Role of Pre-Farrow Natural Planned Exposure of Gilts in Shaping the Passive Antibody Response to Rotavirus A in Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, NPE Material and Sample Collection
2.2. Generation of Rotavirus A VP7 and VP4* Expression Constructs
2.3. Recombinant Protein Expression
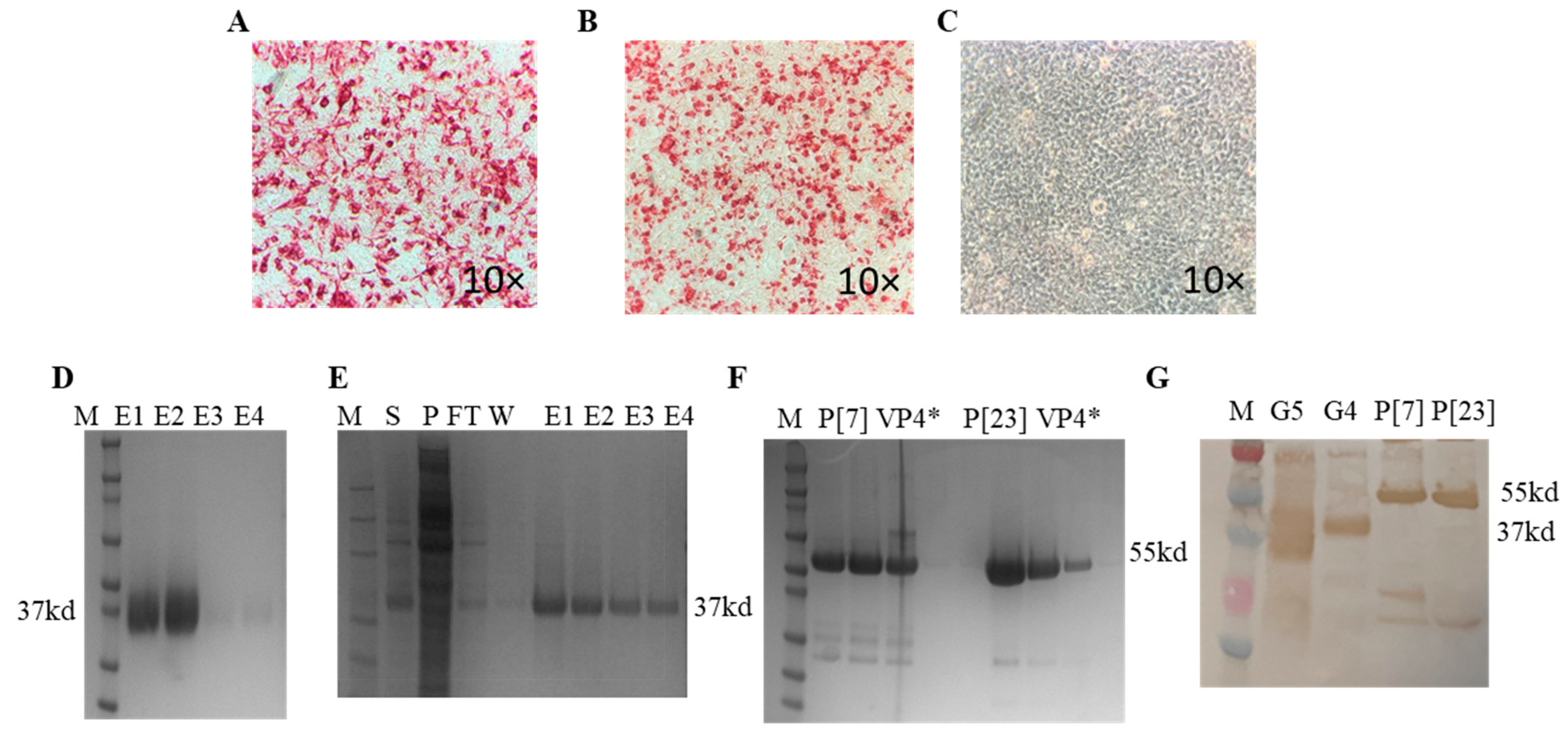
2.4. Protein Purification and Validation
2.5. Development of Recombinant Protein ELISAs to Quantitate RVA Antibodies
2.6. Anti-RVA Antibody Endpoint Titer Determination
2.7. Next-Generation Sequencing of RV Strains in NPE Material and Piglet Feces
2.8. Statistical Analysis
3. Results
3.1. Expression of Recombinant Proteins and Optimization of ELISAs
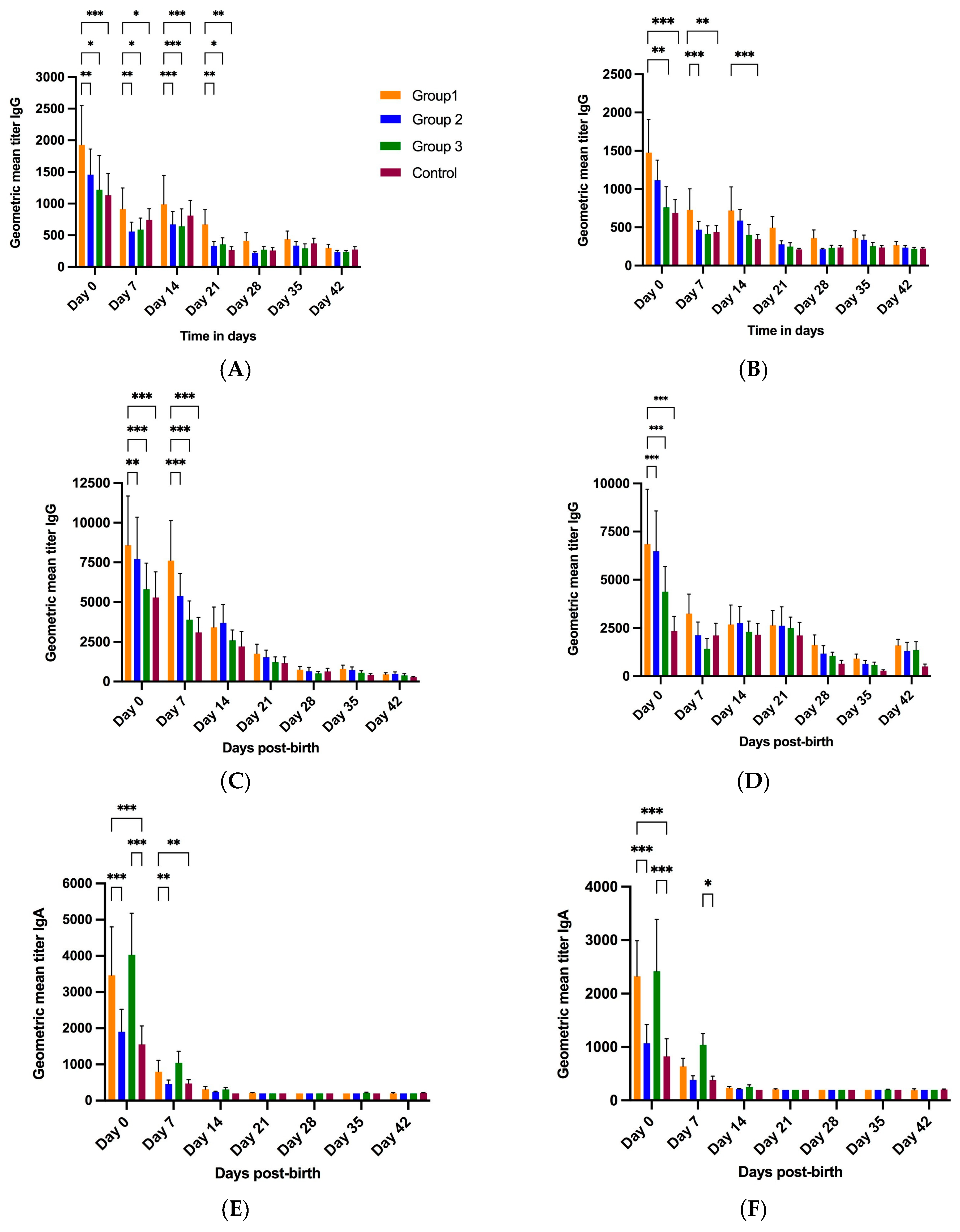
3.2. Antibody Response to RVA NPE
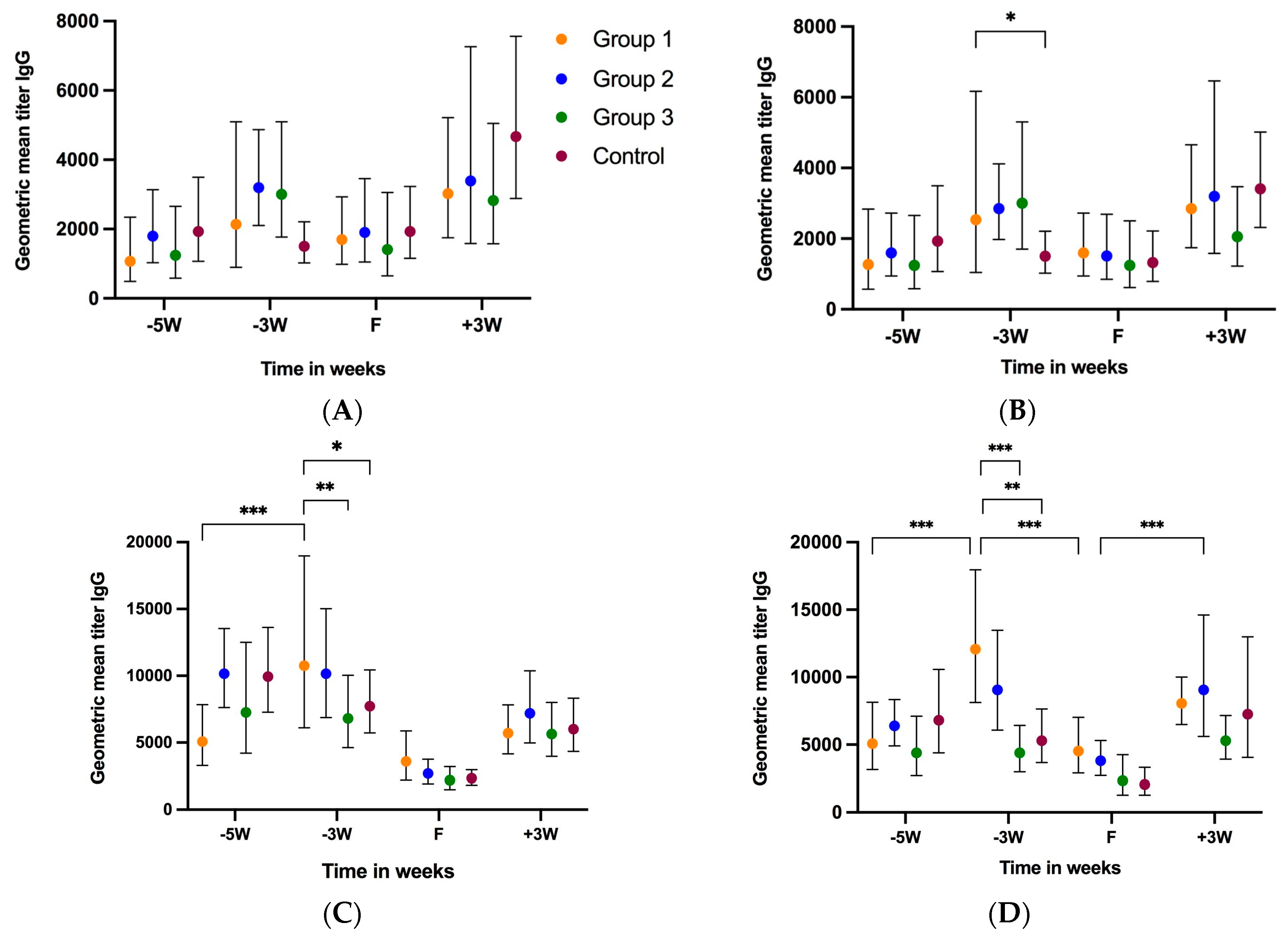
3.2.1. Gilt Serum
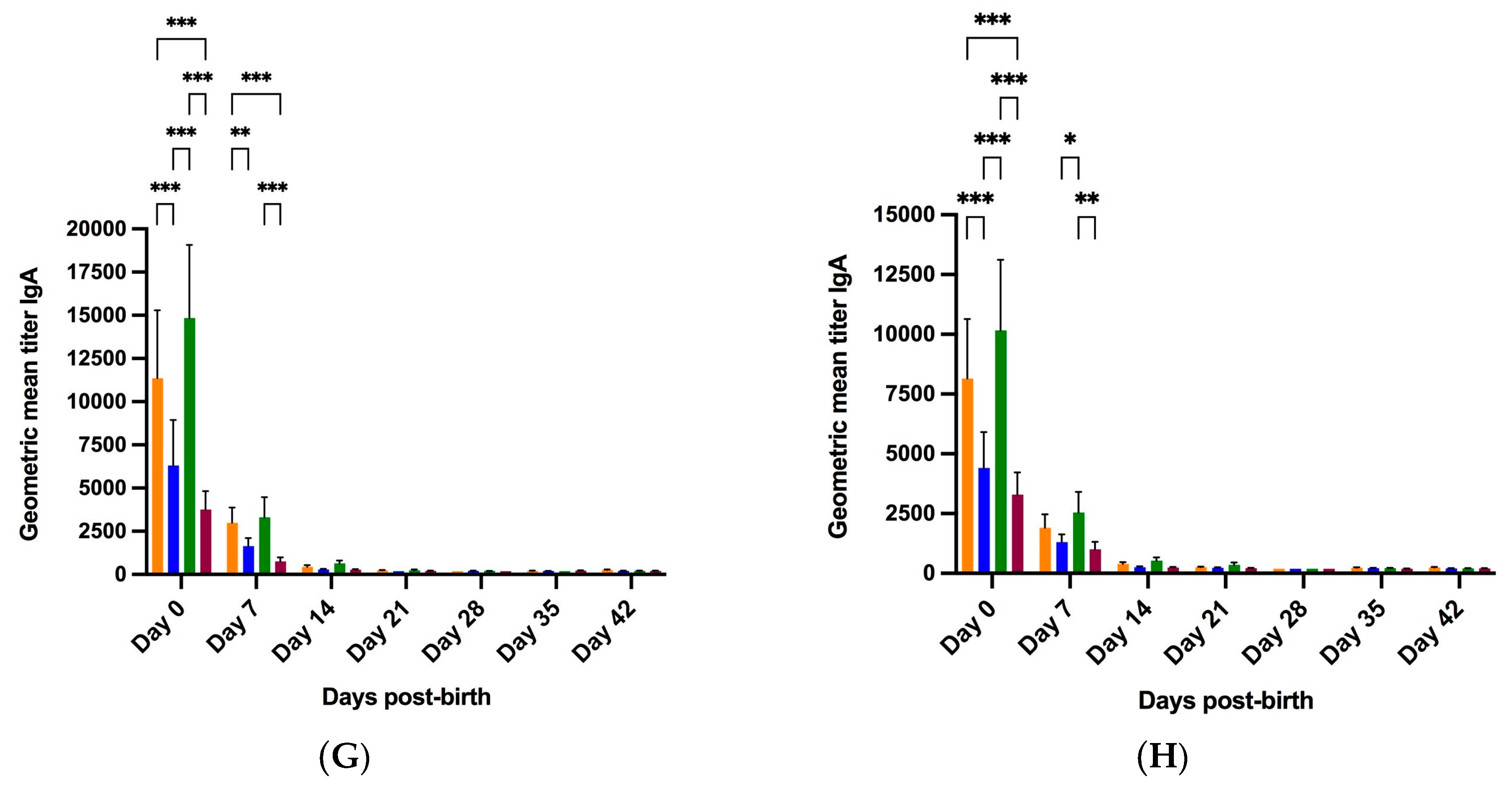
3.2.2. Colostrum and Milk
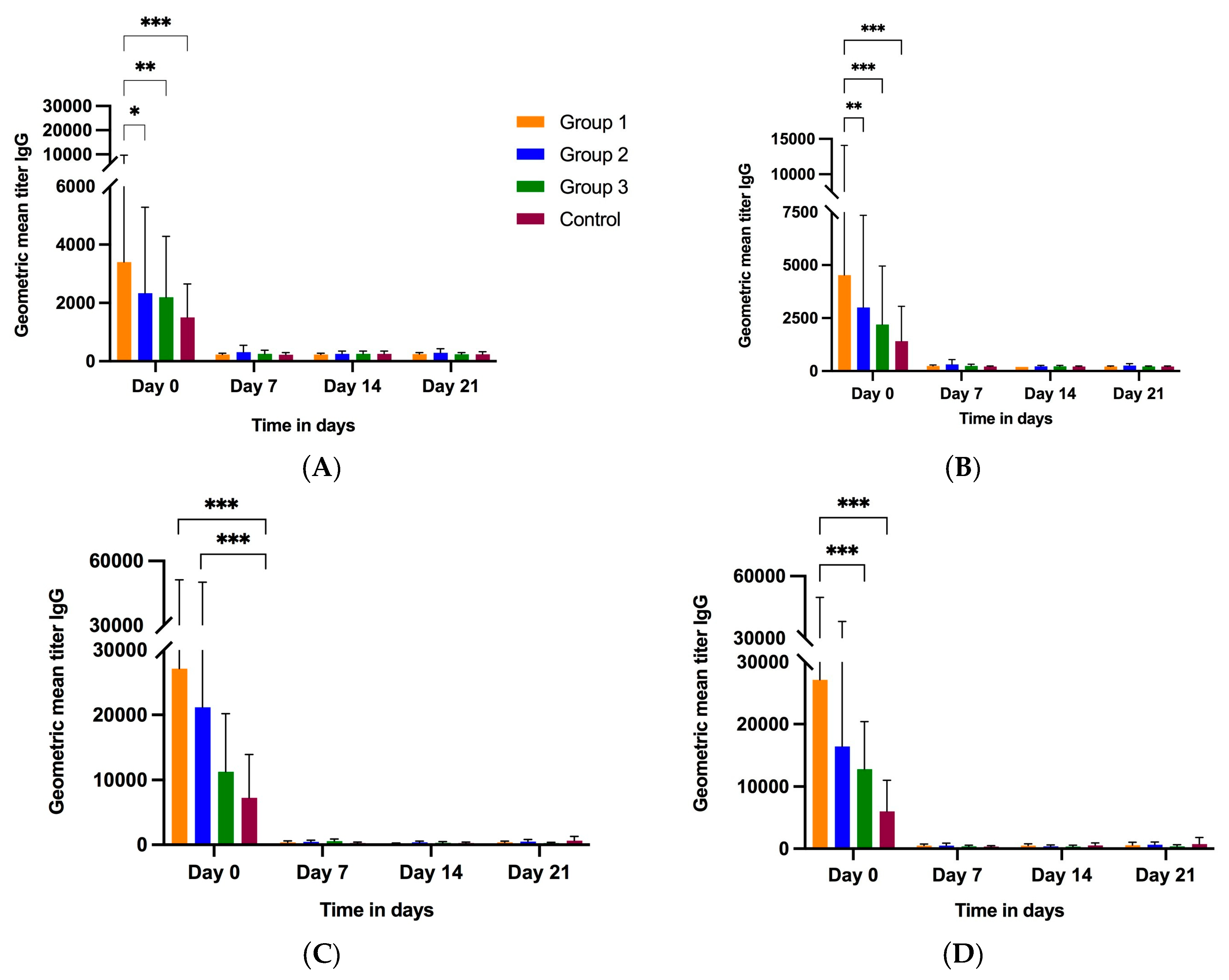
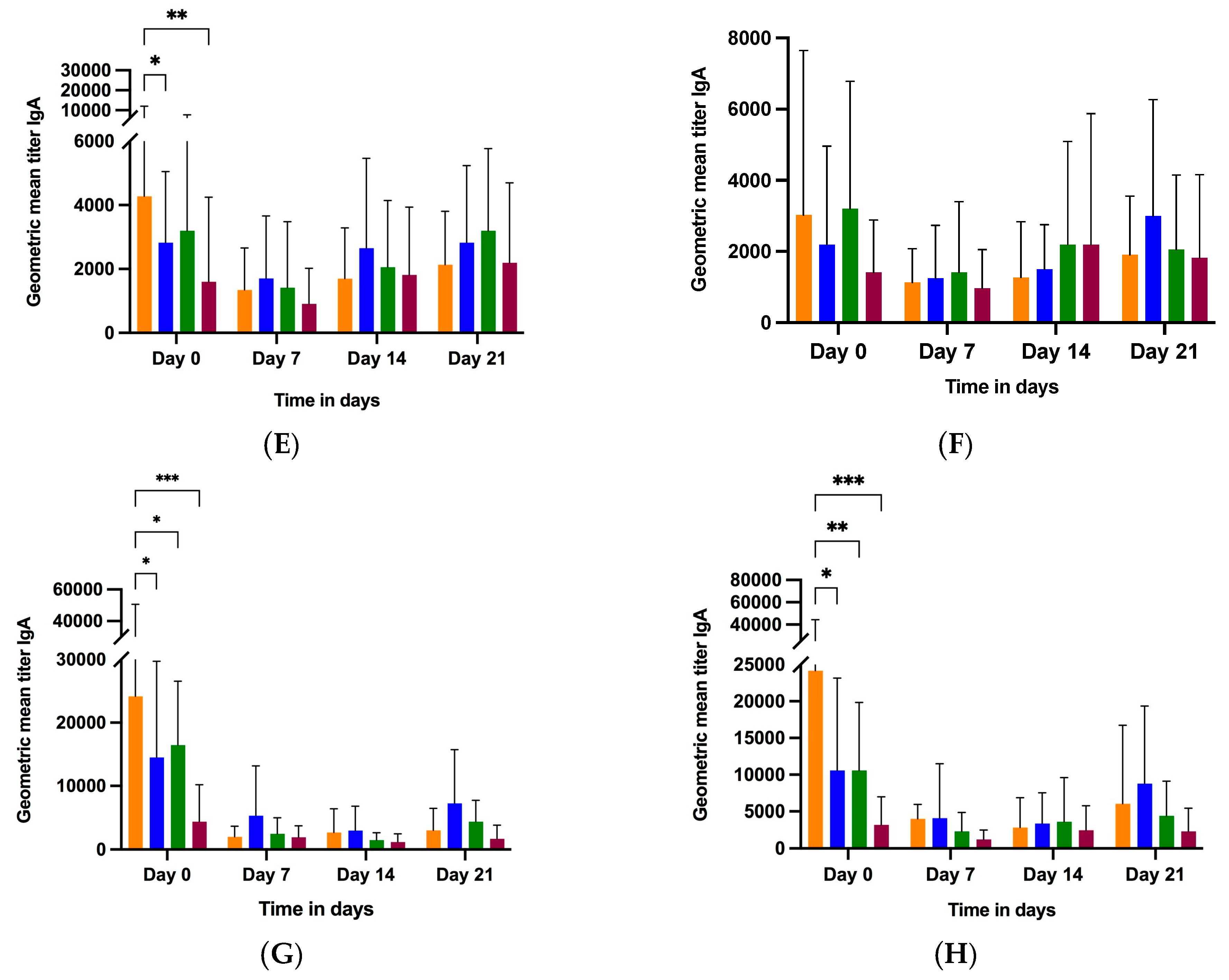
3.2.3. Piglet Serum
3.3. Effects of NPE Administration in Gilts on Piglet Fecal RVA Shedding
3.4. Sequence Analysis and Antigenic Variation among the RVA Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estes, M.; Kapikian, A. Rotaviruses. In Fields Virology Wolters Kluwer Health; Lippincott, Wilams and Wilkins: Philadelphia, PA, USA, 2021; pp. 1917–1975. [Google Scholar]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gómara, M.; Maes, P.; Patton, J.T.; et al. Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 2008, 2, 3204–3219. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Otto, P.H.; Ciarlet, M.; Desselberger, U.; Van Ranst, M.; Johne, R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012, 157, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Kemenesi, G.; Budinski, I.; Földes, F.; Zana, B.; Marton, S.; Varga-Kugler, R.; Oldal, M.; Kurucz, K.; Jakab, F. Candidate new rotavirus species in Schreiber’s bats, Serbia. Infect. Genet. Evol. 2017, 48, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; Rahman, M.; Attoui, H.; Bányai, K.; Estes, M.K.; Gentsch, J.R.; Iturriza-Gómara, M.; Kirkwood, C.D.; Martella, V.; et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008, 153, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Vlasova, A.N.; Saif, L.J. Detection and genetic diversity of porcine group A rotaviruses in historic (2004) and recent (2011 and 2012) swine fecal samples in Ohio: Predominance of the G9P[13] genotype in nursing piglets. J. Clin. Microbiol. 2013, 51, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Vlasova, A.N.; Saif, L.J. Prevalence and genetic heterogeneity of porcine group C rotaviruses in nursing and weaned piglets in Ohio, USA and identification of a potential new VP4 genotype. Vet. Microbiol. 2013, 164, 27–38. [Google Scholar] [CrossRef]
- Marthaler, D.; Rossow, K.; Gramer, M.; Collins, J.; Goyal, S.; Tsunemitsu, H.; Kuga, K.; Suzuki, T.; Ciarlet, M.; Matthijnssens, J. Detection of substantial porcine group B rotavirus genetic diversity in the United States, resulting in a modified classification proposal for G genotypes. Virology 2012, 433, 85–96. [Google Scholar] [CrossRef]
- Marthaler, D.; Homwong, N.; Rossow, K.; Culhane, M.; Goyal, S.; Collins, J.; Matthijnssens, J.; Ciarlet, M. Rapid detection and high occurrence of porcine rotavirus, A.; B, and C by RT-qPCR in diagnostic samples. J. Virol. Methods 2014, 209, 30–34. [Google Scholar] [CrossRef]
- Janke, B.H.; Nelson, J.K.; Benfield, D.A.; Nelson, E.A. Relative prevalence of typical and atypical strains among rotaviruses from diarrheic pigs in conventional swine herds. J. Vet. Diagn. Investig. 1990, 2, 308–311. [Google Scholar] [CrossRef]
- Papp, H.; László, B.; Jakab, F.; Ganesh, B.; De Grazia, S.; Matthijnssens, J.; Ciarlet, M.; Martella, V.; Bányai, K. Review of group A rotavirus strains reported in swine and cattle. Vet. Microbiol. 2013, 165, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F. Enhancing Control of Porcine Rotavirus Through the Identification of Candidate B Cell Epitopes. Ph.D. Thesis, University of Minnesota, Saint Paul, MN, USA, 2020. [Google Scholar]
- Chepngeno, J.; Diaz, A.; Paim, F.C.; Saif, L.J.; Vlasova, A.N. Rotavirus C: Prevalence in suckling piglets and development of virus-like particles to assess the influence of maternal immunity on the disease development. Vet. Res. 2019, 50, 84. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.A.; Rich, E.D.; Besser, T.E. Role of maternally derived circulating antibodies in protection of neonatal swine against porcine group A rotavirus. J. Infect. Dis. 1996, 174, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Gelberg, H.B.; Patterson, J.S.; Woode, G.N. A longitudinal study of rotavirus antibody titers in swine in a closed specific pathogen-free herd. Vet. Microbiol. 1991, 28, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Wang, Q.; Vlasova, A.N.; Saif, L.J. Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates. Pathogens 2020, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Bohl, E.H.; Gupta, R.K.; Olquin, M.V.; Saif, L.J. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect. Immun. 1972, 63, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ludert, J.E.; Ruiz, M.C.; Hidalgo, C.; Liprandi, F. Antibodies to rotavirus outer capsid glycoprotein VP7 neutralize infectivity by inhibiting virion decapsidation. J. Virol. 2002, 76, 6643–6651. [Google Scholar] [CrossRef]
- Nair, N.; Feng, N.; Blum, L.K.; Sanyal, M.; Ding, S.; Jiang, B.; Sen, A.; Morton, J.M.; He, X.S.; Robinson, W.H.; et al. VP4- and VP7-specific antibodies mediate heterotypic immunity to rotavirus in humans. Sci. Transl. Med. 2017, 21, eaam5434. [Google Scholar] [CrossRef]
- Estes, M.K.; Greenberg, H.B. Rotaviruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer, Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 2, pp. 1347–1401. [Google Scholar]
- Settembre, E.C.; Chen, J.Z.; Dormitzer, P.R.; Grigorieff, N.; Harrison, S.C. Atomic model of an infectious rotavirus particle. EMBO J. 2011, 19, 408–416. [Google Scholar] [CrossRef]
- Trask, S.D.; McDonald, S.M.; Patton, J.T. Structural insights into the coupling of virion assembly and rotavirus replication. Nat. Rev. Microbiol. 2012, 23, 165–177. [Google Scholar] [CrossRef]
- Li, Y.; Xue, M.; Yu, L.; Luo, G.; Yang, H.; Jia, L.; Zeng, Y.; Li, T.; Ge, S.; Xia, N. Expression and characterization of a novel truncated rotavirus VP4 for the development of a recombinant rotavirus vaccine. Vaccine 2018, 36, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.S. Field Experiences with Interventions for Rotavirus Control. In ISU Swine Disease Conference for Swine Practitioners; Iowa State University: Ames, IA, USA, 2016; pp. 30–36. [Google Scholar]
- Anderson, A.V.; Shepherd, F.; Dominguez, F.; Pittman, J.S.; Marthaler, D.; Karriker, L.A. Evaluating natural planned exposure protocols on rotavirus shedding patterns in gilts and the impact on their suckling pigs. J. Swine Health Prod. 2023, 31, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Anderson, A.V.; Pittman, J.; Springer, N.L.; Marthaler, D.G.; Mwangi, W. Antibody response to Rotavirus C pre-farrow natural planned exposure to gilts and their piglets. Viruses 2022, 14, 2250. [Google Scholar] [CrossRef] [PubMed]
- Agbla, J.M.; Esona, M.D.; Jaimes, J.; Gautam, R.; Agbankpé, A.J.; Katz, E.; Dougnon, T.V.; Capo-Chichi, A.; Ouedraogo, N.; Razack, O.; et al. Whole genome analysis of rotavirus strains circulating in Benin before vaccine introduction, 2016–2018. Virus Res. 2022, 313, 198715. [Google Scholar] [CrossRef] [PubMed]
- Bourne, F.J.; Curtis, J. The transfer of immunoglobins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology 1973, 24, 157–162. [Google Scholar] [PubMed]
- Klobasa, F.; Habe, F.; Werhahn, E.; Butler, J.E. Changes in the concentrations of serum IgG, IgA and IgM of sows throughout the reproductive cycle. Vet. Immunol. Immunopathol. 1985, 10, 341–353. [Google Scholar] [CrossRef]
- Fu, Z.F.; Hampson, D.J.; Wilks, C.R. Transfer of maternal antibody against group A rotavirus from sows to piglets and serological responses following natural infection. Res. Vet. Sci. 1990, 48, 365–373. [Google Scholar] [CrossRef]
- Weström, B.R.; Svendsen, J.; Ohlsson, B.G.; Tagesson, C.; Karlsson, B.W. Intestinal transmission of macromolecules (BSA and FITC-labelled dextrans) in the neonatal pig: Influence of age of piglet and molecular weight of markers. Biol. Neonate. 1984, 46, 20–26. [Google Scholar] [CrossRef]
- Sangild, P.T.; Foltmann, B.; Cranwell, P.D. Development of gastric proteases in fetal pigs and pigs from birth to thirty six days of age. The effect of adrenocorticotropin (ACTH). J. Dev. Physiol. 1991, 16, 229–238. [Google Scholar]
- Mankarious, S.; Lee, M.; Fischer, S.; Pyun, K.H.; Ochs, H.D.; Oxelius, V.A.; Wedgwood, R.J. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 1988, 112, 634–640. [Google Scholar]
- Challacombe, S.J.; Russell, M.W. Estimation of the intravascular half-lives of normal rhesus monkey IgG, IgA and IgM. Immunology 1979, 36, 331–338. [Google Scholar] [PubMed]
- Velázquez, F.R.; Matson, D.O.; Guerrero, M.L.; Shults, J.; Calva, J.J.; Morrow, A.L.; Glass, R.I.; Pickering, L.K.; Ruiz-Palacios, G.M. Serum antibody as a marker of protection against natural rotavirus infection and disease. J. Infect. Dis. 2000, 182, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Tô, T.L.; Ward, L.A.; Yuan, L.; Saif, L.J. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Gen. Virol. 1998, 79 Pt 11, 2661–2672. [Google Scholar] [CrossRef]
- Westerman, L.E.; McClure, H.M.; Jiang, B.; Almond, J.W.; Glass, R.I. Serum IgG mediates mucosal immunity against rotavirus infection. Proc. Natl. Acad. Sci. USA 2005, 102, 7268–7273. [Google Scholar] [CrossRef] [PubMed]
- O’Ryan, M.L.; Matson, D.O.; Estes, M.K.; Pickering, L.K. Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J. Infect. Dis. 1994, 169, 504–511. [Google Scholar] [CrossRef]
- Hoshino, Y.; Sereno, M.M.; Midthun, K.; Flores, J.; Kapikian, A.Z.; Chanock, R.M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc. Natl. Acad. Sci. USA 1985, 82, 8701–8704. [Google Scholar] [CrossRef]
- Wen, X.; Cao, D.; Jones, R.W.; Li, J.; Szu, S.; Hoshino, Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine 2012, 30, 6121–6126. [Google Scholar] [CrossRef]
- Ishida, S.; Feng, N.; Tang, B.; Gilbert, J.M.; Greenberg, H.B. Quantification of systemic and local immune responses to individual rotavirus proteins during rotavirus infection in mice. J. Clin. Microbiol. 1996, 34, 1694–1700. [Google Scholar] [CrossRef]
- Yuan, L.; Ishida, S.; Honma, S.; Patton, J.T.; Hodgins, D.C.; Kapikian, A.Z.; Hoshino, Y. Homotypic and heterotypic serum isotype-specific antibody responses to rotavirus nonstructural protein 4 and viral protein (VP) 4, VP6, and VP7 in infants who received selected live oral rotavirus vaccines. J. Infect. Dis. 2004, 189, 1833–1845. [Google Scholar] [CrossRef]
- Shepherd, F.K.; Dvorak, C.M.T.; Murtaugh, M.P.; Marthaler, D.G. Leveraging a Validated in silico Approach to Elucidate Genotype-Specific VP7 Epitopes and Antigenic Relationships of Porcine Rotavirus A. Front. Genet. 2020, 11, 828. [Google Scholar] [CrossRef]


| Litter Id. | Group | Week | RVA Ct |
|---|---|---|---|
| 41049 | 1 | 1 | 15.42 |
| 2 | 18.62 | ||
| 40996 | 3 | 2 | 18.48 |
| 3 | 17.28 | ||
| 41071 | 1 | 4 | 13.07 |
| 41045 | 2 | 4 | 11.29 |
| 40996 | 3 | 4 | 12.62 |
| 41053 | 4 | 4 | 12.15 |
| Group | Colostrum IgA Titer (GMT, Day 0) | Piglet Serum IgA Titer (GMT, Day 0) | ||||||
|---|---|---|---|---|---|---|---|---|
| G4 | G5 | P[7] | P[23] | G4 | G5 | P[7] | P[23] | |
| 1 | 3020 | 4278 | 24,163 | 24,163 | 2170 | 3466 | 11,353 | 8145 |
| 2 | 2193 | 2821 | 14,519 | 10,595 | 1025 | 1903 | 6315 | 4406 |
| 3 | 3200 | 3200 | 15,464 | 10,595 | 2216 | 3966 | 15,097 | 10,500 |
| 4 | 1411 | 1600 | 3408 | 3200 | 893 | 1748 | 4981 | 3820 |
| Litter | Antigen | Sow Colostrum IgA (Day 0) | Piglet Serum IgA Levels at Multiple Time Points | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 (Group GMT) | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |||
| 41049 | P[7] | 12,800 | 11,353 | 25,600 | 1600 | 200 | 200 | 200 | 1600 | 3200 |
| P[23] | 25,600 | 8145 | 12,800 | 1600 | 400 | 200 | 200 | 1600 | 1600 | |
| G5 | 800 | 3466 | 3200 | 200 | 200 | 200 | 200 | 200 | 200 | |
| G4 | 800 | 2275 | 1600 | 200 | 200 | 200 | 200 | 200 | 200 | |
| Fecal RVA shedding (Ct Value) | - | 16.09 | 20.96 | 27.13 | 12.80 | 26.96 | 25.13 | |||
| 40996 | P[7] | 25,600 | 15,097 | 12,800 | 1600 | 1600 | 800 | 200 | 200 | 200 |
| P[23] | 25,600 | 10,500 | 25,600 | 3200 | 800 | 1600 | 200 | 200 | 200 | |
| G5 | 800 | 3966 | 800 | 400 | 200 | 200 | 200 | 200 | 200 | |
| G4 | 1600 | 2340 | 3200 | 400 | 200 | 200 | 200 | 200 | 200 | |
| Fecal RVA shedding (Ct value) | - | 28.09 | 20.09 | 15.5 | 14 | 22.62 | 18.07 | |||
| Litter | Group | Week | RVA Ct | Genome Constellation |
|---|---|---|---|---|
| 40996 | 3 | 2 | 18.49 | G11-P[34]-I5-R1-C1-M1-A8-N1-T7-E9-H1 |
| 4 | 12.62 | G9-P[23]-I5-R1-C1-M1-A8-N1-T7-E1-H1 | ||
| 41071 | 1 | 4 | 13.07 | G9-P[23]-I5-R1-C1-M1-A8-N1-T7-E1-H1 |
| 41045 | 2 | 4 | 11.29 | G9-P[23]-I5-R1-C1-M1-A8-N1-T7-E1-H1 |
| 41053 | 4 | 4 | 12.15 | G9-P[23]-I5-R1-C1-M1-A8-N1-T7-E1-H1 |
| NEM | NEM | NEM | NEM | NEM | NEM | NEM | NEM | |||||||||||||||||||||||||||
| 87 | 90 | 91 | 92 | 94 | 95 | 96 | 97 | 99 | 100 | 103 | 119 | 122 | 123 | 124 | 126 | 143 | 144 | 147 | 152 | 189 | 190 | 192 | 208 | 209 | 210 | 211 | 212 | 213 | 214 | 216 | 221 | 223 | 242 | |
| G4-NPE-Parent | T | R | T | O | N | D | N | E | K | D | S | N | S | N | V | E | K | F | G | I | S | S | T | Q | T | T | N | A | N | T | E | S | K | T |
| G11-Piglet | N | A | R | E | A | . | D | K | . | . | . | K | T | D | I | S | . | Y | N | M | T | . | . | L | . | . | . | S | A | . | . | A | . | A |
| G9-Piglet | N | S | . | O | G | . | T | . | . | N | . | K | T | D | I | S | . | Y | T | M | O | . | . | T | . | . | . | T | A | . | . | N | . | N |
| G9-Piglet | N | S | . | O | G | . | T | . | . | N | . | K | T | D | I | S | . | Y | T | M | O | . | . | T | . | . | . | T | A | . | . | N | . | N |
| G9-Piglet | N | S | . | O | G | . | T | . | . | N | . | K | T | D | I | S | . | Y | T | M | O | . | . | T | . | . | . | T | A | . | . | N | . | N |
| G9-Piglet | N | S | . | O | G | . | T | . | . | N | . | K | T | D | I | S | . | Y | T | M | O | . | . | T | . | . | . | T | A | . | . | N | . | N |
| G5-NPE-Parent | N | A | T | E | A | D | T | K | T | E | S | K | A | D | I | S | K | Y | N | M | T | S | T | S | T | T | D | I | N | S | E | A | K | N |
| G11-Piglet | . | . | R | . | . | . | D | . | K | D | . | . | T | . | . | . | . | . | . | . | . | . | . | L | . | . | N | S | A | T | . | . | . | . |
| G9-Piglet | . | S | . | O | G | . | . | E | K | N | . | . | T | . | . | . | . | . | T | . | O | . | . | T | . | . | N | T | A | T | . | N | . | . |
| G9-Piglet | . | S | . | O | G | . | . | E | K | N | . | . | T | . | . | . | . | . | T | . | O | . | . | T | . | . | N | T | A | T | . | N | . | . |
| G9-Piglet | . | S | . | O | G | . | . | E | K | N | . | . | T | . | . | . | . | . | T | . | O | . | . | T | . | . | N | T | A | T | . | N | . | . |
| G9-Piglet | . | S | . | O | G | . | . | E | K | N | . | . | T | . | . | . | . | . | T | . | O | . | . | T | . | . | N | T | A | T | . | N | . | . |
| VP8* | VP5* | |||||||||||||||||||||||||||||||||
| 87 | 88 | 89 | 100 | 113 | 114 | 115 | 116 | 125 | 131 | 132 | 133 | 135 | 146 | 148 | 150 | 173 | 180 | 183 | 188 | 190 | 192 | 193 | 194 | 195 | 196 | 217 | 385 | 388 | 392 | 393 | 425 | 433 | 458 | |
| P[7]-Parent | T | V | E | D | Q | T | T | N | Q | E | N | T | Q | T | P | G | R | T | N | Y | S | T | N | Y | D | T | T | Y | A | G | A | R | G | Q |
| P[34]-Piglet | K | N | D | . | I | S | . | T | . | S | . | M | T | Q | S | . | L | E | . | Q | T | S | . | . | S | E | . | . | R | . | K | . | L | D |
| P[23]-Piglet | S | N | A | . | P | S | E | S | . | . | . | V | T | . | I | . | K | E | . | F | T | . | . | . | . | . | . | . | R | . | . | . | E | G |
| P[23]-Piglet | S | N | A | . | P | S | E | S | . | . | . | V | T | . | I | . | K | E | . | F | T | . | . | . | . | . | . | . | R | . | . | . | E | G |
| P[23]-Piglet | S | N | A | . | P | S | E | S | . | . | . | V | T | . | I | . | K | E | . | F | T | . | . | . | . | . | . | . | R | . | . | . | E | G |
| P[23]-Piglet | S | N | A | . | P | S | E | S | . | . | . | V | T | . | I | . | K | E | . | F | T | . | . | . | . | . | . | . | R | . | . | . | E | G |
| P[23]-Parent | S | N | A | D | P | S | E | S | Q | E | N | V | T | T | I | G | K | E | N | Y | T | T | N | Y | D | T | T | Y | R | G | A | R | E | G |
| P[34]-Piglet | K | . | D | . | I | . | T | T | . | S | . | M | . | Q | S | . | L | . | . | Q | . | S | . | . | S | E | . | . | . | . | K | . | L | D |
| P[23]-Piglet | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| P[23]-Piglet | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| P[23]-Piglet | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| P[23]-Piglet | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | F | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Anderson Reever, A.V.; Pittman, J.S.; Springer, N.L.; Mallen, K.; Roman-Sosa, G.; Sangewar, N.; Casey-Moore, M.C.; Bowen, M.D.; Mwangi, W.; et al. Role of Pre-Farrow Natural Planned Exposure of Gilts in Shaping the Passive Antibody Response to Rotavirus A in Piglets. Vaccines 2023, 11, 1866. https://doi.org/10.3390/vaccines11121866
Kumar D, Anderson Reever AV, Pittman JS, Springer NL, Mallen K, Roman-Sosa G, Sangewar N, Casey-Moore MC, Bowen MD, Mwangi W, et al. Role of Pre-Farrow Natural Planned Exposure of Gilts in Shaping the Passive Antibody Response to Rotavirus A in Piglets. Vaccines. 2023; 11(12):1866. https://doi.org/10.3390/vaccines11121866
Chicago/Turabian StyleKumar, Deepak, Amanda V. Anderson Reever, Jeremy S. Pittman, Nora L. Springer, Kylynn Mallen, Gleyder Roman-Sosa, Neha Sangewar, Mary C. Casey-Moore, Michael D. Bowen, Waithaka Mwangi, and et al. 2023. "Role of Pre-Farrow Natural Planned Exposure of Gilts in Shaping the Passive Antibody Response to Rotavirus A in Piglets" Vaccines 11, no. 12: 1866. https://doi.org/10.3390/vaccines11121866
APA StyleKumar, D., Anderson Reever, A. V., Pittman, J. S., Springer, N. L., Mallen, K., Roman-Sosa, G., Sangewar, N., Casey-Moore, M. C., Bowen, M. D., Mwangi, W., & Marthaler, D. G. (2023). Role of Pre-Farrow Natural Planned Exposure of Gilts in Shaping the Passive Antibody Response to Rotavirus A in Piglets. Vaccines, 11(12), 1866. https://doi.org/10.3390/vaccines11121866





