A Systematic Evaluation of the SARS-CoV-2 Vaccine-Induced Anti-S-RBD-Ig Response in a Population of Health Care Workers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Acquisition and Assay
2.3. Data Organization and Statistical Analysis
3. Results
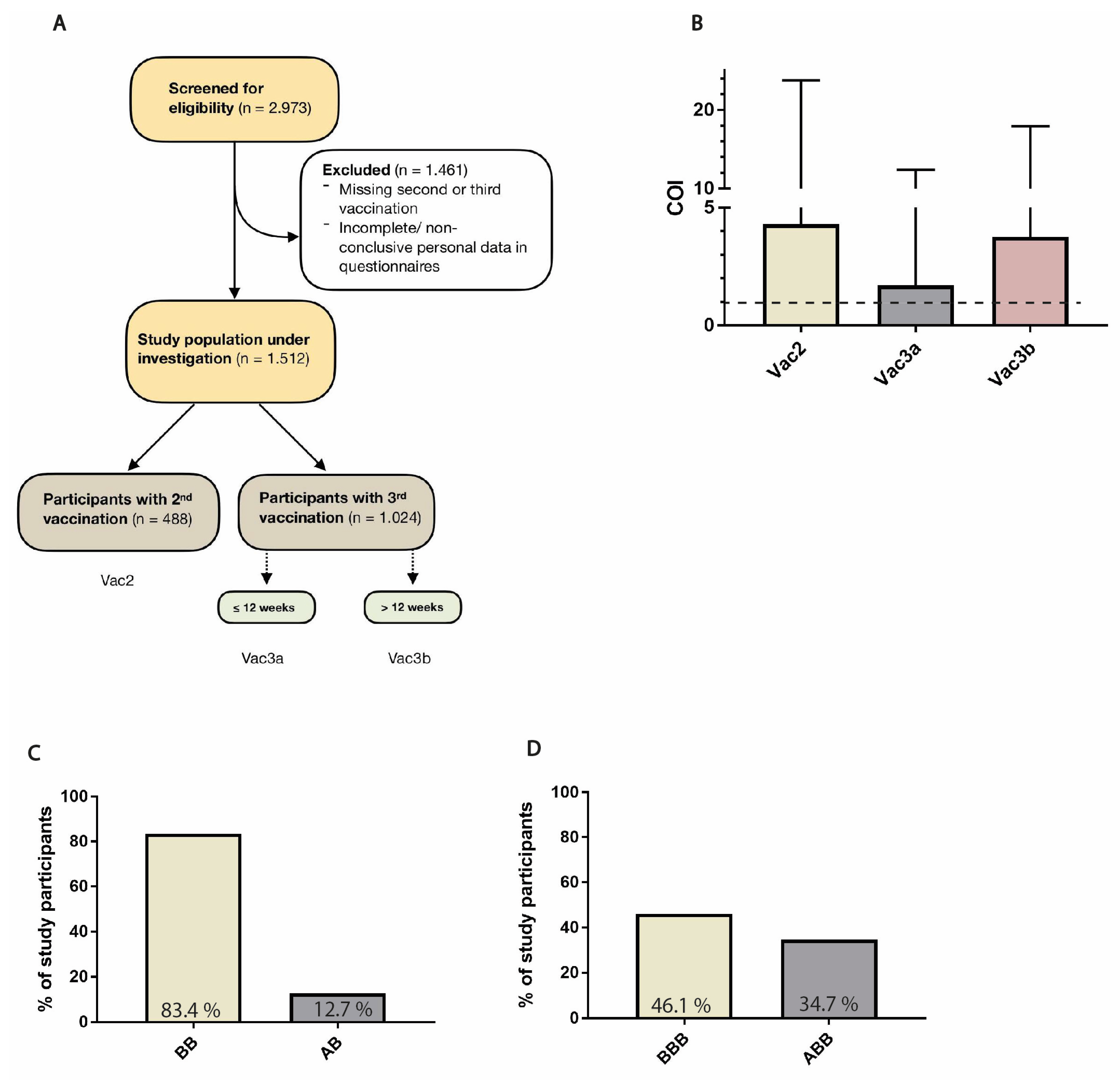
3.1. Description of Study Population
3.2. Sex and BMI
3.3. Smoking
3.4. Age Groups
3.5. Vaccine Combinations and Single Agents
3.6. Previous SARS-CoV-2 Infection
3.7. Household Contacts with a History of SARS-CoV-2 Infection
3.8. Vaccine-Related AE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francis, A.I.; Ghany, S.; Gilkes, T.; Umakanthan, S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad. Med. J. 2022, 98, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Koch-Institut, R. STIKO: 25. Aktualisierung der COVID-19-Impfempfehlung. Aktuelle Daten und Informationen Zu Infekt. Und Public Health Epidemiol. Bull. 2023. Available online: https://www.rki.de/DE/Content/Infekt/Impfen/ImpfungenAZ/COVID-19/Impfempfehlung-Zusfassung.html (accessed on 3 September 2023).
- CDC. Interim COVID-19 Immunization Schedule for Persons 6 Months of Age and Older. 2022. Available online: https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf (accessed on 3 September 2023).
- Deming, M.E.; Lyke, K.E. A ‘mix and match’ approach to SARS-CoV-2 vaccination. Nat. Med. 2021, 27, 1510–1511. [Google Scholar] [CrossRef] [PubMed]
- Pascuale, C.A.; Varese, A.; Ojeda, D.S.; Pasinovich, M.E.; Lopez, L.; Rossi, A.H.; Rodriguez, P.E.; Miglietta, E.A.; Mazzitelli, I.; Garcia, F.D.D.; et al. Immunogenicity and reactogenicity of heterologous immunization against SARS CoV-2 using Sputnik V, ChAdOx1-S, BBIBP-CorV, Ad5-nCoV, and mRNA-1273. Cell Rep. Med. 2022, 3, 100706. [Google Scholar] [CrossRef] [PubMed]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Markewitz, R.; Juhl, D.; Pauli, D.; Görg, S.; Junker, R.; Rupp, J.; Engel, S.; Steinhagen, K.; Herbst, V.; Zapf, D.; et al. Kinetics of the Antibody Response to Boostering with Three Different Vaccines Against SARS-CoV-2. Front. Immunol. 2022, 13, 811020. [Google Scholar] [CrossRef]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-García, J.; et al. Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Markewitz, R.D.H.; Juhl, D.; Pauli, D.; Görg, S.; Junker, R.; Rupp, J.; Engel, S.; Steinhagen, K.; Herbst, V.; Zapf, D.; et al. Differences in Immunogenicity of Three Different Homo- and Heterologous Vaccination Regimens against SARS-CoV-2. Vaccines 2022, 10, 649. [Google Scholar] [CrossRef]
- Ferrara, P.; Gianfredi, V.; Tomaselli, V.; Polosa, R. The Effect of Smoking on Humoral Response to COVID-19 Vaccines: A Systematic Review of Epidemiological Studies. Vaccines 2022, 10, 303. [Google Scholar] [CrossRef]
- Ferrara, P.; Ponticelli, D.; Agüero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, I.; Mantovani, L.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health 2022, 203, 97–99. [Google Scholar] [CrossRef]
- Nasr, M.-J.C.; Geerling, E.; Pinto, A.K. Impact of Obesity on Vaccination to SARS-CoV-2. Front. Endocrinol. 2022, 13, 898810. [Google Scholar] [CrossRef]
- Kumar, M.; James, M.M.; Kumawat, M.; Nabi, B.; Sharma, P.; Pal, N.; Shubham, S.; Tiwari, R.R.; Sarma, D.K.; Nagpal, R. Aging and Microbiome in the Modulation of Vaccine Efficacy. Biomedicines 2022, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Bunders, M.J.; Altfeld, M. Implications of Sex Differences in Immunity for SARS-CoV-2 Pathogenesis and Design of Therapeutic Interventions. Immunity 2020, 53, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Paschall, A.V.; Ozdilek, A.; Briner, S.L.; Brindley, M.A.; Avci, F.Y. Modulation of immunosuppressant drug treatment to improve SARS-CoV-2 vaccine efficacy in mice. Vaccine 2022, 40, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, T.; Pelleau, S.; Anna, F.; Attia, M.; Donnadieu, F.; Gravet, A.; Lohmann, C.; Seraphin, H.; Guiheneuf, R.; Delamare, C.; et al. Humoral immunity to SARS-CoV-2 and seasonal coronaviruses in children and adults in north-eastern France. EBioMedicine 2021, 70, 103495. [Google Scholar] [CrossRef]
- Chaisawangwong, W.; Wang, H.; Kouo, T.; Salathe, S.F.; Isser, A.; Bieler, J.G.; Zhang, M.L.; Livingston, N.K.; Li, S.; Horowitz, J.J.; et al. Cross-reactivity of SARS-CoV-2– and influenza A–specific T cells in individuals exposed to SARS-CoV-2. JCI Insight 2022, 7, 158308. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Nassereldine, H.; Sorensen, R.J.D.; Amlag, J.O.; Bisignano, C.; Byrne, S.; Castro, E.; Coberly, K.; Collins, J.K.; Dalos, J.; et al. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef]
- Chvatal-Medina, M.; Mendez-Cortina, Y.; Patiño, P.J.; Velilla, P.A.; Rugeles, M.T. Antibody Responses in COVID-19: A Review. Front. Immunol. 2021, 12, 633184. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O. COVID-19 vaccination in kidney transplant recipients. Nat. Rev. Nephrol. 2021, 17, 785–787. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Antoni, D.; Manoussopoulos, Y.; Stefanou, P.; Argyropoulou, S.; Vrioni, G.; Tsakris, A. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: A mixed effects model across two vaccination periods. PLoS ONE 2022, 17, e0266958. [Google Scholar] [CrossRef]
- Hägg, S.; Religa, D. COVID vaccination in older adults. Nat. Microbiol. 2022, 7, 1106–1107. [Google Scholar] [CrossRef]
- Newman, J.; Thakur, N.; Peacock, T.P.; Bialy, D.; Elrefaey, A.M.E.; Bogaardt, C.; Horton, D.L.; Ho, S.; Kankeyan, T.; Carr, C.; et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat. Microbiol. 2022, 7, 1180–1188. [Google Scholar] [CrossRef]
- Romero-Olmedo, A.J.; Schulz, A.R.; Hochstätter, S.; Das Gupta, D.; Virta, I.; Hirseland, H.; Staudenraus, D.; Camara, B.; Münch, C.; Hefter, V.; et al. Induction of robust cellular and humoral immunity against SARS-CoV-2 after a third dose of BNT162b2 vaccine in previously unresponsive older adults. Nat. Microbiol. 2022, 7, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Richards, N.E.; Keshavarz, B.; Workman, L.J.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Comparison of SARS-CoV-2 Antibody Response by Age Among Recipients of the BNT162b2 vs the mRNA-1273 Vaccine. JAMA Netw. Open 2021, 4, e2124331. [Google Scholar] [CrossRef] [PubMed]
- Bánki, Z.; Mateus, J.; Rössler, A.; Schäfer, H.; Bante, D.; Riepler, L.; Grifoni, A.; Sette, A.; Simon, V.; Falkensammer, B.; et al. Heterologous ChAdOx1/BNT162b2 vaccination induces stronger immune response than homologous ChAdOx1 vaccination: The pragmatic, multi-center, three-arm, partially randomized HEVACC trial. EBioMedicine 2022, 80, 104073. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Ramos, G.M.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Kim, D.-I.; Lee, S.J.; Park, S.; Kim, P.; Lee, S.M.; Lee, N.; Shum, D.; Kim, D.H.; Kim, E.H. Immunogenicity and Durability of Antibody Responses to Homologous and Heterologous Vaccinations with BNT162b2 and ChAdOx1 Vaccines for COVID-19. Vaccines 2022, 10, 1864. [Google Scholar] [CrossRef]
- Gareayaghi, N.; Demirci, M.; Ozbey, D.; Dasdemir, F.; Dinc, H.O.; Balkan, I.I.; Saribas, S.; Saltoglu, N.; Kocazeybek, B. Comparison of SARS-CoV-2 Antibody Levels after a Third Heterologous and Homologous BNT162b2 Booster Dose. Vaccines 2022, 10, 1672. [Google Scholar] [CrossRef]
- Brunner, W.M.; Freilich, D.; Victory, J.; Krupa, N.; Scribani, M.B.; Jenkins, P.; Lasher, E.G.; Fink, A.; Shah, A.; Cross, P.; et al. Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines in healthcare workers. Int. J. Infect. Dis. 2022, 123, 183–191. [Google Scholar] [CrossRef]
- Havervall, S.; Marking, U.; Greilert-Norin, N.; Gordon, M.; Ng, H.; Christ, W.; Phillipson, M.; Nilsson, P.; Hober, S.; Blom, K.; et al. Impact of SARS-CoV-2 infection on vaccine-induced immune responses over time. Clin. Transl. Immunol. 2022, 11, e1388. [Google Scholar] [CrossRef]
- Uprichard, S.L.; O’brien, A.; Evdokimova, M.; Rowe, C.L.; Joyce, C.; Hackbart, M.; Cruz-Pulido, Y.E.; Cohen, C.A.; Rock, M.L.; Dye, J.M.; et al. Antibody Response to SARS-CoV-2 Infection and Vaccination in COVID-19-naïve and Experienced Individuals. Viruses 2022, 14, 370. [Google Scholar] [CrossRef] [PubMed]
- Krutikov, M.; Palmer, T.; Tut, G.; Fuller, C.; Azmi, B.; Giddings, R.; Shrotri, M.; Kaur, N.; Sylla, P.; Lancaster, T.; et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): Prospective cohort study in England. Lancet Health Longev. 2022, 3, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Töllner, M.; Hidmark, A.; Schaier, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Buylaert, M.; Grenz, J.; Ponath, G.; et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 Prime-Boost Vaccination Induces Strong Humoral Responses among Health Care Workers. Vaccines 2021, 9, 857. [Google Scholar] [CrossRef]
- Groß, R.; Zanoni, M.; Seidel, A.; Conzelmann, C.; Gilg, A.; Krnavek, D.; Erdemci-Evin, S.; Mayer, B.; Hoffmann, M.; Pöhlmann, S.; et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine 2022, 75, 103761. [Google Scholar] [CrossRef]
- Mori, Y.; Tanaka, M.; Kozai, H.; Hotta, K.; Aoyama, Y.; Shigeno, Y.; Aoike, M.; Kawamura, H.; Tsurudome, M.; Ito, M. Antibody response of smokers to the COVID-19 vaccination: Evaluation based on cigarette dependence. Drug Discov. Ther. 2022, 16, 78–84. [Google Scholar] [CrossRef]
- Ou, X.; Jiang, J.; Lin, B.; Liu, Q.; Lin, W.; Chen, G.; Wen, J. Antibody responses to COVID-19 vaccination in people with obesity: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2023, 17, e13078. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes/Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef] [PubMed]

| All (n = 488) | Linear Regression Analysis, Univariable | Linear Regression Analysis, Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Data Available, n | Anti-S-RBD Ig Antibody Value (U/mL) (Median, IQR) | Estimate | T Value | p-Value | Estimate | T Value | p-Value |
| Age (year-old) | ||||||||
| 83 | 1291 (800–2012) | ||||||
| 115 | 941 (594–1435) | −1281 | −0.001 | 0.999 | |||
| 109 | 706 (471–1369) | −1072.374 | −1.235 | 0.217 | |||
| 117 | 881 (382.75–1603.5) | 466.211 | 0.546 | 0.585 | |||
| 64 | 736 (247.5–1299.5) | −519.517 | −0.526 | 0.599 | |||
| Sex | ||||||||
| 126 | 866 (497.5–1498.5) | ||||||
| 362 | 932.5 (500.25–1.583.75) | 160.7 | 0.262 | 0.794 | |||
| BMI | ||||||||
| 12 | 741 (655–1016) | ||||||
| 296 | 938.5 (533.5–1631.5) | 1650 | 0.946 | 0.345 | |||
| 123 | 890 (482.75–1442) | 1774 | 0.990 | 0.323 | |||
| 56 | 669 (425.75–1344) | 3069 | 1.629 | 0.104 | |||
| Smoking | ||||||||
| 189 | 1086 (592.5–2849.5) | ||||||
| 23 | 1022 (451–2248) | 1078.0 | 0.621 | 0.535 | |||
| Previous SARS-CoV-2 infection | ||||||||
| 55 | 15,230 (4853–25,000) | ||||||
| 433 | 795 (481–1295) | 13,360.5 | 22.122 | <0.000 (***) | 17,317.7 | 19.759 | <0.000 (***) |
| Contact with infected household members | ||||||||
| 44 | 1.805 (720–22,289.5) | ||||||
| 113 | 987.5 (486.25–2085.25) | 5243.8 | 3.596 | <0.000 (***) | −969.3 | −1.167 | 0.245 |
| Vaccine combination | ||||||||
| 407 | 816 (481–1.394) | ||||||
| 62 | 1687 (850–4971.5) | 4221.7 | 5.674 | <0.000 (***) | |||
| First vaccine | ||||||||
| 407 | 816 (481–1394) | ||||||
| 78 | 1435 (754–6888) | 4318.6 | 6.192 | <0.000 (***) | 855.4 | 1.130 | 0.2602 |
| Second vaccine | ||||||||
| 470 | 888 (499.25–1555) | ||||||
| 16 | 946 (428.25–14,071.5) | 4121.3 | 2.788 | 0.006 (**) | 195.2 | 0.150 | 0.8807 |
| Vaccine-related adverse events | ||||||||
| 397 | 951 (544.5–1571.5) | ||||||
| 91 | 706 (388.5–1391) | −81.5 | −0.118 | 0.906 | |||
| All (n = 653) | Linear Regression Analysis, Univariable | Linear Regression Analysis, Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Data Available, n | Anti-S-RBD Ig Antibody Value (U/mL) (Median, IQR) | Estimate | T Value | p-Value | Estimate | T Value | p-Value |
| Age (year-old) | ||||||||
| 129 | 16,189 (10,184.5–25,000) | ||||||
| 128 | 13,597 (8880–21,164.5) | −1848.2 | −2.000 | 0.046 (*) | −1852.9 | −2.050 | 0.041 (*) |
| 148 | 13,795,5 (7869.25–21,996.25) | −1910.0 | −2.140 | 0.033 (*) | −2860.3 | −3.199 | 0.001 (**) |
| 172 | 16,060 (9526.5–23,179.5) | −468.9 | −0.543 | 0.587 | −1111.5 | −1.268 | 0.205 |
| 76 | 12,889 (9439.5–20,558.5) | −1713.1 | −1.600 | 0.110 | −2042.1 | −1.925 | 0.055 |
| Sex | ||||||||
| 116 | 16,675 (11,293.5–24,408.5) | ||||||
| 537 | 14,383 (8789.5–23,407.5) | −1088.4 | −1.435 | 0.152 | |||
| BMI | ||||||||
| 13 | 9937 (5252–15,135) | ||||||
| 364 | 14,121 (8465.5–21,915.5) | 4416 | 2.143 | 0.033 (*) | 4610.5 | 2.277 | 0.023 (*) |
| 175 | 14,984.5 (9955.75–22,838.75) | 5182 | 2.468 | 0.014 (*) | 5224.3 | 2.523 | 0.012 (*) |
| 98 | 18,813 (11,715–25,000) | 7250 | 3.364 | 0.001 (***) | 7598.2 | 3.561 | <0.000 (***) |
| Smoking | ||||||||
| 579 | 14,739 (9315–23,596) | ||||||
| 74 | 14,403.5 (7837.5–19,939.25) | −832.9 | −0.91 | 0.363 | |||
| Previous SARS-CoV−2 infection | ||||||||
| 50 | 18,937 (12,770.75–25,000) | ||||||
| 603 | 14,427 (8800–22,600) | 3288.4 | 3.033 | 0.003 (**) | 2173.7 | 1.950 | 0.052 |
| Contact with infected household members | ||||||||
| 119 | 16,868 (9667–25,000) | ||||||
| 534 | 14,383 (9010–22,234.5) | 1658.4 | 2.213 | 0.027 (*) | 1076.6 | 1.389 | 0.165 |
| Vaccine combination | ||||||||
| 271 | 13,641 (7985.75–19,337.75) | ||||||
| 226 | 15,793 (9677–25,000) | −1754.2 | −2.672 | 0.008 (**) | |||
| First vaccine | ||||||||
| 251 | 16,187 (9879.75–25,000) | ||||||
| 391 | 14,007 (8758–20,626.5) | −1665.7 | −2.792 | 0.005 (**) | −1775.5 | −3.048 | 0.002 (**) |
| 11 | 20,813 (11,825–25,000) | 1588.0 | 0.699 | 0.485 | −626.4 | −0.283 | 0.778 |
| Second vaccine | ||||||||
| 557 | 14,638 (9253.5–23,180.75) | ||||||
| 75 | 14,565.5 (8758–21,181.25) | −691.8 | −0.758 | 0.449 | |||
| 21 | 17,056 (9480–25,000) | 1041.4 | 0.631 | 0.528 | |||
| Third vaccine | ||||||||
| 569 | 14,310 (8758–21,728) | ||||||
| 2 | 10,489.5 (8463.25–12,515.75) | −4152.0 | −0.803 | 0.423 | −2461.7 | −0.487 | 0.626 |
| 82 | 20,797.5 (11,477.5–25,000) | 3950.1 | 4.578 | <0.000 (***) | 3921.2 | 4.524 | <0.000 (***) |
| Vaccine-related adverse events | ||||||||
| 620 | 14,800 (9315–23,182) | 1135 | 0.857 | 0.392 | |||
| 33 | 12,275,5 (6372.5–21,725.75) | ||||||
| All (n = 371) | Linear Regression Analysis, Univariable | Linear Regression Analysis, Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Data Available, n | Anti-S-RBD Ig Antibody Value (U/mL) (Median, IQR) | Estimate | T Value | p-Value | Estimate | T Value | p-Value |
| Age (year-old) | ||||||||
| 67 | 8645 (6210–15,693) | ||||||
| 88 | 7986 (4291.25–18,578.75) | 16.27 | 0.013 | 0.990 | |||
| 73 | 9138 (5139–19,087) | 681.07 | 0.506 | 0.613 | |||
| 103 | 9465 (4164–17,568.75) | 285.93 | 0.229 | 0.819 | |||
| 40 | 8300 (4384–15,453) | −798.58 | −0.502 | 0.616 | |||
| Sex | ||||||||
| 76 | 9009 (5805–14,394.5) | ||||||
| 295 | 8546.5 (4432.5–17,568.75) | 25.26 | 0.025 | 0.980 | |||
| BMI | ||||||||
| 6 | 10,609.5 (5559.25–20,610.5) | ||||||
| 210 | 7772.5 (3782–16,267.25) | −2797 | −0.853 | 0.394 | |||
| 99 | 9126.5 (5346.5–18,072.75) | −1453 | −0.436 | 0.663 | |||
| 55 | 10,224 (6834–17,765) | −636 | −0.187 | 0.852 | |||
| Smoking | ||||||||
| 327 | 8595 (4825–17,608.5) | ||||||
| 44 | 8214 (3949–13,985) | −1452 | −1.141 | 0.254 | |||
| Previous SARS-CoV-2 infection | ||||||||
| 55 | 23,307 (12,215–25,000) | ||||||
| 316 | 7644 (4145–14,323) | 8726.3 | 8.181 | <0.000 (***) | 6347.2 | 4.066 | <0.000 (***) |
| Contact with infected household members | ||||||||
| 86 | 12,313.5 (5749.25–23,699.25) | ||||||
| 285 | 7917.5 (4341.5–15,827.75) | 2977.1 | 3.089 | 0.002 (**) | −741.5 | −0.692 | 0.490 |
| Vaccine combination | ||||||||
| 84 | 6798.5 (4056.25–14,611.5) | ||||||
| 246 | 9011 (4525.5–16,621.5) | −1175.8 | −1.182 | 0.238 | |||
| First vaccine | ||||||||
| 257 | 9126.5 (4692.75–18,358.75) | ||||||
| 110 | 7297 (4127.75–15,608.25) | −1010.7 | −1.119 | 0.264 | |||
| 4 | 8439 (6891.75–10,111.75) | −2865.4 | −0.717 | 0.474 | |||
| Second vaccine | ||||||||
| 346 | 8631 (4487.5–17,608.5) | ||||||
| 18 | 7837 (3980.5–14,496.75) | −674.9 | −0.352 | 0.725 | |||
| 7 | 7174 (6844.5–10,284.5) | −2460.4 | −0.812 | 0.417 | |||
| Third vaccine | ||||||||
| 348 | 8295 (4404–16,226) | ||||||
| 3 | 4333 (2167–5947) | −6825.9 | −1.515 | 0.131 | −5761.4 | 3.296 | 0.167 |
| 20 | 18,608.5 (11,554–25,000) | 6745.2 | 3.775 | <0.000 (***) | 5492.3 | −1.384 | 0.001 (**) |
| Vaccine-related adverse events | ||||||||
| 369 | 8589.5 (4485.75–16,663.25) | ||||||
| 2 | 15,448.5 (10,771.75–20,125.25) | −4373 | −0.778 | 0.437 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hentschel, V.; Horsch, C.; Mayer, B.; Thies, A.; Qian, W.; Kroschel, J.; Seufferlein, T.; Perkhofer, L.; Müller, M. A Systematic Evaluation of the SARS-CoV-2 Vaccine-Induced Anti-S-RBD-Ig Response in a Population of Health Care Workers. Vaccines 2023, 11, 1467. https://doi.org/10.3390/vaccines11091467
Hentschel V, Horsch C, Mayer B, Thies A, Qian W, Kroschel J, Seufferlein T, Perkhofer L, Müller M. A Systematic Evaluation of the SARS-CoV-2 Vaccine-Induced Anti-S-RBD-Ig Response in a Population of Health Care Workers. Vaccines. 2023; 11(9):1467. https://doi.org/10.3390/vaccines11091467
Chicago/Turabian StyleHentschel, Viktoria, Cornelia Horsch, Benjamin Mayer, Annsophie Thies, Will Qian, Joris Kroschel, Thomas Seufferlein, Lukas Perkhofer, and Martin Müller. 2023. "A Systematic Evaluation of the SARS-CoV-2 Vaccine-Induced Anti-S-RBD-Ig Response in a Population of Health Care Workers" Vaccines 11, no. 9: 1467. https://doi.org/10.3390/vaccines11091467
APA StyleHentschel, V., Horsch, C., Mayer, B., Thies, A., Qian, W., Kroschel, J., Seufferlein, T., Perkhofer, L., & Müller, M. (2023). A Systematic Evaluation of the SARS-CoV-2 Vaccine-Induced Anti-S-RBD-Ig Response in a Population of Health Care Workers. Vaccines, 11(9), 1467. https://doi.org/10.3390/vaccines11091467







