Correction: Wang et al. A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Potentially Protective Immune Responses in M. tuberculosis H37Ra Infected Mice. Vaccines 2023, 11, 941
- We changed the title of the paper from “A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Protective Immune Responses in M. tuberculosis H37Ra Infected Mice” to “A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Potentially Protective Immune Responses in M. tuberculosis H37Ra Infected Mice”.
- We added the content “and reduced the bacterial load in the lungs of mice infected with M. tuberculosis H37Ra” to the second-to-last sentence in the Abstract, so the sentence should be changed to: “We demonstrated that LV20 combined with the adjuvant composed of DDA and Poly I: C (DP) elicited significantly higher antigen-specific antibodies and CD4+/CD8+ T cell responses than PBS and BCG vaccination in mice, and reduced the bacterial load in the lungs of mice infected with M. tuberculosis H37Ra.”
- We added a second paragraph to Section 2.7:
- 4.
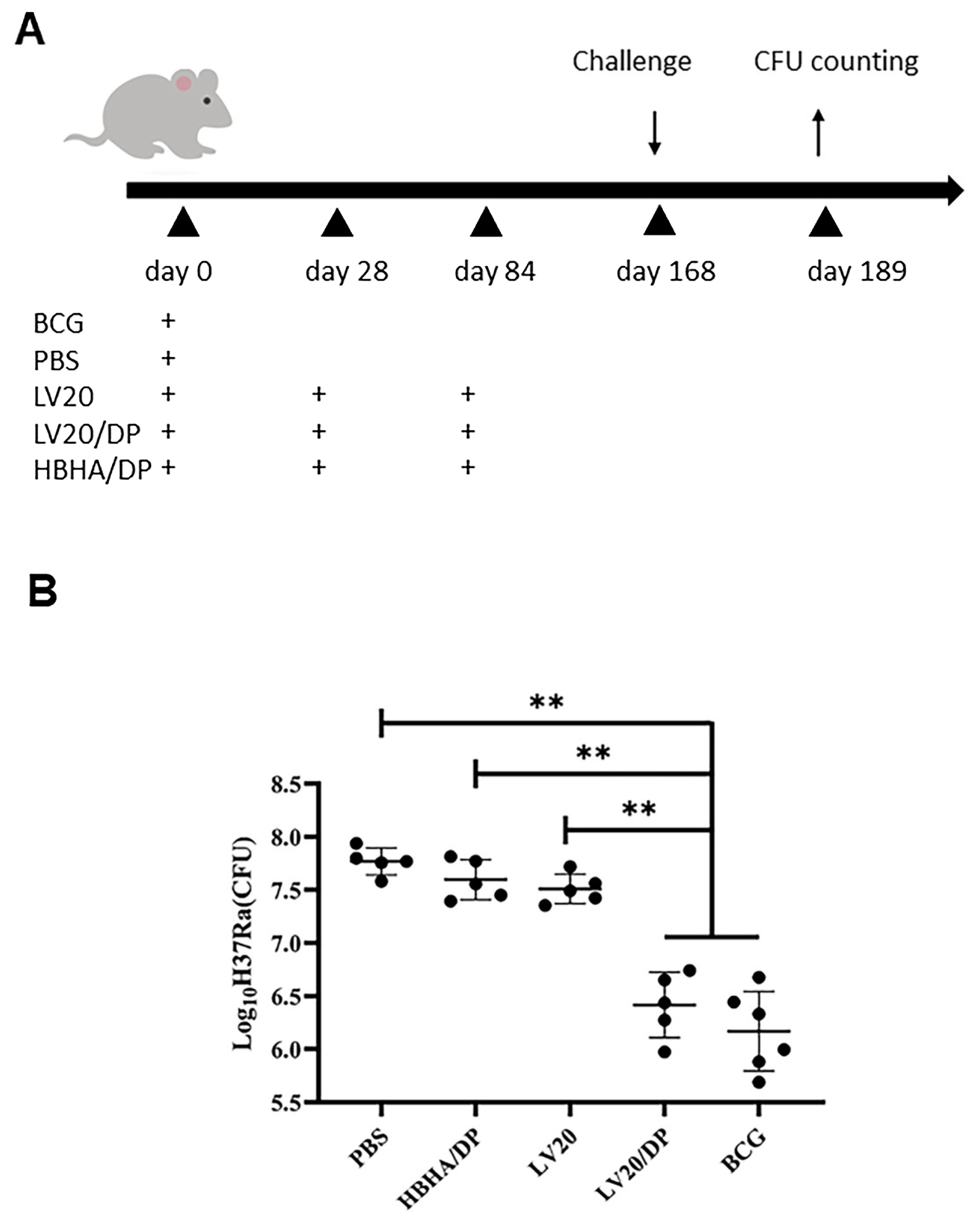
- We added Section 3.6, which includes a new paragraph and the original Figure S2 from the Supplementary Material as Figure 7:
Reference
- Wang, J.; Xie, T.; Ullah, I.; Mi, Y.; Li, X.; Gong, Y.; He, P.; Liu, Y.; Li, F.; Li, J.; et al. A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Potentially Protective Immune Responses in M. tuberculosis H37Ra Infected Mice. Vaccines 2023, 11, 941. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xie, T.; Ullah, I.; Mi, Y.; Li, X.; Gong, Y.; He, P.; Liu, Y.; Li, F.; Li, J.; et al. Correction: Wang et al. A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Potentially Protective Immune Responses in M. tuberculosis H37Ra Infected Mice. Vaccines 2023, 11, 941. Vaccines 2023, 11, 1454. https://doi.org/10.3390/vaccines11091454
Wang J, Xie T, Ullah I, Mi Y, Li X, Gong Y, He P, Liu Y, Li F, Li J, et al. Correction: Wang et al. A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Potentially Protective Immune Responses in M. tuberculosis H37Ra Infected Mice. Vaccines 2023, 11, 941. Vaccines. 2023; 11(9):1454. https://doi.org/10.3390/vaccines11091454
Chicago/Turabian StyleWang, Juan, Tao Xie, Inayat Ullah, Youjun Mi, Xiaoping Li, Yang Gong, Pu He, Yuqi Liu, Fei Li, Jixi Li, and et al. 2023. "Correction: Wang et al. A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Potentially Protective Immune Responses in M. tuberculosis H37Ra Infected Mice. Vaccines 2023, 11, 941" Vaccines 11, no. 9: 1454. https://doi.org/10.3390/vaccines11091454
APA StyleWang, J., Xie, T., Ullah, I., Mi, Y., Li, X., Gong, Y., He, P., Liu, Y., Li, F., Li, J., Lu, Z., & Zhu, B. (2023). Correction: Wang et al. A VLP-Based Vaccine Displaying HBHA and MTP Antigens of Mycobacterium tuberculosis Induces Potentially Protective Immune Responses in M. tuberculosis H37Ra Infected Mice. Vaccines 2023, 11, 941. Vaccines, 11(9), 1454. https://doi.org/10.3390/vaccines11091454




