Comparison of Humoral Response between Third and Fourth Doses of COVID-19 Vaccine in Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics and COVID-19 Infection
3.2. Anti-RBD IgG Levels and Neutralizing Tests
3.3. Anti-RBD IgG Levels and Disease Severity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexiewicz, J.M.; Smogorzewski, M.; Fadda, G.Z.; Massry, S.G. Impaired Phagocytosis in Dialysis Patients: Studies on Mechanisms. Am. J. Nephrol. 2008, 11, 102–111. [Google Scholar] [CrossRef]
- Sester, U.; Sester, M.; Hauk, M.; Kaul, H.; Köhler, H.; Girndt, M. T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol. Dial. Transplant. 2000, 15, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, P.; Elahimehr, R.; Pahl, M.V.; Vaziri, N.D. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol. Dial. Transplant. 2010, 25, 737–746. [Google Scholar] [CrossRef]
- Pahl, M.V.; Gollapudi, S.; Sepassi, L.; Gollapudi, P.; Elahimehr, R.; Vaziri, N.D. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol. Dial. Transplant. 2010, 25, 205–212. [Google Scholar] [CrossRef]
- Gollapudi, P.; Yoon, J.W.; Gollapudi, S.; Pahl, M.V.; Vaziri, N.D. Leukocyte Toll-Like Receptor Expression in End-Stage Kidney Disease. Am. J. Nephrol. 2010, 31, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Pahl, M.V.; Vaziri, N.D. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 2007, 71, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Kliger, A.S. Minimizing the risk of COVID-19 among patients on dialysis. Nat. Rev. Nephrol. 2020, 16, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Zieliński, M.; Tylicki, L.; Biedunkiewicz, B.; Kubanek, A.; Ślizień, Z.; Polewska, K.; Tylicki, P.; Muchlado, M.; Sakowska, J.; et al. Local and Systemic Immunity Are Impaired in End-Stage-Renal-Disease Patients Treated with Hemodialysis, Peritoneal Dialysis and Kidney Transplant Recipients Immunized with BNT162b2 Pfizer-BioNTech SARS-CoV-2 Vaccine. Front. Immunol. 2022, 13, 832924. [Google Scholar] [CrossRef]
- Swai, J.; Gui, M.; Long, M.; Wei, Z.; Hu, Z.; Liu, S. Humoral and cellular immune response to severe acute respiratory syndrome coronavirus-2 vaccination in haemodialysis and kidney transplant patients. Nephrology 2022, 27, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Talisa, V.B.; Yan, P.; Shaikh, O.S.; Omer, S.B.; Mayr, F.B. Real-World Effectiveness of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) mRNA Vaccines in Preventing Confirmed Infection in Patients on Chronic Hemodialysis. Clin. Infect. Dis. 2022, 75, e617–e622. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Minn, D.; Hong, S.; Jeong, S.; Kim, S.; Lee, C.H.; Kim, B. Immunogenicity of COVID-19 Vaccination in Patients with End-Stage Renal Disease Undergoing Maintenance Hemodialysis: The Efficacy of a Mix-and-Match Strategy. J. Korean Med. Sci. 2022, 37, e180. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Lesny, P.; Anderson, M.; Cloherty, G.; Stec, M.; Haase-Fielitz, A.; Haarhaus, M.; Santos-Araújo, C.; Veiga, P.M.; Macario, F. Humoral immunogenicity and tolerability of heterologous ChAd/BNT compared with homologous BNT/BNT and ChAd/ChAd SARS-CoV-2 vaccination in hemodialysis patients: A multicenter prospective observational study. J. Nephrol. 2022, 35, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Jung, S.W.; Moon, J.Y.; Jeong, K.H.; Hwang, H.S.; Kim, J.S.; Lee, S.H.; Kang, S.Y.; Kim, Y.G. Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Response after Heterologous Immunizations with ChAdOx1/BNT162b2 in End-Stage Renal Disease Patients on Hemodialysis. Front. Immunol. 2022, 13, 894700. [Google Scholar] [CrossRef] [PubMed]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Weiner, D.E.; Manley, H.J.; Aweh, G.N.; Ladik, V.; Frament, J.; Miskulin, D.; Argyropoulos, C.; Abreo, K.; Chin, A.; et al. Seroresponse to SARS-CoV-2 Vaccines among Maintenance Dialysis Patients over 6 Months. Clin. J. Am. Soc. Nephrol. 2022, 17, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Bassi, J.; Giannini, O.; Silacci-Fregni, C.; Pertusini, L.; Hitz, P.; Terrot, T.; Franzosi, Y.; Muoio, F.; Saliba, C.; Meury, M.; et al. Poor neutralization and rapid decay of antibodies to SARS-CoV-2 variants in vaccinated dialysis patients. PLoS ONE 2022, 17, e0263328. [Google Scholar] [CrossRef]
- Wand, O.; Nacasch, N.; Fadeela, A.; Shashar, M.; Grupper, A.; Benchetrit, S.; Erez, D.; Shitrit, P.; Cohen-Hagai, K. Humoral response and breakthrough infections with SARS-CoV-2 B.1.617.2 variant in vaccinated maintenance hemodialysis patients. J. Nephrol. 2022, 35, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Yoshifuji, A.; Kikuchi, K.; Koinuma, M.; Komatsu, M.; Fujii, K.; Kato, A.; Kikuchi, T.; Nakazawa, A.; Ryuzaki, M. Factors associated with SARS-CoV-2 antibody titers and prognosis of breakthrough infection in hemodialysis patients. Clin. Exp. Nephrol. 2022, 26, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Sibbel, S.; McKeon, K.; Luo, J.; Wendt, K.; Walker, A.G.; Kelley, T.; Lazar, R.; Zywno, M.L.; Connaire, J.J.; Tentori, F.; et al. Real-World Effectiveness and Immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Patients on Hemodialysis. J. Am. Soc. Nephrol. 2022, 33, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, H.; Fairley, C.K.; Zou, Z.; Xie, L.; Li, X.; Shen, M.; Li, Y.; Zhang, L. Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States. Int. J. Infect. Dis. 2022, 119, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Hagai, K.; Hornik-Lurie, T.; Benchetrit, S.; Nacasch, N.; Grupper, A.; Einbinder, Y.; Wand, O.; Shashar, M. Clinical efficacy of the fourth dose of the BNT162b2 vaccine in maintenance dialysis patients. J. Nephrol. 2023, 36, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Housset, P.; Kubab, S.; Hanafi, L.; Pardon, A.; Vittoz, N.; Bozman, D.-F.; Caudwell, V.; Faucon, A.-L. Humoral response after a fourth “booster” dose of a Coronavirus disease 2019 vaccine following a 3-dose regimen of mRNA-based vaccination in dialysis patients. Kidney Int. 2022, 101, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mähr, H.; Lhotta, K.; Zitt, E. Humoral and Cellular Immune Response after a 3-Dose Heterologous SARS-CoV-2 Vaccination Using the mRNA-BNT162b2 and Viral Vector Ad26COVS1 Vaccine in Hemodialysis Patients. Front. Immunol. 2022, 13, 907615. [Google Scholar] [CrossRef] [PubMed]
- Doernberg, S.B.; Holubar, M.A.-O.; Jain, V.; Weng, Y.; Lu, D.; Bollyky, J.B.; Sample, H.; Huang, B.; Craik, C.S.; Desai, M.; et al. Incidence and Prevalence of Coronavirus Disease 2019 within a Healthcare Worker Cohort during the First Year of the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic. Clin. Infect Dis. 2022, 75, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Lioulios, G.; Fylaktou, A.; Asouchidou, D.; Xochelli, A.; Nikolaidou, V.; Stai, S.; Christodoulou, M.; Giamalis, P.; Tsouchnikas, I.; Papagianni, A.; et al. Effect of Lymphocyte Phenotypic Alterations on the Humoral Response to Vaccination Against SARS-CoV-2 in Dialysis Patients. Ann. Lab. Med. 2023, 43, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.; Krüger, T.; Seikrit, C.; Mühlfeld, A.S.; Kunter, U.; Werner, C.; Kleines, M.; Schulze-Hagen, M.; Dreher, M.; Kersten, A.; et al. Time on previous renal replacement therapy is associated with worse outcomes of COVID-19 in a regional cohort of kidney transplant and dialysis patients. Medicine 2021, 100, e24893. [Google Scholar] [CrossRef]
- Manley, H.J.; Li, N.C.; Aweh, G.N.; Hsu, C.M.; Weiner, D.E.; Miskulin, D.; Harford, A.M.; Johnson, D.; Lacson, E., Jr. SARS-CoV-2 Vaccine Effectiveness and Breakthrough Infections among Patients Receiving Maintenance Dialysis. Am. J. Kidney Dis. 2023, 81, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Einbinder, Y.; Perl, J.; Nacasch, N.; Bnaya, A.; Shavit, L.; Erez, D.; Shashar, M.; Halperin, T.; Grupper, A.; Benchetrit, S.; et al. Humoral Response and SARS-CoV-2 Infection Risk following the Third and Fourth Doses of the BNT162b2 Vaccine in Dialysis Patients. Am. J. Nephrol. 2022, 53, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Füessl, L.; Lau, T.; Rau, S.; Regenauer, R.; Paal, M.; Hasmann, S.; Arend, F.M.; Bruegel, M.; Teupser, D.; Fischereder, M.; et al. Humoral response after SARS-CoV-2 booster vaccination in haemodialysis patients with and without prior infection. Clin. Kidney J. 2022, 15, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Löfström, E.; Eringfält, A.; Kötz, A.; Wickbom, F.; Tham, J.; Lingman, M.; Nygren, J.M.; Undén, J. Dynamics of IgG-avidity and antibody levels after COVID-19. J. Clin. Virol. 2021, 144, 104986. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Khan, S.R.; Sasson, S.C.; Kent, S.J.; Khoury, D.S.; Davenport, M.P. Predicting vaccine effectiveness against severe COVID-19 over time and against variants: A meta-analysis. Nat. Commun. 2023, 14, 1633. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, H.; Bao, W.; Fu, S.; Jin, H. Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2070. [Google Scholar] [CrossRef] [PubMed]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Faucon, A.L.; Housset, P. SARS-CoV-2 Antibody Response after a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2022, 79, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Then, E.; Lucas, C.; Monteiro, V.S.; Miric, M.; Brache, V.; Cochon, L.; Vogels, C.B.F.; Malik, A.A.; De la Cruz, E.; Jorge, A.; et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022, 28, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; He, P.; Li, J.; Liu, H.; Shi, M.; Yu, J.; Wei, H. Steep Decline in Binding Capability of SARS-CoV-2 Omicron Variant (B.1.1.529) RBD to the Antibodies in Early COVID-19 Convalescent Sera and Inactivated Vaccine Sera. Viruses 2022, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Bekliz, M.; Adea, K.; Vetter, P.; Eberhardt, C.S.; Hosszu-Fellous, K.; Vu, D.-L.; Puhach, O.; Essaidi-Laziosi, M.; Waldvogel-Abramowski, S.; Stephan, C.; et al. Neutralization capacity of antibodies elicited through homologous or heterologous infection or vaccination against SARS-CoV-2 VOCs. Nat. Commun. 2022, 13, 3840. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A.; et al. Previous COVID-19 Infection and Antibody Levels After Vaccination. Front. Public Health 2021, 9, 778243. [Google Scholar] [CrossRef] [PubMed]
- Khong, K.-W.; Zhang, R.; Hung, I.F. The Four Ws of the Fourth Dose COVID-19 Vaccines: Why, Who, When and What. Vaccines 2022, 10, 1924. [Google Scholar] [CrossRef]
- Affeldt, P.; Koehler, F.C.; Brensing, K.A.; Gies, M.; Platen, E.; Adam, V.; Butt, L.; Grundmann, F.; Heger, E.; Hinrichs, S.; et al. Immune Response to Third and Fourth COVID-19 Vaccination in Hemodialysis Patients and Kidney Transplant Recipients. Viruses 2022, 14, 2646. [Google Scholar] [CrossRef]
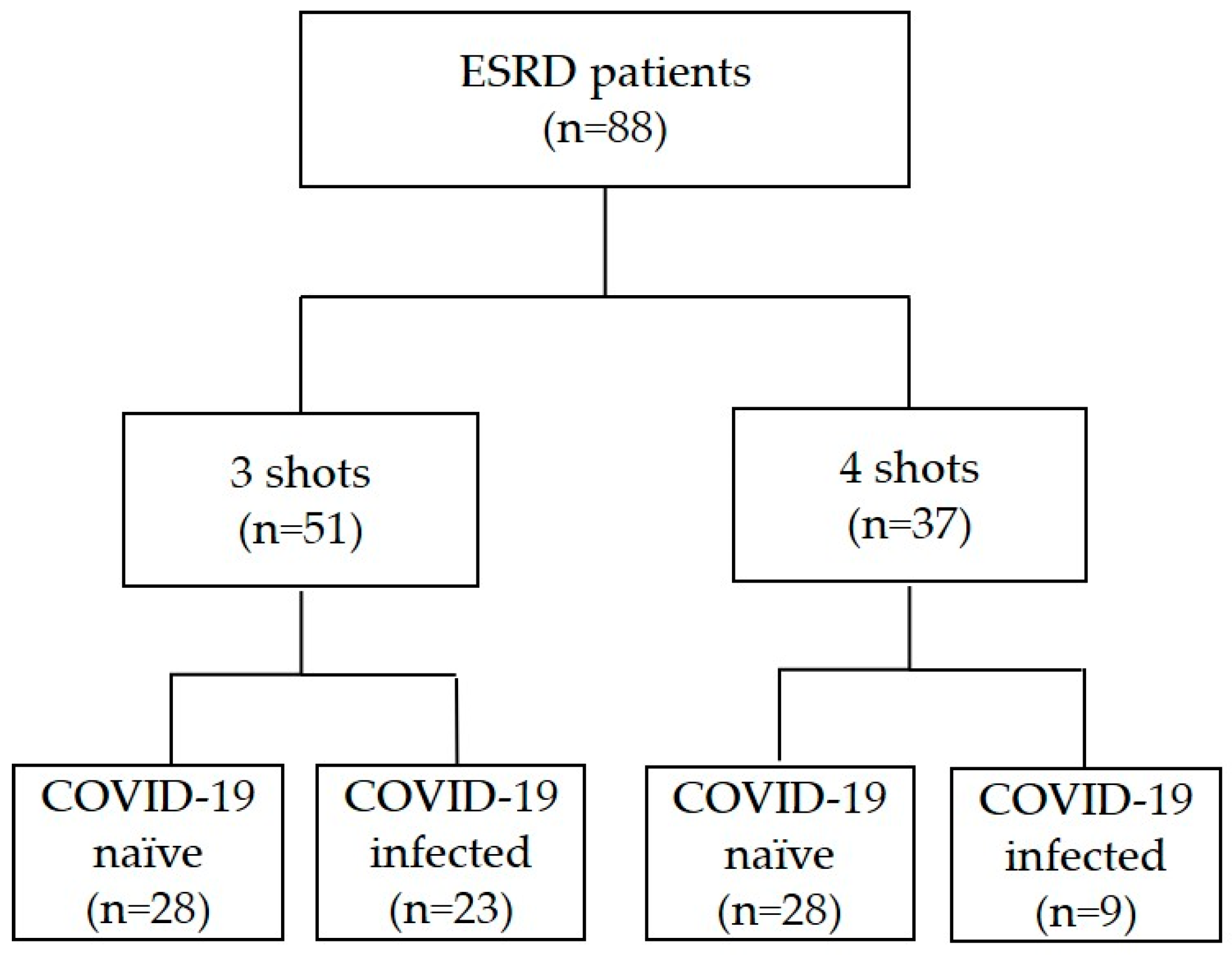



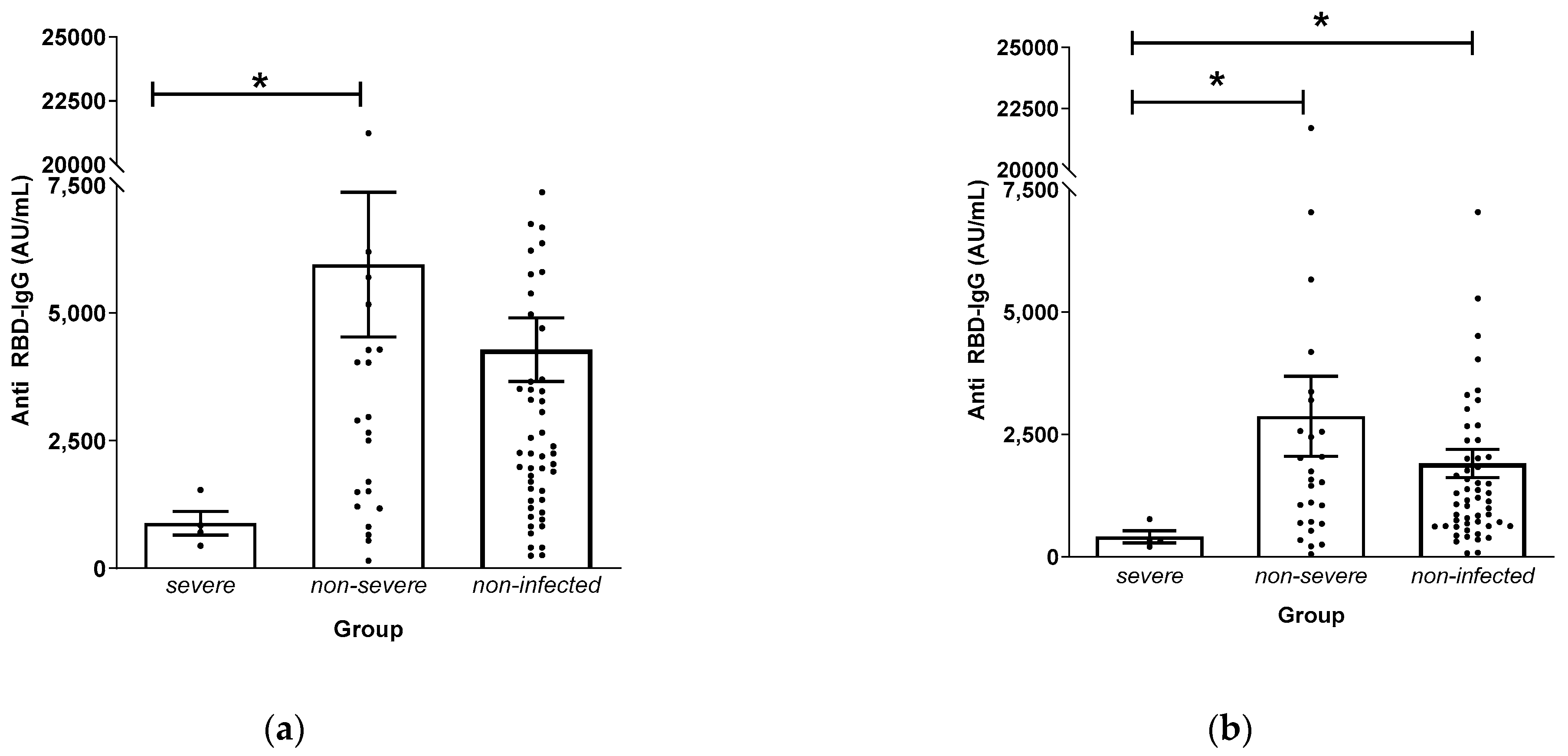
| Date | Event | Definition |
|---|---|---|
| 30 April 2021 | 1st vaccination | |
| 6–7 July 2021 | Anti-RBD 1 IgG measurement | T1 |
| 13–16 July 2021 | 2nd vaccination | |
| 6–7 September 2021 | Anti-RBD IgG measurement PRNT 2 | T2 |
| 8 November 2021 | Anti-RBD IgG measurement | T3 |
| 22 November~23 December 2021 | 3rd vaccination | |
| 28 March 2022 | Anti-RBD IgG measurement PRNT | T4 |
| 25 April~8 November 2022 | 4th vaccination | |
| 7 December 2022 | Anti-RBD IgG measurement | T5 |
| Parameter | Total (n = 88) | Naïve, 3 Doses (n = 28) | Infected, 3 Doses (n = 23) | Naïve, 4 Doses (n = 28) | Infected, 4 Doses (n = 9) | p-Value |
|---|---|---|---|---|---|---|
| Age (yr) | 59.1 ± 10.6 | 55.0 ± 8.9 | 57.5 ± 13.7 | 64.7 ± 7.7 | 58.6 ± 8.2 | 0.005 |
| Male, n (%) | 56 (63.6) | 14 (50.0) | 14 (60.9) | 20 (71.4) | 8 (88.9) | 0.136 |
| BMI (kg/m2) | 23.7 ± 4.7 | 23.0 ± 4.2 | 26.2 ± 5.9 | 22.4 ± 3.4 | 22.9 ± 4.2 | 0.019 |
| Dialysis | ||||||
| vintage (mo) | 76.3 ± 54.8 | 83.9 ± 57.8 | 76.7 ± 54.9 | 73.6 ± 53.5 | 60.3 ± 54.0 | 0.719 |
| Hemoglobin (g/dL) | 10.9 ± 1.0 | 10.9 ± 1.0 | 11.1 ± 1.0 | 10.8 ± 1.0 | 10.7 ± 1.2 | 0.722 |
| Albumin (g/dL) | 4.1 ± 0.8 | 4.0 ± 0.3 | 3.9 ± 0.3 | 4.2 ± 1.3 | 4.1 ± 0.3 | 0.692 |
| Cholesterol (mg/dL) | 129.8 ± 29.8 | 134.7 ± 33.7 | 127.1 ± 31.1 | 125.2 ± 26.8 | 135.1 ± 22.4 | 0.606 |
| hs-CRP 1 (mg/L) | 1.1 ± 3.6 | 1.1 ± 2.0 | 1.7 ± 6.4 | 0.8 ± 1.4 | 0.9 ± 0.8 | 0.813 |
| Kt/V | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.7 ± 0.4 | 1.6 ± 0.2 | 0.342 |
| Intact PTH 2 (pmol/L) | 409.3 ± 362.9 | 492.2 ± 457.1 | 442.5 ± 372.6 | 352.2 ± 280.5 | 253.5 ± 154.7 | 0.269 |
| Hemoglobin A1c 3 (%) | 6.3 ± 1.3 | 6.1 ± 0.9 | 6.4 ± 1.5 | 5.9 ± 1.1 | 7.5 ± 1.5 | 0.071 |
| Anti-RBD IgG Levels (AU/mL) | Total (n = 88) | Naïve, 3 Doses (n = 28) | Infected, 3 Doses (n = 23) | Naïve, 4 Doses (n = 28) | Infected, 4 Doses (n = 9) | p Value |
|---|---|---|---|---|---|---|
| T1 | 83.3 (37.6–176.6) | 129.7 (51.5–296.3) | 87.1 (34.4–212.7) | 75.1 (44.4–156.2) | 23.6 (14.8/127.3) | 0.064 |
| T2 | 2777.6 (381.1–6064.3) | 3485.0 (1915.6–6727.7) | 2966.1 (1174.6–7598.1) | 2220.6 (1031.8–4906.5) | 2897.0 (1080.2–4155.3) | 0.360 |
| T3 | 1255.7 (640.2–2433.4) | 1438.0 (894.0–2934.2) | 1523.6 (672.7–3201.9) | 806.0 (484.2–2009.9) | 1049.8 (391.9–2067.3) | 0.304 |
| T4 | 5148.0 (2193.5–31,096.1) | 4288.2 (2510.0–25,827.4) | 29,537.7 (3309.3–81,815.4) | 2758.6 (1523.9–9752.2) | 4402.7 (2819.0–30,290.6) | 0.039 1 |
| T5 | 13,221.9 (3717.74–31,375.2) | 2492.5 (851.2–17,086.3) | 28,883.4 (14,987.0–36,717.6) | 14,075.3 (4527.1–30,460.0) | 13,107.5 (9110.3–48,038.3) | 0.000 2 |
| Co− Variates | Time | ||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | |
| Age 1 (years) | −0.007 (0.951) | −0.162 (0.131) | −0.113 (0.293) | −0.104 (0.341) | 0.212 (0.051) |
| BMI 1 (kg/m2) | 0.002 (0.984) | −0.159 (0.140) | −0.079 (0.464) | 0.072 (0.515) | 0.199 (0.069) |
| Dialysis vintage 1 (months) | 0.033 (0.757) | 0.116 (0.284) | 0.059 (0.584) | 0.073 (0.506) | −0.080 (0.465) |
| Sex 2 | 0.314 (0.003) | 0.071 (0.511) | 0.012 (0.909) | 0.049 (0.657) | −0.048 (0.664) |
| Diabetes 2 | −0.063 (0.559) | 0.083 (0.440) | 0.105 (0.332) | −0.012 (0.911) | −0.061 (0.579) |
| Description | Date of Vaccination 1 (3rd Dose) | Date of Infection 1 | Date of Death (If Applicable) 1 | Antibody Level Prior to Infection (AU/mL) |
|---|---|---|---|---|
| 72-year-old male patient with hypertension on dialysis for 4 years | 2021-12-02 | 2022-09-19 | N/A | 553.2 |
| 61-year-old male patient with hypertension on dialysis for 8 years | 2021-12-14 | 2022-07-21 | N/A | 788.8 |
| 59-year-old female patient with diabetes on dialysis for 16.8 years | 2021-12-08 | 2022-03-11 | 2022-03-22 | 342 |
| 69-year-old female patient with diabetes on dialysis for 11.5 years | 2021-12-03 | 2022-03-15 | 2022-03-28 | 317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, Y.; Kim, D.K.; Jeon, Y.G.; Kim, A.-R.; Do, H.N.; Yoon, S.-Y.; Kim, J.S.; Jung, S.W.; Hwang, H.S.; Moon, J.-Y.; et al. Comparison of Humoral Response between Third and Fourth Doses of COVID-19 Vaccine in Hemodialysis Patients. Vaccines 2023, 11, 1584. https://doi.org/10.3390/vaccines11101584
Joo Y, Kim DK, Jeon YG, Kim A-R, Do HN, Yoon S-Y, Kim JS, Jung SW, Hwang HS, Moon J-Y, et al. Comparison of Humoral Response between Third and Fourth Doses of COVID-19 Vaccine in Hemodialysis Patients. Vaccines. 2023; 11(10):1584. https://doi.org/10.3390/vaccines11101584
Chicago/Turabian StyleJoo, Yoosun, Dae Kyu Kim, Yun Gi Jeon, Ah-Ra Kim, Hyeon Nam Do, Soo-Young Yoon, Jin Sug Kim, Su Woong Jung, Hyeon Seok Hwang, Ju-Young Moon, and et al. 2023. "Comparison of Humoral Response between Third and Fourth Doses of COVID-19 Vaccine in Hemodialysis Patients" Vaccines 11, no. 10: 1584. https://doi.org/10.3390/vaccines11101584
APA StyleJoo, Y., Kim, D. K., Jeon, Y. G., Kim, A.-R., Do, H. N., Yoon, S.-Y., Kim, J. S., Jung, S. W., Hwang, H. S., Moon, J.-Y., Jeong, K. H., Lee, S.-H., Kang, S.-Y., & Kim, Y. G. (2023). Comparison of Humoral Response between Third and Fourth Doses of COVID-19 Vaccine in Hemodialysis Patients. Vaccines, 11(10), 1584. https://doi.org/10.3390/vaccines11101584






