vaccinated Pigs after Challenge with Type A Viruses
3.5. Comparison of Virus-Neutralizing Antibody Titers in Vaccinated Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, B.P.; Rodriguez, L.L.; Hammond, J.M.; Pinto, J.; Perez, A.M. Review of the Global Distribution of Foot-and-Mouth Disease Virus from 2007 to 2014. Transbound. Emerg. Dis. 2017, 64, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Lyons, N.A.; Ludi, A.B.; Wilsden, G.; Hamblin, P.; Qasim, I.A.; Gubbins, S.; King, D.P. Evaluation of a polyvalent foot-and-mouth disease virus vaccine containing A Saudi-95 against field challenge on large-scale dairy farms in Saudi Arabia with the emerging A/ASIA/G-VII viral lineage. Vaccine 2017, 35, 6850–6857. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Parida, S. Foot and mouth disease vaccine strain selection: Current approaches and future perspectives. Expert Rev. Vaccines 2018, 17, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Cho, G.; Kim, H.; Lee, G.; Lim, T.G.; Kwak, H.Y.; Park, J.H.; Park, S.H. Immunogenicity and Protection against Foot-and-Mouth Disease Virus in Swine Intradermally Vaccinated with a Bivalent Vaccine of Foot-and-Mouth Disease Virus Type O and A. Vaccines 2023, 11, 815. [Google Scholar] [CrossRef] [PubMed]
- Bachanek-Bankowska, K.; Di Nardo, A.; Wadsworth, J.; Henry, E.K.M.; Parlak, Ü.; Timina, A.; Mischenko, A.; Qasim, I.A.; Abdollahi, D.; Sultana, M.; et al. Foot-and-Mouth Disease in the Middle East Caused by an A/ASIA/G-VII Virus Lineage, 2015–2016. Emerg. Infect. Dis. 2018, 24, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Ludi, A.B.; Fowler, V.L.; Wilsden, G.; Browning, C.; Gubbins, S.; Statham, B.; Bin-Tarif, A.; Mioulet, V.; King, D.J.; et al. Efficacy of a high-potency multivalent foot-and-mouth disease virus vaccine in cattle against heterologous challenge with a field virus from the emerging A/ASIA/G-VII lineage. Vaccine 2018, 36, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Statham, B.; Li, Y.; Hammond, J.; Paton, D.; Parida, S. Emergence of antigenic variants within serotype A FMDV in the Middle East with antigenically critical amino acid substitutions. Vaccine 2016, 34, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, Y.J.; Kim, R.H.; Park, J.N.; Park, M.E.; Ko, M.K.; Choi, J.H.; Chu, J.Q.; Lee, K.N.; Kim, S.M.; et al. Rapid Engineering of Foot-and-Mouth Disease Vaccine and Challenge Viruses. J. Virol. 2017, 91, e00155-17. [Google Scholar] [CrossRef]
- Ko, M.K.; Jo, H.E.; Choi, J.H.; You, S.H.; Shin, S.H.; Jo, H.; Lee, M.J.; Kim, S.M.; Kim, B.; Park, J.H. Chimeric vaccine strain of type O foot-and-mouth disease elicits a strong immune response in pigs against ME-SA and SEA topotypes. Vet. Microbiol. 2019, 229, 124–129. [Google Scholar] [CrossRef]
- Ito, N.; Takayama-Ito, M.; Yamada, K.; Hosokawa, J.; Sugiyama, M.; Minamoto, N. Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol. Immunol. 2003, 47, 613–617. [Google Scholar] [CrossRef]
- Shin, S.H.; Jo, H.; Ko, M.K.; Choi, J.H.; You, S.H.; Jo, H.E.; Lee, M.J.; Kim, S.M.; Kim, B.; Park, J.H. Antigenic properties of a novel vaccine strain for type Asia1 foot-and-mouth disease in pigs. Vet. Microbiol. 2020, 248, 108802. [Google Scholar] [CrossRef] [PubMed]
- Longjam, N.; Tayo, T. Antigenic variation of foot and mouth disease virus—An overview. Vet. World 2011, 4, 475–479. [Google Scholar] [CrossRef]
- Fernandez-Sainz, I.; Gavitt, T.D.; Koster, M.; Ramirez-Medina, E.; Rodriguez, Y.Y.; Wu, P.; Silbart, L.K.; de Los Santos, T.; Szczepanek, S.M. The VP1 G-H loop hypervariable epitope contributes to protective immunity against Foot and Mouth Disease Virus in swine. Vaccine 2019, 37, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Seeyo, K.B.; Nishi, T.; Kawaguchi, R.; Ungvanijban, S.; Udon, R.; Fukai, K.; Yamakawa, M.; Rukkwamsuk, T. Evolution of antigenic and genetic characteristics of foot-and-mouth disease virus serotype A circulating in Thailand, 2007–2019. Virus Res. 2020, 290, 198166. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Alkheraije, K.A. The prevalence of foot-and-mouth disease in Asia. Front. Vet. Sci. 2023, 10, 1201578. [Google Scholar] [CrossRef]
- Knowles, N.J.; Samuel, A.R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003, 91, 65–80. [Google Scholar] [CrossRef]
- Knowles, N.J.; Nazem Shirazi, M.H.; Wadsworth, J.; Swabey, K.G.; Stirling, J.M.; Statham, R.J.; Li, Y.; Hutchings, G.H.; Ferris, N.P.; Parlak, U.; et al. Recent spread of a new strain (A-Iran-05) of foot-and-mouth disease virus type A in the Middle East. Transbound. Emerg. Dis. 2009, 56, 157–169. [Google Scholar] [CrossRef]
- Mattion, N.; König, G.; Seki, C.; Smitsaart, E.; Maradei, E.; Robiolo, B.; Duffy, S.; León, E.; Piccone, M.; Sadir, A.; et al. Reintroduction of foot-and-mouth disease in Argentina: Characterisation of the isolates and development of tools for the control and eradication of the disease. Vaccine 2004, 22, 4149–4162. [Google Scholar] [CrossRef]
- Bari, F.D.; Parida, S.; Tekleghiorghis, T.; Dekker, A.; Sangula, A.; Reeve, R.; Haydon, D.T.; Paton, D.J.; Mahapatra, M. Genetic and antigenic characterisation of serotype A FMD viruses from East Africa to select new vaccine strains. Vaccine 2014, 32, 5794–5800. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Kim, H.H.; Park, J.H.; Park, C.K. Heterologous Prime-Boost Vaccination with Commercial FMD Vaccines Elicits a Broader Immune Response than Homologous Prime-Boost Vaccination in Pigs. Vaccines 2023, 11, 551. [Google Scholar] [CrossRef]
- Brehm, K.E.; Kumar, N.; Thulke, H.H.; Haas, B. High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 2008, 26, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Gubbins, S.; Paton, D.J.; Dekker, A.; Ludi, A.B.; Wilsden, G.; Browning, C.F.J.; Eschbaumer, M.; Barnabei, J.; Duque, H.; Pauszek, L.L.; et al. Predicting cross-protection against foot-and-mouth disease virus strains by serology after vaccination. Front. Vet. Sci. 2022, 9, 1027006. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.J.; Aggarwal, N.; Statham, R.J.; Barnett, P.V. Longevity of antibody and cytokine responses following vaccination with high potency emergency FMD vaccines. Vaccine 2003, 21, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Oh, Y.; Reid, S.M.; Cox, S.J.; Statham, R.J.; Mahapatra, M.; Anderson, J.; Barnett, P.V.; Charleston, B.; Paton, D.J. Interferon-gamma production in vitro from whole blood of foot-and-mouth disease virus (FMDV) vaccinated and infected cattle after incubation with inactivated FMDV. Vaccine 2006, 24, 964–969. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Jin, Y.; Ma, J.; Cai, H.; Zhang, X. Immunoprotective mechanisms in swine within the “grey zone” in antibody response after immunization with foot-and-mouth disease vaccine. Virus Res. 2016, 220, 39–46. [Google Scholar] [CrossRef]
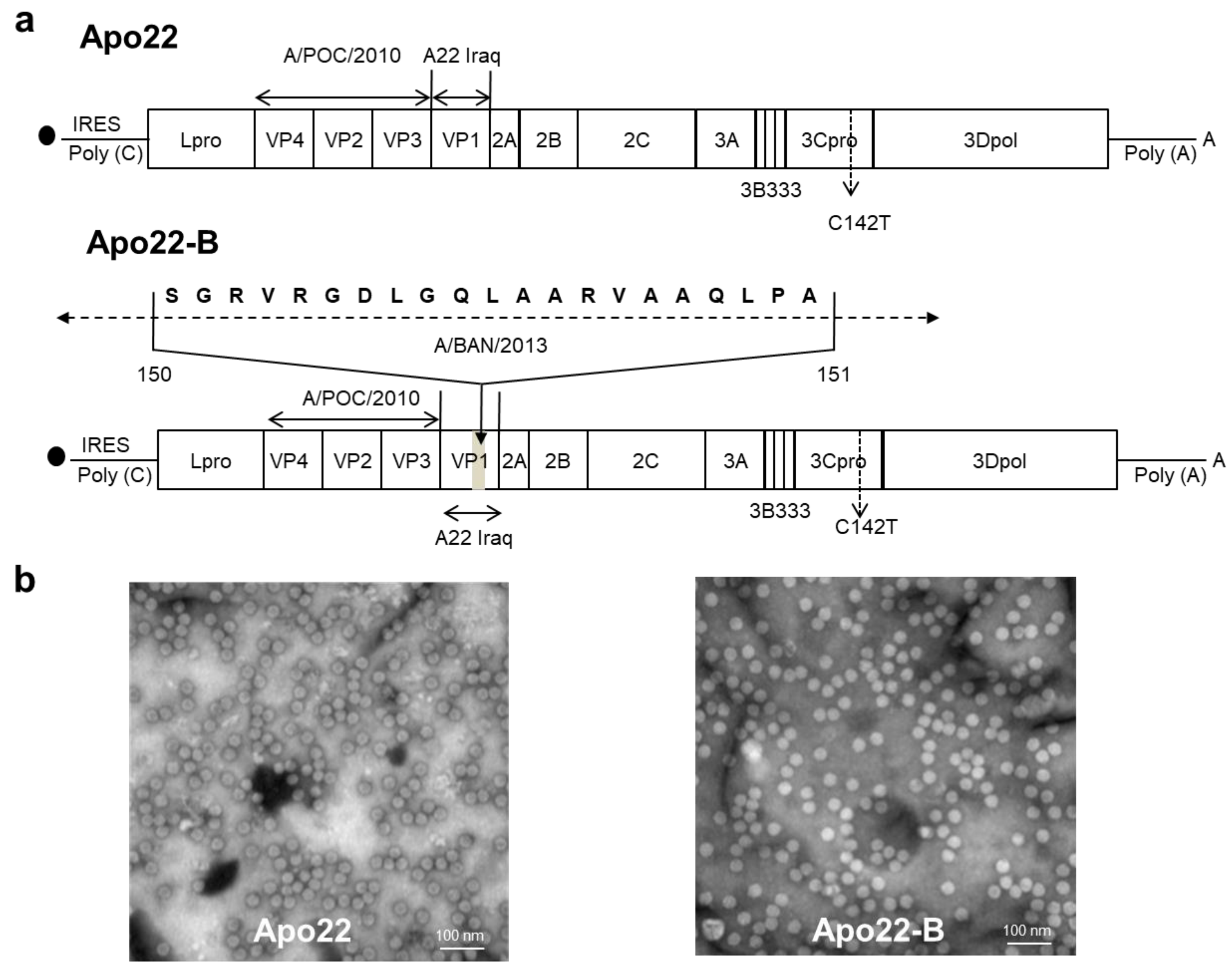


| Vaccine Candidates | Challenge Viruses (PD50) | |||
|---|---|---|---|---|
| G1 (A/POC/2010) | G2 (A/GP/2018) | A22 Iraq | GVII (A/NEP/2017) | |
| Apo22 | >128.0 | >128.0 | >128.0 | 73.5 |
| Apo22-B | >128.0 | >128.0 | >128.0 | 97.0 |
| Group (Cage) | No. of Animal | Experimental Group | Challenge Virus (1 × 105 TCID50/0.1 mL) | Days of Blood Collection (dpc) | Days of Oral Swab Collection (dpc) | Comments |
|---|---|---|---|---|---|---|
| 1 (1) | 4 | Apo22-B | A/POC/2010 | −28, −21, −14, −7, 0, 2, 4, 6, 8, 10, 12 | 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 | If symptoms were observed after the challenge, they were isolated in empty cage. |
| 2 (1) | 2 | Negative group | ||||
| 3 (2) | 4 | Apo22-B | A/GP/2018 | |||
| 4 (2) | 2 | Negative group | ||||
| 5 (3) | 4 | Apo22-B | A/NEP/2017 | −28, −21, −14, −7, 0, 2, 4, 6, 8 | 0, 1, 2, 3, 4, 5, 6, 7, 8 | |
| 6 (3) | 2 | Negative group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.H.; Hwang, S.Y.; Kim, H.-M.; Shin, S.H.; Ko, M.-K.; Lee, M.J.; Kim, S.-M.; Park, J.-H. Evaluation of a Vaccine Candidate Designed for Broad-Spectrum Protection against Type A Foot-and-Mouth Disease in Asia. Vaccines 2024, 12, 64. https://doi.org/10.3390/vaccines12010064
Shin SH, Hwang SY, Kim H-M, Shin SH, Ko M-K, Lee MJ, Kim S-M, Park J-H. Evaluation of a Vaccine Candidate Designed for Broad-Spectrum Protection against Type A Foot-and-Mouth Disease in Asia. Vaccines. 2024; 12(1):64. https://doi.org/10.3390/vaccines12010064
Chicago/Turabian StyleShin, Sung Ho, Seong Yun Hwang, Hyun-Mi Kim, Se Hee Shin, Mi-Kyeong Ko, Min Ja Lee, Su-Mi Kim, and Jong-Hyeon Park. 2024. "Evaluation of a Vaccine Candidate Designed for Broad-Spectrum Protection against Type A Foot-and-Mouth Disease in Asia" Vaccines 12, no. 1: 64. https://doi.org/10.3390/vaccines12010064
APA StyleShin, S. H., Hwang, S. Y., Kim, H.-M., Shin, S. H., Ko, M.-K., Lee, M. J., Kim, S.-M., & Park, J.-H. (2024). Evaluation of a Vaccine Candidate Designed for Broad-Spectrum Protection against Type A Foot-and-Mouth Disease in Asia. Vaccines, 12(1), 64. https://doi.org/10.3390/vaccines12010064








