Exploring the Effects of Vitamin D and Vitamin A Levels on the Response to COVID-19 Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Vitamins and Antibody Measurement and Classification
2.3. Antibody Avidity Assay
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
3.2. Effect of the Demographic/Clinical Characteristics on the Response to the BNT162b2 Vaccine
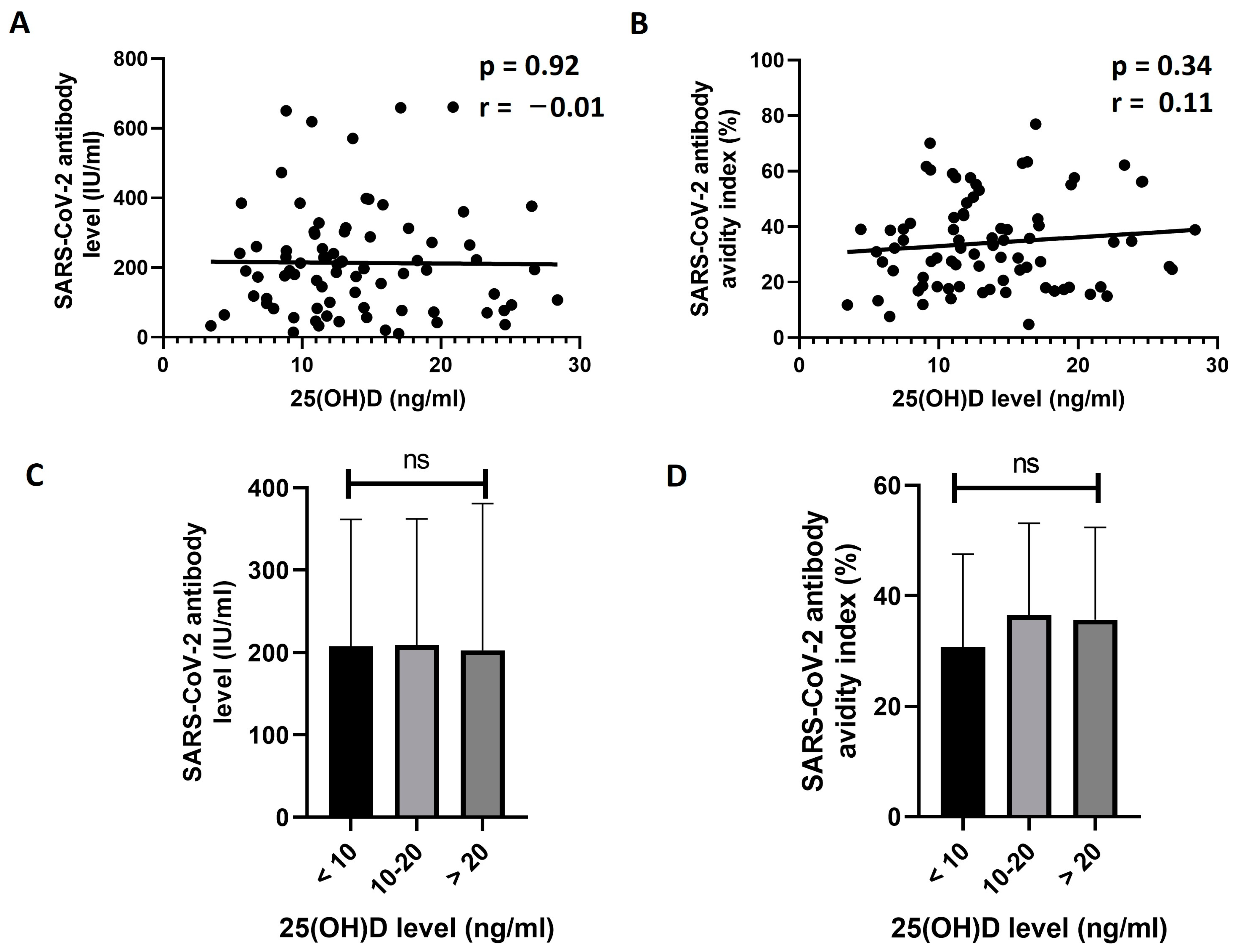
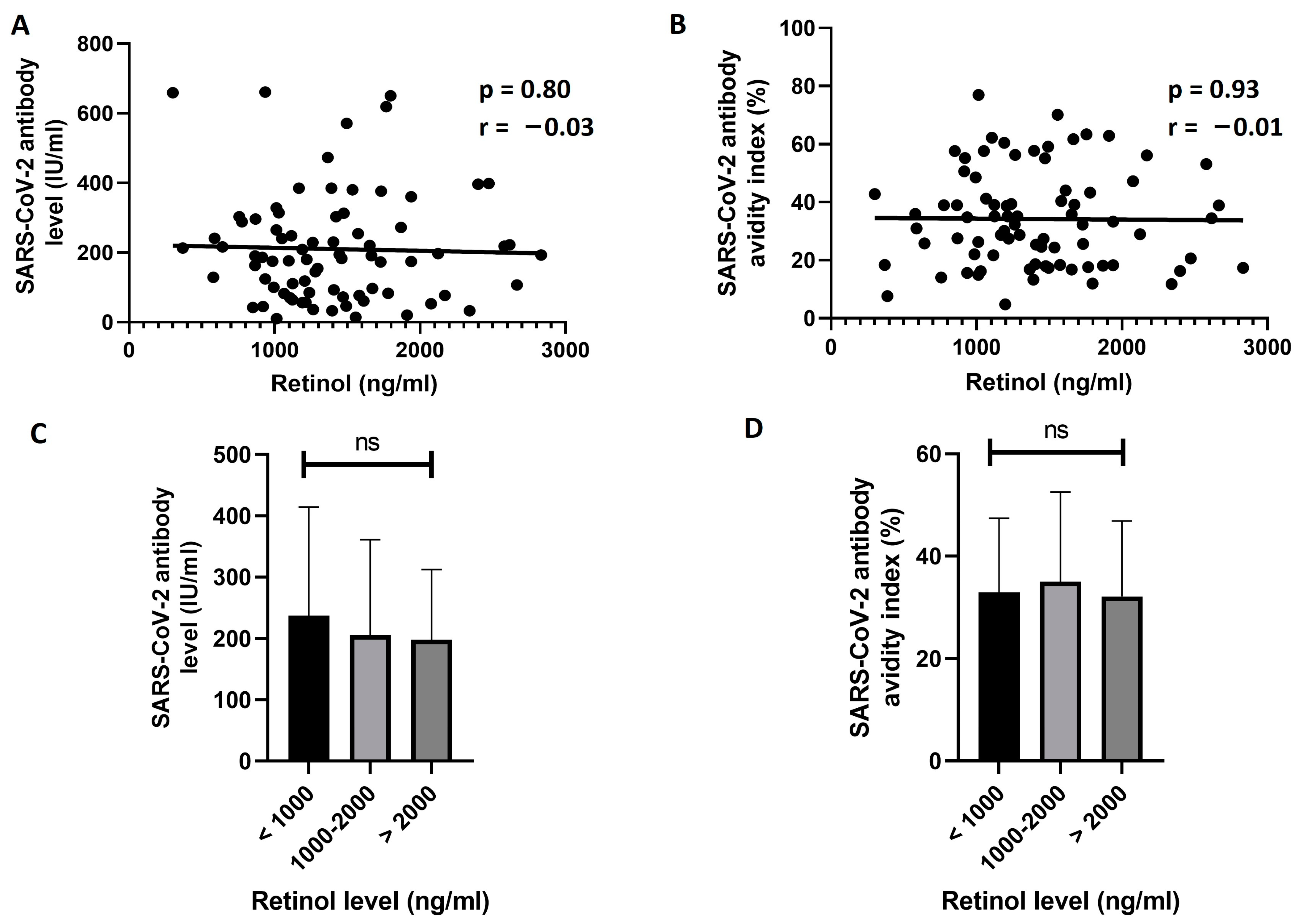
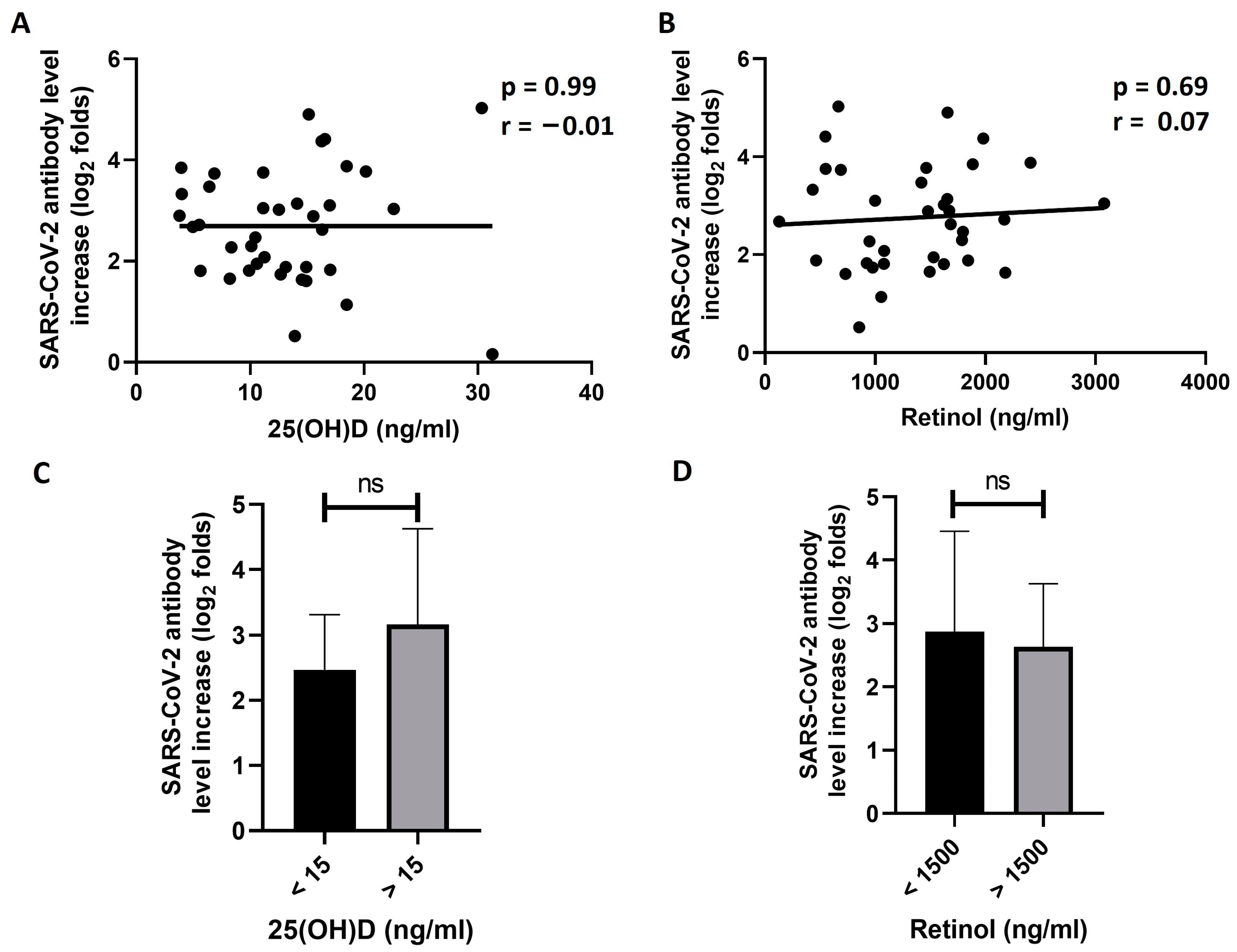
3.3. Exploring the Effect of 25(OH)D and Retinol Levels on the Response to BNT162b2 Vaccine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 9 August 2023).
- Silver Spring (MD): Food and Drug Administration (US). Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations (EUAs). Available online: https://www.ncbi.nlm.nih.gov/books/NBK570900/ (accessed on 9 August 2023).
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Hewison, M. Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 2011, 7, 337–345. [Google Scholar] [CrossRef]
- Heine, G.; Drozdenko, G.; Lahl, A.; Unterwalder, N.; Mei, H.; Volk, H.D.; Dorner, T.; Radbruch, A.; Worm, M. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur. J. Clin. Nutr. 2011, 65, 329–334. [Google Scholar] [CrossRef]
- Zitt, E.; Sprenger-Mahr, H.; Knoll, F.; Neyer, U.; Lhotta, K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine 2012, 30, 931–935. [Google Scholar] [CrossRef]
- Slawin, A.; Brydak, L.B.; Doniec, Z.; Bujnowska-Fedak, M.; Mastalerz-Migas, A. Serum Vitamin D and Immunogenicity of Influenza Vaccination in the Elderly. Adv. Exp. Med. Biol. 2021, 1324, 21–28. [Google Scholar] [CrossRef]
- Sundaram, M.E.; Talbot, H.K.; Zhu, Y.; Griffin, M.R.; Spencer, S.; Shay, D.K.; Coleman, L.A. Vitamin D is not associated with serologic response to influenza vaccine in adults over 50 years old. Vaccine 2013, 31, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Crum-Cianflone, N.F.; Won, S.; Lee, R.; Lalani, T.; Ganesan, A.; Burgess, T.; Agan, B.K. Vitamin D levels and influenza vaccine immunogenicity among HIV-infected and HIV-uninfected adults. Vaccine 2016, 34, 5040–5046. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.D.; Lin, C.H.; Lei, W.T.; Chang, H.Y.; Lee, H.C.; Yeung, C.Y.; Chiu, N.C.; Chi, H.; Liu, J.M.; Hsu, R.J.; et al. Does Vitamin D Deficiency Affect the Immunogenic Responses to Influenza Vaccination? A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Vivaldi, G.; Chambers, E.S.; Cai, W.; Li, W.; Faustini, S.E.; Gibbons, J.M.; Pade, C.; Coussens, A.K.; Richter, A.G.; et al. Vitamin D Supplementation Does Not Influence SARS-CoV-2 Vaccine Efficacy or Immunogenicity: Sub-Studies Nested within the CORONAVIT Randomised Controlled Trial. Nutrients 2022, 14, 3821. [Google Scholar] [CrossRef]
- Meyers, E.; De Smet, E.; Vercruysse, H.; Callens, S.; Padalko, E.; Heytens, S.; Vandekerckhove, L.; Cools, P.; Witkowski, W. No Significant Association between 25-OH Vitamin D Status and SARS-CoV-2 Antibody Response after COVID-19 Vaccination in Nursing Home Residents and Staff. Vaccines 2023, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Chillon, T.S.; Demircan, K.; Heller, R.A.; Hirschbil-Bremer, I.M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults. Biomedicines 2021, 9, 1714. [Google Scholar] [CrossRef] [PubMed]
- Zelini, P.; d’Angelo, P.; Cereda, E.; Klersy, C.; Sabrina, P.; Albertini, R.; Grugnetti, G.; Grugnetti, A.M.; Marena, C.; Cutti, S.; et al. Association between Vitamin D Serum Levels and Immune Response to the BNT162b2 Vaccine for SARS-CoV-2. Biomedicines 2022, 10, 1993. [Google Scholar] [CrossRef]
- Di Filippo, L.; Frara, S.; Terenzi, U.; Nannipieri, F.; Locatelli, M.; Ciceri, F.; Giustina, A. Lack of vitamin D predicts impaired long-term immune response to COVID-19 vaccination. Endocrine 2023. [Google Scholar] [CrossRef]
- Piec, I.; Cook, L.; Dervisevic, S.; Fraser, W.D.; Ruetten, S.; Berman, M.; English, E.; John, W.G. Age and vitamin D affect the magnitude of the antibody response to the first dose of the SARS-CoV-2 BNT162b2 vaccine. Curr. Res. Transl. Med. 2022, 70, 103344. [Google Scholar] [CrossRef]
- Takahashi, N. Inhibitory Effects of Vitamin A and Its Derivatives on Cancer Cell Growth Not Mediated by Retinoic Acid Receptors. Biol. Pharm. Bull. 2022, 45, 1213–1224. [Google Scholar] [CrossRef]
- Semba, R.D. The role of vitamin A and related retinoids in immune function. Nutr. Rev. 1998, 56, S38–S48. [Google Scholar] [CrossRef]
- Carazo, A.; Macakova, K.; Matousova, K.; Krcmova, L.K.; Protti, M.; Mladenka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Clagett-Dame, M.; Knutson, D. Vitamin A in reproduction and development. Nutrients 2011, 3, 385–428. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, E.M.; Bar-El Dadon, S.; Reifen, R. The vicious cycle of vitamin a deficiency: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Fawzi, W.W. Vitamin A supplementation: Implications for morbidity and mortality in children. J. Infect. Dis. 2000, 182 (Suppl. S1), S122–S133. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef]
- Villamor, E.; Fawzi, W.W. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin. Microbiol. Rev. 2005, 18, 446–464. [Google Scholar] [CrossRef]
- Sinopoli, A.; Caminada, S.; Isonne, C.; Santoro, M.M.; Baccolini, V. What Are the Effects of Vitamin A Oral Supplementation in the Prevention and Management of Viral Infections? A Systematic Review of Randomized Clinical Trials. Nutrients 2022, 14, 4081. [Google Scholar] [CrossRef]
- Semba, R.D. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 1999, 58, 719–727. [Google Scholar] [CrossRef]
- Penkert, R.R.; Rowe, H.M.; Surman, S.L.; Sealy, R.E.; Rosch, J.; Hurwitz, J.L. Influences of Vitamin A on Vaccine Immunogenicity and Efficacy. Front. Immunol. 2019, 10, 1576. [Google Scholar] [CrossRef]
- Bhaskaram, P.; Rao, K.V. Enhancement in seroconversion to measles vaccine with simultaneous administration of vitamin A in 9-months-old Indian infants. Indian J. Pediatr. 1997, 64, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.S.; Balde, A.; George, E.; Kidd, M.; Whittle, H.; Lisse, I.M.; Aaby, P. Effect of vitamin A supplementation on measles-specific antibody levels in Guinea-Bissau. Lancet 2002, 359, 1313–1314. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.S.; Aaby, P.; Bale, C.; Olsen, J.; Michaelsen, K.F.; George, E.; Whittle, H. Randomised trial of effect of vitamin A supplementation on antibody response to measles vaccine in Guinea-Bissau, west Africa. Lancet 1997, 350, 101–105. [Google Scholar] [CrossRef]
- Bahl, R.; Bhandari, N.; Kant, S.; Molbak, K.; Ostergaard, E.; Bhan, M.K. Effect of vitamin A administered at Expanded Program on Immunization contacts on antibody response to oral polio vaccine. Eur. J. Clin. Nutr. 2002, 56, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Muhilal; Scott, A.L.; Natadisastra, G.; Wirasasmita, S.; Mele, L.; Ridwan, E.; West, K.P., Jr.; Sommer, A. Depressed immune response to tetanus in children with vitamin A deficiency. J. Nutr. 1992, 122, 101–107. [Google Scholar] [CrossRef]
- Newton, S.; Owusu-Agyei, S.; Ampofo, W.; Zandoh, C.; Adjuik, M.; Adjei, G.; Tchum, S.; Filteau, S.; Kirkwood, B.R. Vitamin A supplementation enhances infants’ immune responses to hepatitis B vaccine but does not affect responses to Haemophilus influenzae type b vaccine. J. Nutr. 2007, 137, 1272–1277. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Gianfagna, F.; Veronesi, G.; Baj, A.; Dalla Gasperina, D.; Siclari, S.; Drago Ferrante, F.; Maggi, F.; Iacoviello, L.; Ferrario, M.M. Anti-SARS-CoV-2 antibody levels and kinetics of vaccine response: Potential role for unresolved inflammation following recovery from SARS-CoV-2 infection. Sci. Rep. 2022, 12, 385. [Google Scholar] [CrossRef]
- Panel, E. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch. Intern Med. 1998, 158, 1855–1867. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]
- Sharif, Y.; Sadeghi, O.; Dorosty, A.; Siassi, F.; Jalali, M.; Djazayery, A.; Shokri, A.; Mohammad, K.; Parsaeian, M.; Abdollahi, Z.; et al. Association of vitamin D, retinol and zinc deficiencies with stunting in toddlers: Findings from a national study in Iran. Public Health 2020, 181, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pichler, D.; Baumgartner, M.; Kimpel, J.; Rossler, A.; Riepler, L.; Bates, K.; Fleischer, V.; von Laer, D.; Borena, W.; Wurzner, R.J. Marked Increase in Avidity of SARS-CoV-2 Antibodies 7–8 Months after Infection Is Not Diminished in Old Age. J. Infect. Dis. 2021, 224, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J. Too many digits: The presentation of numerical data. Arch. Dis. Child. 2015, 100, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Einollahi, B.; Beiraghdar, F.; Darvishi, M.; Fathi, S.; Javanbakht, M.; Shafiee, S.; Akhavan-Sigari, R. Fully understanding the efficacy profile of the COVID-19 vaccination and its associated factors in multiple real-world settings. Front. Immunol. 2022, 13, 947602. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, A.S.; Licciardi, P.V.; Binks, M.J. Vitamin D Modulation of the Innate Immune Response to Paediatric Respiratory Pathogens Associated with Acute Lower Respiratory Infections. Nutrients 2021, 13, 276. [Google Scholar] [CrossRef]
- Greiller, C.L.; Suri, R.; Jolliffe, D.A.; Kebadze, T.; Hirsman, A.G.; Griffiths, C.J.; Johnston, S.L.; Martineau, A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2019, 187, 152–159. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef]
- Di Filippo, L.; Frara, S.; Doga, M.; Giustina, A. The osteo-metabolic phenotype of COVID-19: An update. Endocrine 2022, 78, 247–254. [Google Scholar] [CrossRef]
- Di Filippo, L.; Frara, S.; Giustina, A. The emerging osteo-metabolic phenotype of COVID-19: Clinical and pathophysiological aspects. Nat. Rev. Endocrinol. 2021, 17, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.B.; Norton, E.C.; McCullough, J.S.; Meltzer, D.O.; Lavigne, J.; Fiedler, V.C.; Gibbons, R.D. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci. Rep. 2022, 12, 19397. [Google Scholar] [CrossRef] [PubMed]
- Nimer, R.M.; Khabour, O.F.; Swedan, S.F.; Kofahi, H.M. The impact of vitamin and mineral supplements usage prior to COVID-19 infection on disease severity and hospitalization. Bosn. J. Basic Med. Sci. 2022, 22, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Sirisinha, S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac. J. Allergy Immunol. 2015, 33, 71–89. [Google Scholar]
- Tian, Y.; Tian, Q.; Wu, Y.; Peng, X.; Chen, Y.; Li, Q.; Zhang, G.; Tian, X.; Ren, L.; Luo, Z. Vitamin A supplement after neonatal Streptococcus pneumoniae pneumonia inhibits the progression of experimental asthma by altering CD4(+)T cell subsets. Sci. Rep. 2020, 10, 4214. [Google Scholar] [CrossRef]
- Xing, Y.; Sheng, K.; Xiao, X.; Li, J.; Wei, H.; Liu, L.; Zhou, W.; Tong, X. Vitamin A deficiency is associated with severe Mycoplasma pneumoniae pneumonia in children. Ann. Transl. Med. 2020, 8, 120. [Google Scholar] [CrossRef]
- Hu, N.; Li, Q.B.; Zou, S.Y. Effect of vitamin A as an adjuvant therapy for pneumonia in children: A Meta analysis. Zhongguo Dang Dai Er Ke Za Zhi 2018, 20, 146–153. [Google Scholar] [CrossRef]
- Fiorino, S.; Gallo, C.; Zippi, M.; Sabbatani, S.; Manfredi, R.; Moretti, R.; Fogacci, E.; Maggioli, C.; Travasoni Loffredo, F.; Giampieri, E.; et al. Cytokine storm in aged people with CoV-2: Possible role of vitamins as therapy or preventive strategy. Aging Clin. Exp. Res. 2020, 32, 2115–2131. [Google Scholar] [CrossRef]
- Li, R.; Wu, K.; Li, Y.; Liang, X.; Tse, W.K.F.; Yang, L.; Lai, K.P. Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19. Aging 2020, 12, 15784–15796. [Google Scholar] [CrossRef]
- Li, R.; Zhao, W.; Wang, H.; Toshiyoshi, M.; Zhao, Y.; Bu, H. Vitamin A in children’s pneumonia for a COVID-19 perspective: A systematic review and meta-analysis of 15 trials. Medicine 2022, 101, e31289. [Google Scholar] [CrossRef]
- Ivanov, A.P.; Dragunsky, E.M.; Chumakov, K.M. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J. Infect. Dis. 2006, 193, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Daynes, R.A.; Enioutina, E.Y.; Butler, S.; Mu, H.H.; McGee, Z.A.; Araneo, B.A. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect. Immun. 1996, 64, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Enioutina, E.Y.; Visic, D.; McGee, Z.A.; Daynes, R.A. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: Characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine 1999, 17, 3050–3064. [Google Scholar] [CrossRef] [PubMed]
- Daynes, R.A.; Araneo, B.A. The development of effective vaccine adjuvants employing natural regulators of T-cell lymphokine production in vivo. Ann. N. Y. Acad. Sci. 1994, 730, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, S.P.; Whitaker, J.A.; Poland, G.A. “Let there be light”: The role of vitamin D in the immune response to vaccines. Expert Rev. Vaccines 2015, 14, 1427–1440. [Google Scholar] [CrossRef]
- Siddiqui, F.Q.; Ahmad, M.M.; Kakar, F.; Akhtar, S.; Dil, A.S. The role of vitamin A in enhancing humoral immunity produced by antirabies vaccine. East Mediterr. Health J. 2001, 7, 799–804. [Google Scholar] [CrossRef]
- El-Khateeb, M.; Khader, Y.; Batieha, A.; Jaddou, H.; Hyassat, D.; Khawaja, N.; Abujbara, M.; Ajlouni, K. Vitamin D deficiency and associated factors in Jordan. SAGE Open Med. 2019, 7, 2050312119876151. [Google Scholar] [CrossRef]
- Abu-Samak, M.S.; AbuRuz, M.E.; Masa’Deh, R.; Khuzai, R.; Jarrah, S. Correlation of selected stress associated factors with vitamin D deficiency in Jordanian men and women. Int. J. Gen. Med. 2019, 12, 225–233. [Google Scholar] [CrossRef]
- Al-Horani, H.; Abu Dayyih, W.; Mallah, E.; Hamad, M.; Mima, M.; Awad, R.; Arafat, T. Nationality, Gender, Age, and Body Mass Index Influences on Vitamin D Concentration among Elderly Patients and Young Iraqi and Jordanian in Jordan. Biochem. Res. Int. 2016, 2016, 8920503. [Google Scholar] [CrossRef]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: A longitudinal observational cohort study in western Greece. BMJ Open 2022, 12, e057084. [Google Scholar] [CrossRef]
- Pavel-Tanasa, M.; Constantinescu, D.; Cianga, C.M.; Anisie, E.; Mereuta, A.I.; Tuchilus, C.G.; Cianga, P. Adipokines, and not vitamin D, associate with antibody immune responses following dual BNT162b2 vaccination within individuals younger than 60 years. Front. Immunol. 2022, 13, 1000006. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Vicenti, I.; Gatti, F.; Scaggiante, R.; Boccuto, A.; Zago, D.; Basso, M.; Dragoni, F.; Zazzi, M.; Parisi, S.G. Single-dose BNT162b2 mRNA COVID-19 vaccine significantly boosts neutralizing antibody response in health care workers recovering from asymptomatic or mild natural SARS-CoV-2 infection. Int. J. Infect. Dis. 2021, 108, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Henry, B.M.; di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.L.; Lippi, G. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Somi, M.H.; Faghih Dinevari, M.; Taghizadieh, A.; Varshochi, M.; Sadeghi Majd, E.; Abbasian, S.; Nikniaz, Z. Effect of vitamin A supplementation on the outcome severity of COVID-19 in hospitalized patients: A pilot randomized clinical trial. Nutr. Health 2022, 2601060221129144. [Google Scholar] [CrossRef]

| Characteristic | Value |
|---|---|
| Number | 124 |
| Age (mean ± SD) | 43.8 ± 9.2 |
| Sex | |
| Female | 59 (48%) |
| Male | 65 (52%) |
| BMI category | |
| Normal | 42 (34%) |
| Overweight | 43 (35%) |
| Obese | 39 (34%) |
| Chronic diseases | |
| One or more chronic disease | 26 (21%) |
| Hypertension | 20 (16%) |
| Diabetes | 14 (11%) |
| Cardiac diseases | 6 (4.8%) |
| Exposure to SARS-CoV-2 virus prior to vaccination | |
| Exposed | 39 (32%) |
| Unexposed | 85 (69%) |
| 25(OH)D level (ng/mL) (mean ± SD) | 14.3 ± 7.4 |
| Deficient | 108 (85%) |
| Insufficient | 15 (12%) |
| Normal | 4 (3.1%) |
| Retinol (ng/mL) (mean ± SD) | 1450 ± 720 |
| Deficient | 1 (0.8%) |
| Normal | 123 (99.2%) |
| Characteristic | Number | SARS-CoV-2 Ab Titer (Mean ± SD) | p Value | Avidity Index (%) (Mean ± SD) | p Value |
|---|---|---|---|---|---|
| Sex | 0.88 | 0.28 | |||
| Male | 47 | 210 ± 170 | 32 ± 17 | ||
| Female | 38 | 210 ± 140 | 36 ± 15 | ||
| BMI category | 0.93 | 0.63 | |||
| Normal | 30 | 220 ± 190 | 36 ± 18 | ||
| Overweight | 31 | 200 ± 120 | 34 ± 15 | ||
| Obese | 24 | 210 ± 140 | 32 ± 17 | ||
| Chronic diseases | 0.48 | 0.19 | |||
| No | 65 | 220 ± 160 | 34 ± 16 | ||
| Yes | 20 | 190 ± 130 | 35 ± 19 |
| Characteristic | Number | SARS-CoV-2 Ab Titer Increase (log2 Folds) | p Value |
|---|---|---|---|
| Gender | 0.63 | ||
| Male | 17 | 2.8 ± 1.1 | |
| Female | 21 | 2.6 ± 1.1 | |
| BMI category | 0.54 | ||
| Normal | 12 | 3.0 ± 1.2 | |
| Overweight | 11 | 2.7 ± 1.1 | |
| Obese | 15 | 2.5 ± 1.1 | |
| Chronic diseases | 0.93 | ||
| No | 31 | 2.7 ± 1.2 | |
| Yes | 6 | 2.7 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kofahi, H.M.; Badran, B.R.; Nimer, R.M.; Atoom, A.M.; Al Hersh, S.M. Exploring the Effects of Vitamin D and Vitamin A Levels on the Response to COVID-19 Vaccine. Vaccines 2023, 11, 1509. https://doi.org/10.3390/vaccines11091509
Kofahi HM, Badran BR, Nimer RM, Atoom AM, Al Hersh SM. Exploring the Effects of Vitamin D and Vitamin A Levels on the Response to COVID-19 Vaccine. Vaccines. 2023; 11(9):1509. https://doi.org/10.3390/vaccines11091509
Chicago/Turabian StyleKofahi, Hassan M., Baha’ R. Badran, Refat M. Nimer, Ali M. Atoom, and Shefa’ M. Al Hersh. 2023. "Exploring the Effects of Vitamin D and Vitamin A Levels on the Response to COVID-19 Vaccine" Vaccines 11, no. 9: 1509. https://doi.org/10.3390/vaccines11091509
APA StyleKofahi, H. M., Badran, B. R., Nimer, R. M., Atoom, A. M., & Al Hersh, S. M. (2023). Exploring the Effects of Vitamin D and Vitamin A Levels on the Response to COVID-19 Vaccine. Vaccines, 11(9), 1509. https://doi.org/10.3390/vaccines11091509






