Multiplex Assays for Analysis of Antibody Responses to South Asian Plasmodium falciparum and Plasmodium vivax Malaria Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sample Collection
2.3. Antigen Constructs
2.4. In Vitro Transcription
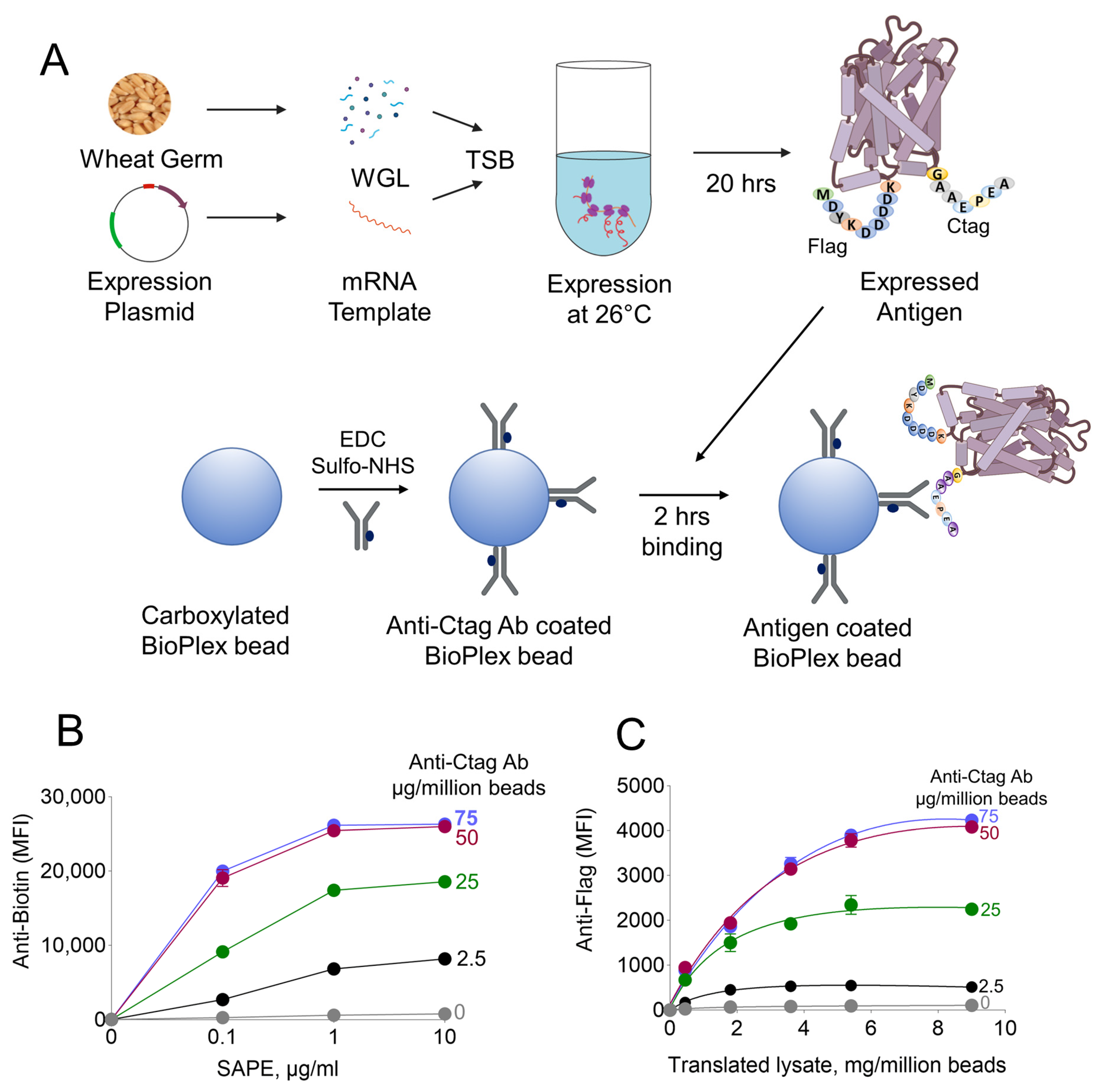
2.5. Cell-Free Expression of Malarial Antigens
2.6. Functionalization of Luminex Beads with Anti-Ctag Antibodies
2.7. Adsorption of Ctagged PfMSP1-42 on Functionalized Beads from Wheat-Translated Lysates
2.8. Validation of Adsorbed PfMSP1-42 on Anti-Ctag Antibody Functionalized Beads
2.9. Patient Antibody Binding Studies in Multiplex Assay
3. Results
3.1. Antigen Selection
3.2. Rapid Generation of Malaria Antigen-Coated BioPlex Beads
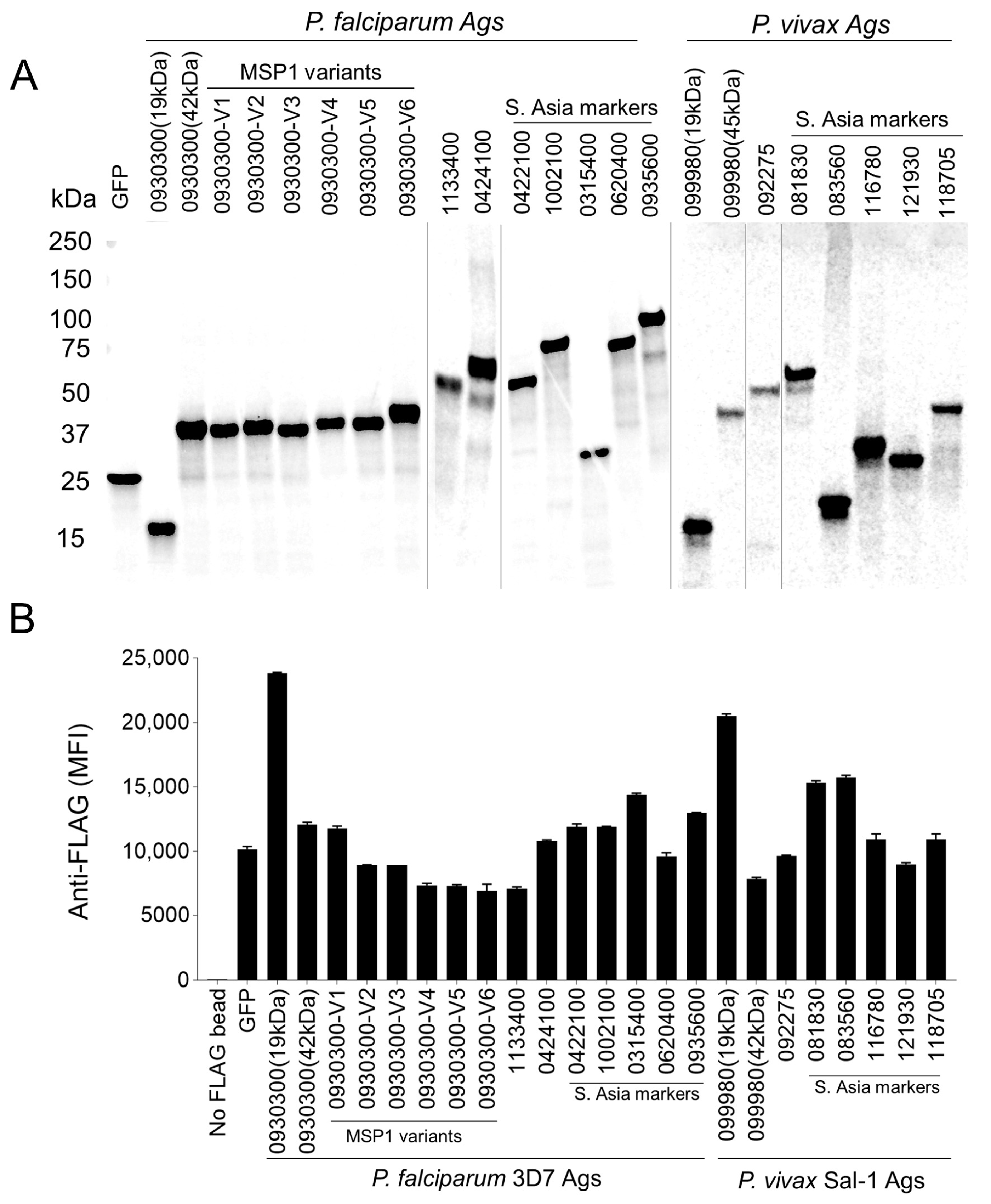
3.3. Parallel Adsorption of Malaria Antigens on Functionalized Beads and Their Validation
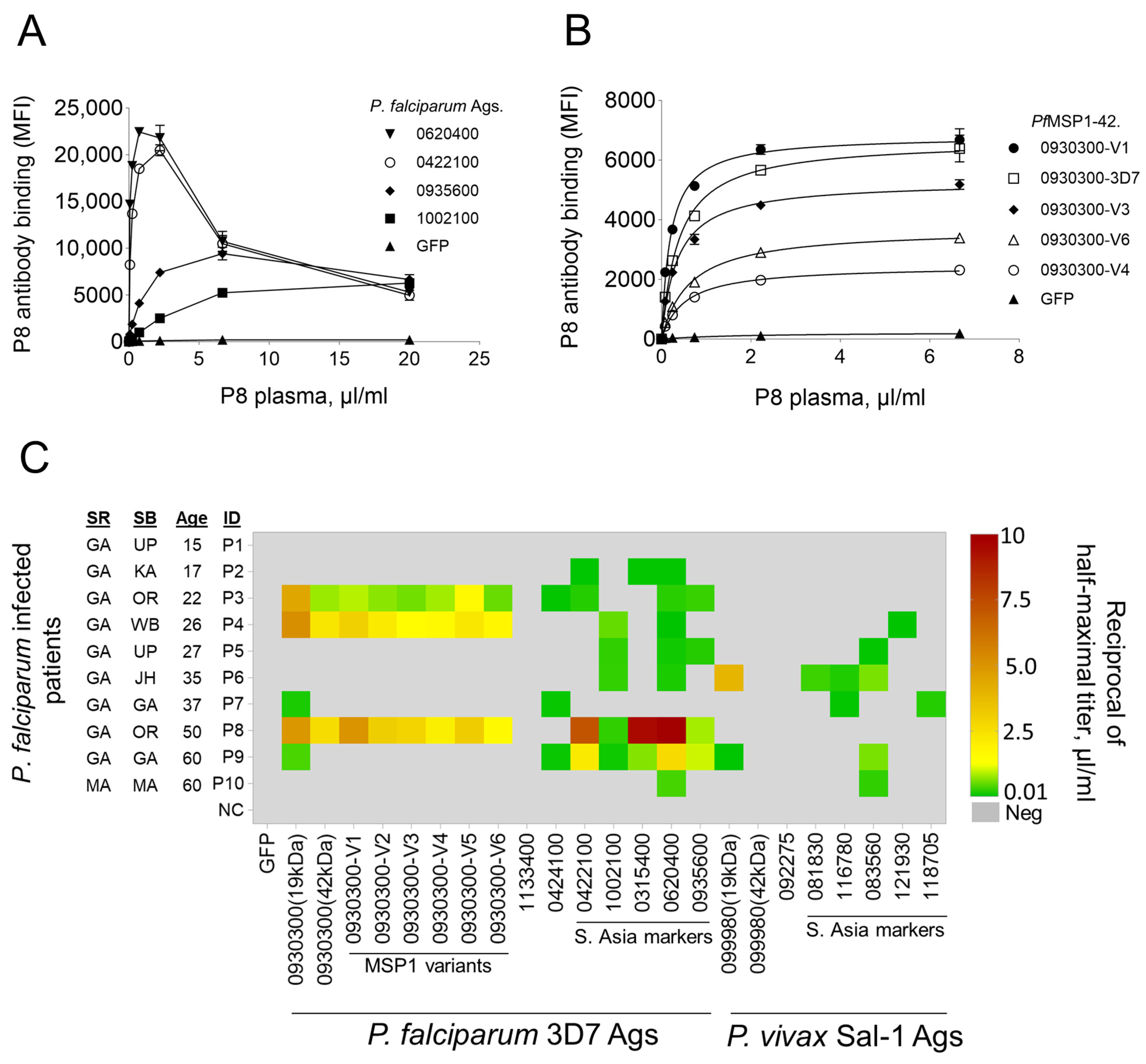
3.4. Patient Antibody Levels to P. falciparum and P. vivax Antigens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Tse, E.G.; Korsik, M.; Todd, M.H. The past, present and future of anti-malarial medicines. Malar. J. 2019, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Day, N.P.J.; Ashley, E.A.; Smithuis, F.M.; Nosten, F.H. Have we really failed to roll back malaria? Lancet 2022, 399, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Doolan, D.L.; Dobano, C.; Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef]
- Cohen, S.; McGregor, I.A.; Carrington, S. Gamma-globulin and acquired immunity to human malaria. Nature 1961, 192, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Crompton, P.D.; Kayala, M.A.; Traore, B.; Kayentao, K.; Ongoiba, A.; Weiss, G.E.; Molina, D.M.; Burk, C.R.; Waisberg, M.; Jasinskas, A.; et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. USA 2010, 107, 6958–6963. [Google Scholar] [CrossRef] [PubMed]
- Emili, A.Q.; Cagney, G. Large-scale functional analysis using peptide or protein arrays. Nat. Biotechnol. 2000, 18, 393–397. [Google Scholar] [CrossRef] [PubMed]
- MacBeath, G.; Schreiber, S.L. Printing proteins as microarrays for high-throughput function determination. Science 2000, 289, 1760–1763. [Google Scholar] [CrossRef]
- Haab, B.B.; Dunham, M.J.; Brown, P.O. Protein microarrays for highly parallel detection and quantitation of specific proteins and antibodies in complex solutions. Genome Biol. 2001, 2, 4. [Google Scholar] [CrossRef]
- Davies, D.H.; Wyatt, L.S.; Newman, F.K.; Earl, P.L.; Chun, S.; Hernandez, J.E.; Molina, D.M.; Hirst, S.; Moss, B.; Frey, S.E.; et al. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax. J. Virol. 2008, 82, 652–663. [Google Scholar] [CrossRef]
- Doolan, D.L.; Mu, Y.; Unal, B.; Sundaresh, S.; Hirst, S.; Valdez, C.; Randall, A.; Molina, D.; Liang, X.; Freilich, D.A.; et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 2008, 8, 4680–4694. [Google Scholar] [CrossRef]
- Venkatesh, A.; Jain, A.; Davies, H.; Periera, L.; Maki, J.N.; Gomes, E.; Felgner, P.L.; Srivastava, S.; Patankar, S.; Rathod, P.K. Hospital-derived antibody profiles of malaria patients in Southwest India. Malar. J. 2019, 18, 138. [Google Scholar] [CrossRef]
- Stillman, B.A.; Tonkinson, J.L. FAST slides: A novel surface for microarrays. Biotechniques 2000, 29, 630–635. [Google Scholar] [CrossRef] [PubMed]
- King, C.L.; Davies, D.H.; Felgner, P.; Baum, E.; Jain, A.; Randall, A.; Tetteh, K.; Drakeley, C.J.; Greenhouse, B. Biosignatures of Exposure/Transmission and Immunity. Am. J. Trop. Med. Hyg. 2015, 93, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Herrera, M.; Lopez-Perez, M.; Dotsey, E.; Jain, A.; Rubiano, K.; Felgner, P.L.; Davies, D.H.; Herrera, S. Antibody Profiling in Naive and Semi-immune Individuals Experimentally Challenged with Plasmodium vivax Sporozoites. PLoS Negl. Trop. Dis. 2016, 10, e0004563. [Google Scholar] [CrossRef] [PubMed]
- Uplekar, S.; Rao, P.N.; Ramanathapuram, L.; Awasthi, V.; Verma, K.; Sutton, P.; Ali, S.Z.; Patel, A.; Priya, G.S.L.; Ravishankaran, S.; et al. Characterizing Antibody Responses to Plasmodium vivax and Plasmodium falciparum Antigens in India Using Genome-Scale Protein Microarrays. PLoS. Negl. Trop. Dis. 2017, 11, e0005323. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chen, S.B.; Wang, Y.; Ju, C.; Zhang, T.; Xu, B.; Shen, H.M.; Mo, X.J.; Molina, D.M.; Eng, M.; et al. An immunomics approach for the analysis of natural antibody responses to Plasmodium vivax infection. Mol. Biosyst. 2015, 11, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Roestenberg, M.; Liang, L.; Hung, C.; Jain, A.; Pablo, J.; Nakajima-Sasaki, R.; Molina, D.; Teelen, K.; Hermsen, C.C.; et al. Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci. Rep. 2013, 3, 3549. [Google Scholar] [CrossRef]
- Baum, E.; Sattabongkot, J.; Sirichaisinthop, J.; Kiattibutr, K.; Davies, D.H.; Jain, A.; Lo, E.; Lee, M.C.; Randall, A.Z.; Molina, D.M.; et al. Submicroscopic and asymptomatic Plasmodium falciparum and Plasmodium vivax infections are common in western Thailand—Molecular and serological evidence. Malar. J. 2015, 14, 95. [Google Scholar] [CrossRef]
- Stone, W.J.R.; Campo, J.J.; Ouedraogo, A.L.; Meerstein-Kessel, L.; Morlais, I.; Da, D.; Cohuet, A.; Nsango, S.; Sutherland, C.J.; van de Vegte-Bolmer, M.; et al. Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity. Nat. Commun. 2018, 9, 558. [Google Scholar] [CrossRef]
- Liu, E.W.; Skinner, J.; Tran, T.M.; Kumar, K.; Narum, D.L.; Jain, A.; Ongoiba, A.; Traore, B.; Felgner, P.L.; Crompton, P.D. Protein-Specific Features Associated with Variability in Human Antibody Responses to Plasmodium falciparum Malaria Antigens. Am. J. Trop. Med. Hyg. 2018, 98, 57–66. [Google Scholar] [CrossRef]
- Vigil, A.; Davies, D.H.; Felgner, P.L. Defining the humoral immune response to infectious agents using high-density protein microarrays. Future Microbiol. 2010, 5, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Baum, E.; Badu, K.; Molina, D.M.; Liang, X.; Felgner, P.L.; Yan, G. Protein microarray analysis of antibody responses to Plasmodium falciparum in western Kenyan highland sites with differing transmission levels. PLoS ONE 2013, 8, e82246. [Google Scholar] [CrossRef] [PubMed]
- Kamuyu, G.; Tuju, J.; Kimathi, R.; Mwai, K.; Mburu, J.; Kibinge, N.; Chong Kwan, M.; Hawkings, S.; Yaa, R.; Chepsat, E.; et al. KILchip v1.0: A Novel Plasmodium falciparum Merozoite Protein Microarray to Facilitate Malaria Vaccine Candidate Prioritization. Front. Immunol. 2018, 9, 2866. [Google Scholar] [CrossRef] [PubMed]
- Oulton, T.; Obiero, J.; Rodriguez, I.; Ssewanyana, I.; Dabbs, R.A.; Bachman, C.M.; Greenhouse, B.; Drakeley, C.; Felgner, P.L.; Stone, W.; et al. Plasmodium falciparum serology: A comparison of two protein production methods for analysis of antibody responses by protein microarray. PLoS ONE 2022, 17, e0273106. [Google Scholar] [CrossRef] [PubMed]
- Kanoi, B.N.; Takashima, E.; Morita, M.; White, M.T.; Palacpac, N.M.; Ntege, E.H.; Balikagala, B.; Yeka, A.; Egwang, T.G.; Horii, T.; et al. Antibody profiles to wheat germ cell-free system synthesized Plasmodium falciparum proteins correlate with protection from symptomatic malaria in Uganda. Vaccine 2017, 35, 873–881. [Google Scholar] [CrossRef]
- Wilson, M.R.; Wotherspoon, J.S. A new microsphere immunofluorescence assay using flow cytometry. J. Immunol. Methods 1988, 107, 225–230. [Google Scholar] [CrossRef]
- Fulwyler, M.J.; McHugh, T.M. Flow microsphere immunoassay for the quantitative and simultaneous detection of multiple soluble analytes. Methods Cell Biol. 1990, 33, 613–629. [Google Scholar]
- Graham, H.; Chandler, D.J.; Dunbar, S.A. The genesis and evolution of bead-based multiplexing. Methods 2019, 158, 2–11. [Google Scholar] [CrossRef]
- Fouda, G.G.; Leke, R.F.; Long, C.; Druilhe, P.; Zhou, A.; Taylor, D.W.; Johnson, A.H. Multiplex assay for simultaneous measurement of antibodies to multiple Plasmodium falciparum antigens. Clin. Vaccine Immunol. 2006, 13, 1307–1313. [Google Scholar] [CrossRef]
- Fernandez-Becerra, C.; Sanz, S.; Brucet, M.; Stanisic, D.I.; Alves, F.P.; Camargo, E.P.; Alonso, P.L.; Mueller, I.; del Portillo, H.A. Naturally-acquired humoral immune responses against the N- and C-termini of the Plasmodium vivax MSP1 protein in endemic regions of Brazil and Papua New Guinea using a multiplex assay. Malar. J. 2010, 9, 29. [Google Scholar] [CrossRef]
- Dodoo, D.; Atuguba, F.; Bosomprah, S.; Ansah, N.A.; Ansah, P.; Lamptey, H.; Egyir, B.; Oduro, A.R.; Gyan, B.; Hodgson, A.; et al. Antibody levels to multiple malaria vaccine candidate antigens in relation to clinical malaria episodes in children in the Kasena-Nankana district of Northern Ghana. Malar. J. 2011, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.M.; Quinto, L.; Jimenez, A.; Gonzalez, R.; Bardaji, A.; Maculuve, S.; Dobano, C.; Ruperez, M.; Vala, A.; Aponte, J.J.; et al. Multiplexing detection of IgG against Plasmodium falciparum pregnancy-specific antigens. PLoS ONE 2017, 12, e0181150. [Google Scholar] [CrossRef] [PubMed]
- Yman, V.; White, M.T.; Asghar, M.; Sundling, C.; Sonden, K.; Draper, S.J.; Osier, F.H.A.; Farnert, A. Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: Kinetics and longevity in absence of re-exposure. BMC Med. 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Ondigo, B.N.; Park, G.S.; Gose, S.O.; Ho, B.M.; Ochola, L.A.; Ayodo, G.O.; Ofulla, A.V.; John, C.C. Standardization and validation of a cytometric bead assay to assess antibodies to multiple Plasmodium falciparum recombinant antigens. Malar. J. 2012, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Priest, J.W.; Plucinski, M.M.; Huber, C.S.; Rogier, E.; Mao, B.; Gregory, C.J.; Candrinho, B.; Colborn, J.; Barnwell, J.W. Specificity of the IgG antibody response to Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale MSP119 subunit proteins in multiplexed serologic assays. Malar. J. 2018, 17, 417. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.; Ahmed, A.A.; Deressa, W.; Sime, H.; Mohammed, H.; Kebede, A.; Solomon, H.; Teka, H.; Gurrala, K.; Matei, B.; et al. Multiplex serology demonstrate cumulative prevalence and spatial distribution of malaria in Ethiopia. Malar. J. 2019, 18, 246. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.; van den Hoogen, L.; Herman, C.; Gurrala, K.; Joseph, V.; Stresman, G.; Presume, J.; Romilus, I.; Mondelus, G.; Elisme, T.; et al. High-throughput malaria serosurveillance using a one-step multiplex bead assay. Malar. J. 2019, 18, 402. [Google Scholar] [CrossRef]
- Chery, L.; Maki, J.N.; Mascarenhas, A.; Walke, J.T.; Gawas, P.; Almeida, A.; Fernandes, M.; Vaz, M.; Ramanan, R.; Shirodkar, D.; et al. Demographic and clinical profiles of Plasmodium falciparum and Plasmodium vivax patients at a tertiary care centre in southwestern India. Malar. J. 2016, 15, 569. [Google Scholar] [CrossRef]
- Mudeppa, D.G.; Rathod, P.K. Expression of functional Plasmodium falciparum enzymes using a wheat germ cell-free system. Eukaryot. Cell 2013, 12, 1653–1663. [Google Scholar] [CrossRef]
- Sawasaki, T.; Morishita, R.; Gouda, M.D.; Endo, Y. Methods for high-throughput materialization of genetic information based on wheat germ cell-free expression system. Methods Mol. Biol. 2007, 375, 95–106. [Google Scholar]
- Kumar, S.; Mudeppa, D.G.; Sharma, A.; Mascarenhas, A.; Dash, R.; Pereira, L.; Shaik, R.B.; Maki, J.N.; White, J., 3rd; Zuo, W.; et al. Distinct genomic architecture of Plasmodium falciparum populations from South Asia. Mol. Biochem. Parasitol. 2016, 210, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.J.; White, M.T.; Takashima, E.; Morita, M.; Kanoi, B.N.; Li Wai Suen, C.S.N.; Betuela, I.; Kuehn, A.; Sripoorote, P.; Franca, C.T.; et al. Naturally acquired antibody responses to more than 300 Plasmodium vivax proteins in three geographic regions. PLoS Negl. Trop. Dis. 2017, 11, e0005888. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.J.; White, M.T.; Takashima, E.; Brewster, J.; Morita, M.; Harbers, M.; Obadia, T.; Robinson, L.J.; Matsuura, F.; Liu, Z.S.J.; et al. Development and validation of serological markers for detecting recent Plasmodium vivax infection. Nat. Med. 2020, 26, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Helb, D.A.; Tetteh, K.K.; Felgner, P.L.; Skinner, J.; Hubbard, A.; Arinaitwe, E.; Mayanja-Kizza, H.; Ssewanyana, I.; Kamya, M.R.; Beeson, J.G.; et al. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc. Natl. Acad. Sci. USA 2015, 112, E4438–E4447. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.S.; Duncan, E.H.; Mease, R.M.; Angov, E. Impact of pre-existing MSP1(42)-allele specific immunity on potency of an erythrocytic Plasmodium falciparum vaccine. Malar. J. 2012, 11, 315. [Google Scholar] [CrossRef]
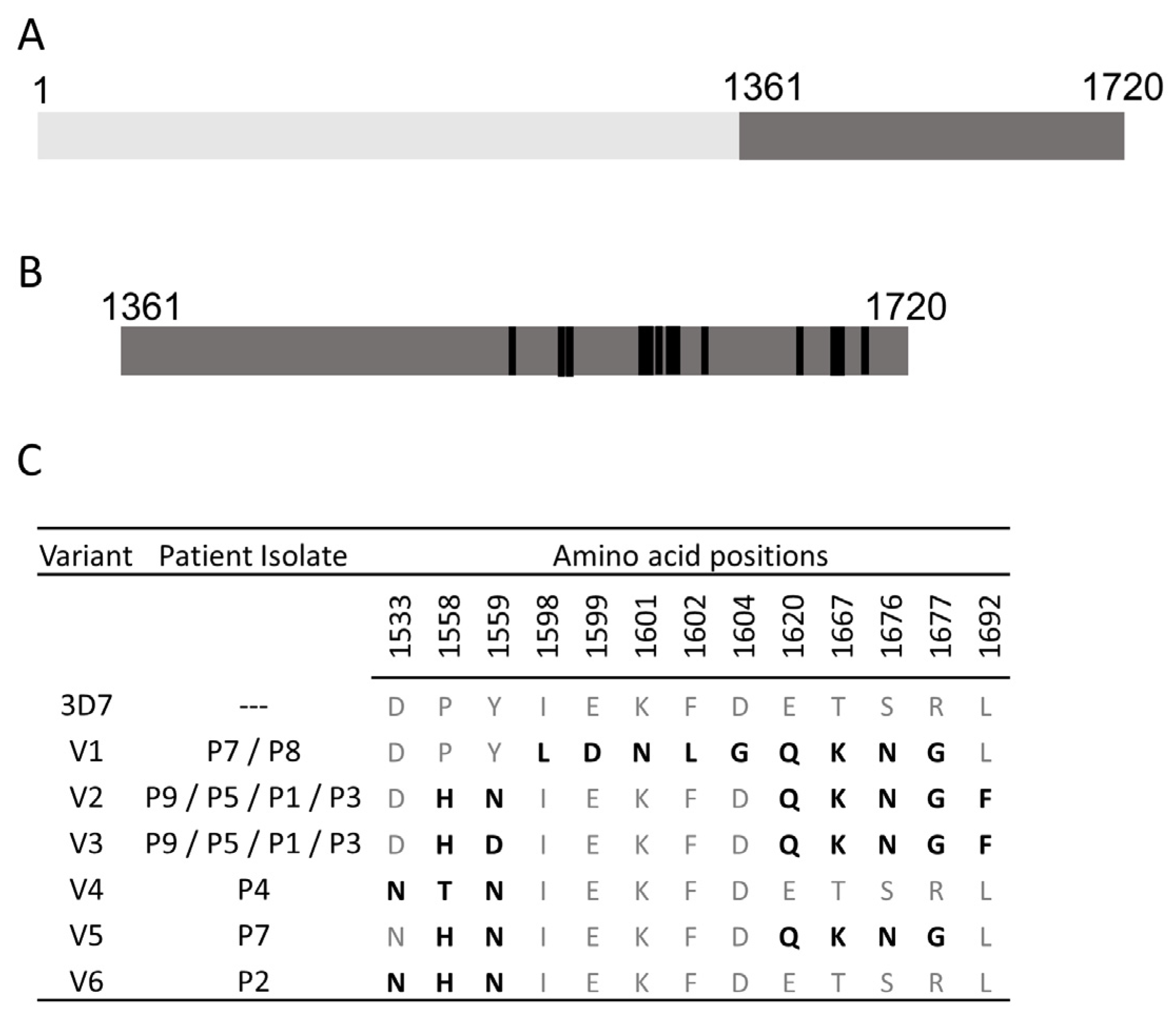
| Sl. No. | Antigen ID | Common Name | ORF Fragment, (AAs) | MW, (kDa) | |
|---|---|---|---|---|---|
| P. falciparum antigens | 1 | - | Green fluorescent protein (GFP) | 1–238 (238) | 26.9 |
| 2 | PF3D7_0930300 | Merozoite surface protein1 (MSP1-19) | 1612–1720 (108) | 12.1 | |
| 3 | PF3D7_0930300 | Merozoite surface protein1 (MSP1-42) | 1361–1720 (360) | 41.5 | |
| 4 | PF3D7_0930300 | MSP1-42 V1 | 1361–1720 (360) | 41.4 | |
| 5 | PF3D7_0930300 | MSP1-42 V2 | 1361–1720 (360) | 41.6 | |
| 6 | PF3D7_0930300 | MSP1-42 V3 | 1361–1720 (360) | 41.4 | |
| 7 | PF3D7_0930300 | MSP1-42 V4 | 1361–1720 (360) | 41.4 | |
| 8 | PF3D7_0930300 | MSP1-42 V5 | 1361–1720 (360) | 41.4 | |
| 9 | PF3D7_0930300 | MSP1-42 V6 | 1361–1720 (360) | 41.3 | |
| 10 | PF3D7_1133400 | Apical membrane antigen 1 (AMA1) | 22–544 (523) | 60.5 | |
| 11 | PF3D7_0424100 | Reticulocyte binding protein homologue 5 | 1–526 (526) | 63.0 | |
| South Asia P. falciparum top reactive serological markers [6] | 12 | PF3D7_0422100 | Transmembrane emp24 domain-containing protein | 1–385 (385) | 45.0 |
| 13 | PF3D7_1002100 | EMP1-trafficking protein | 1–631 (631) | 69.7 | |
| 14 | PF3D7_0315400 | Conserved protein, unknown function | 1–256 (256) | 30.5 | |
| 15 | PF3D7_0620400 | Merozoite surface protein10 | 1–525 (525) | 61.2 | |
| 16 | PF3D7_0935600 | Gametocytogenesis-implicated protein | 1–512 (512) | 58.2 | |
| P. vivax antigens | 17 | PVX_099980 | MSP1-19 | 1622–1729 (108) | 11.8 |
| 18 | PVX_099980 | MSP1-42 | 1325–1729 (428) | 42.0 | |
| 19 | PVX_092275 | Apical membrane antigen 1 (AMA1) | 43–487 (444) | 50.1 | |
| South Asia P. vivax top reactive serological markers [6] | 20 | PVX_081830 | Exported protein, unknown function | 1–494 (496) | 56.7 |
| 21 | PVX_116780 | Protein transport protein SFT2, putative | 1–268 (269) | 29.0 | |
| 22 | PVX_083560 | Exported protein, unknown function | 1–310 (311) | 34.0 | |
| 23 | PVX_121930 | Exported protein, unknown function | 1–289 (290) | 32.4 | |
| 24 | PVX_118705 | Hypothetical protein, conserved | 1–438 (439) | 50.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momoh, E.O.; Ghag, S.K.; White, J.; Mudeppa, D.G.; Rathod, P.K. Multiplex Assays for Analysis of Antibody Responses to South Asian Plasmodium falciparum and Plasmodium vivax Malaria Infections. Vaccines 2024, 12, 1. https://doi.org/10.3390/vaccines12010001
Momoh EO, Ghag SK, White J, Mudeppa DG, Rathod PK. Multiplex Assays for Analysis of Antibody Responses to South Asian Plasmodium falciparum and Plasmodium vivax Malaria Infections. Vaccines. 2024; 12(1):1. https://doi.org/10.3390/vaccines12010001
Chicago/Turabian StyleMomoh, Elizabeth O., Sonam K. Ghag, John White, Devaraja G. Mudeppa, and Pradipsinh K. Rathod. 2024. "Multiplex Assays for Analysis of Antibody Responses to South Asian Plasmodium falciparum and Plasmodium vivax Malaria Infections" Vaccines 12, no. 1: 1. https://doi.org/10.3390/vaccines12010001
APA StyleMomoh, E. O., Ghag, S. K., White, J., Mudeppa, D. G., & Rathod, P. K. (2024). Multiplex Assays for Analysis of Antibody Responses to South Asian Plasmodium falciparum and Plasmodium vivax Malaria Infections. Vaccines, 12(1), 1. https://doi.org/10.3390/vaccines12010001






