Humoral Immunity in Immunosuppressed IBD Patients after the Third SARS-CoV-2 Vaccination: A Comparison with Healthy Control Subjects
Abstract
:1. Introduction
2. Methods
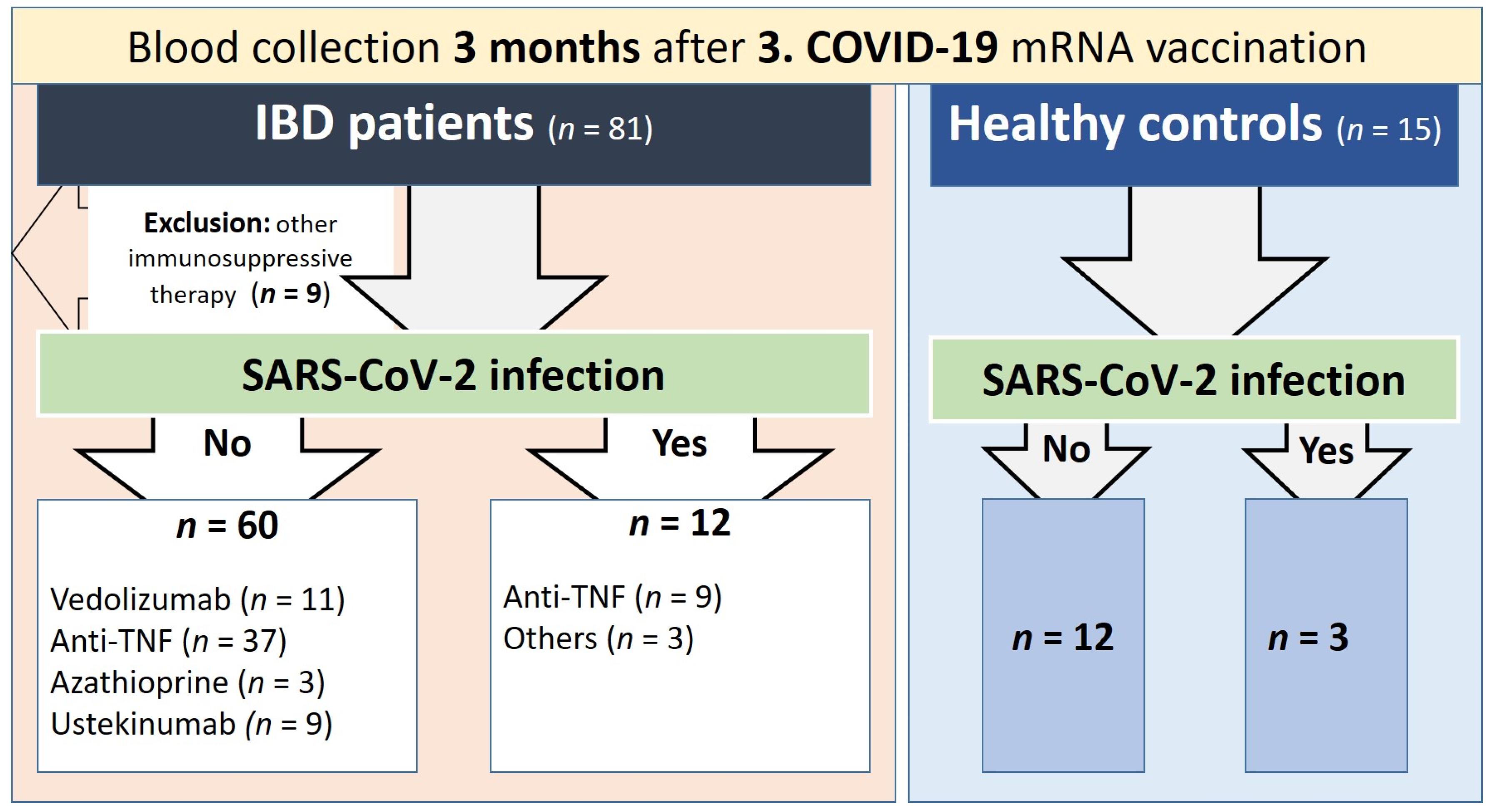
2.1. Study Subjects and Samples
2.2. Quantification of Serum Markers
2.3. Statistical Examination
3. Results
3.1. Cohort Characteristics
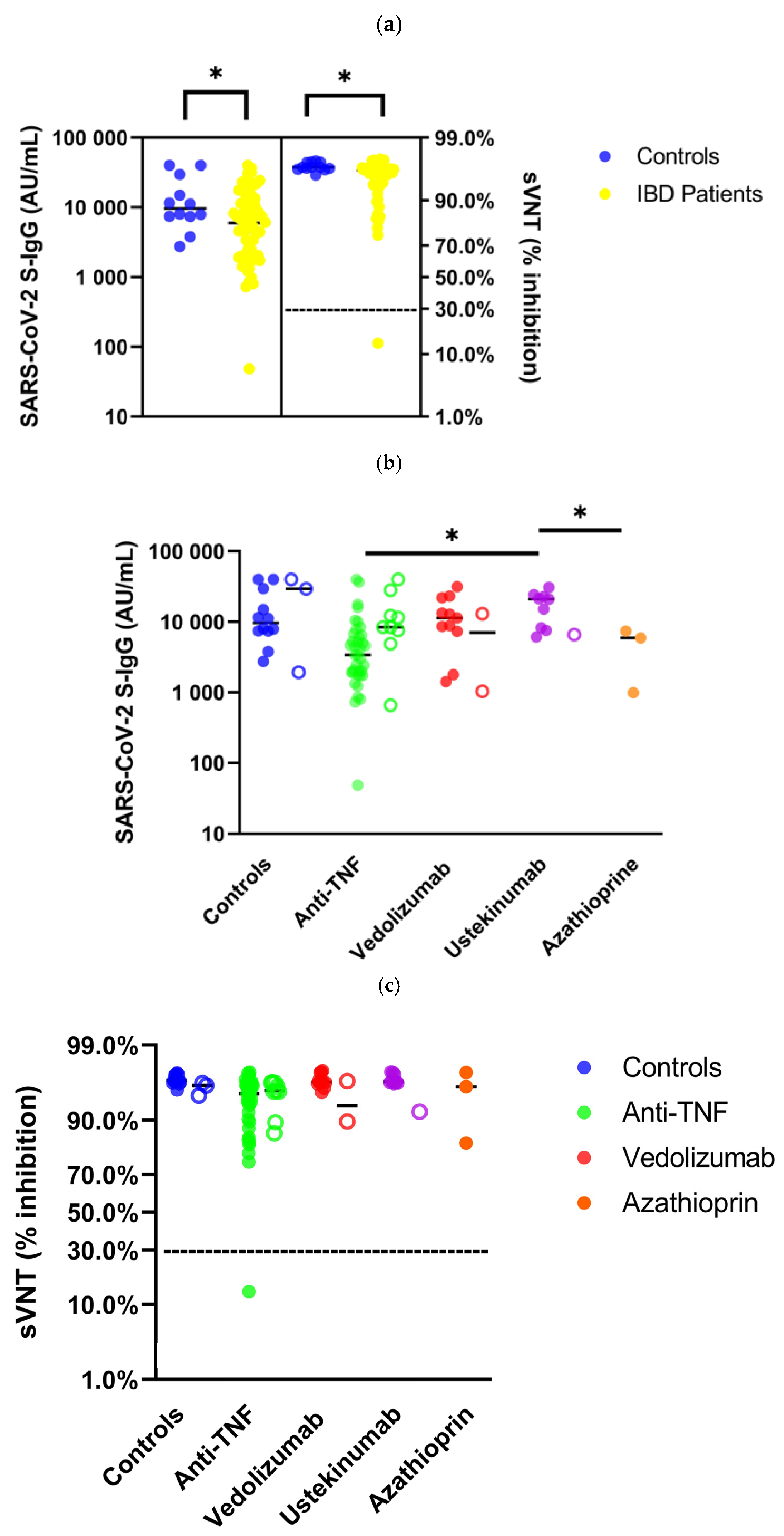
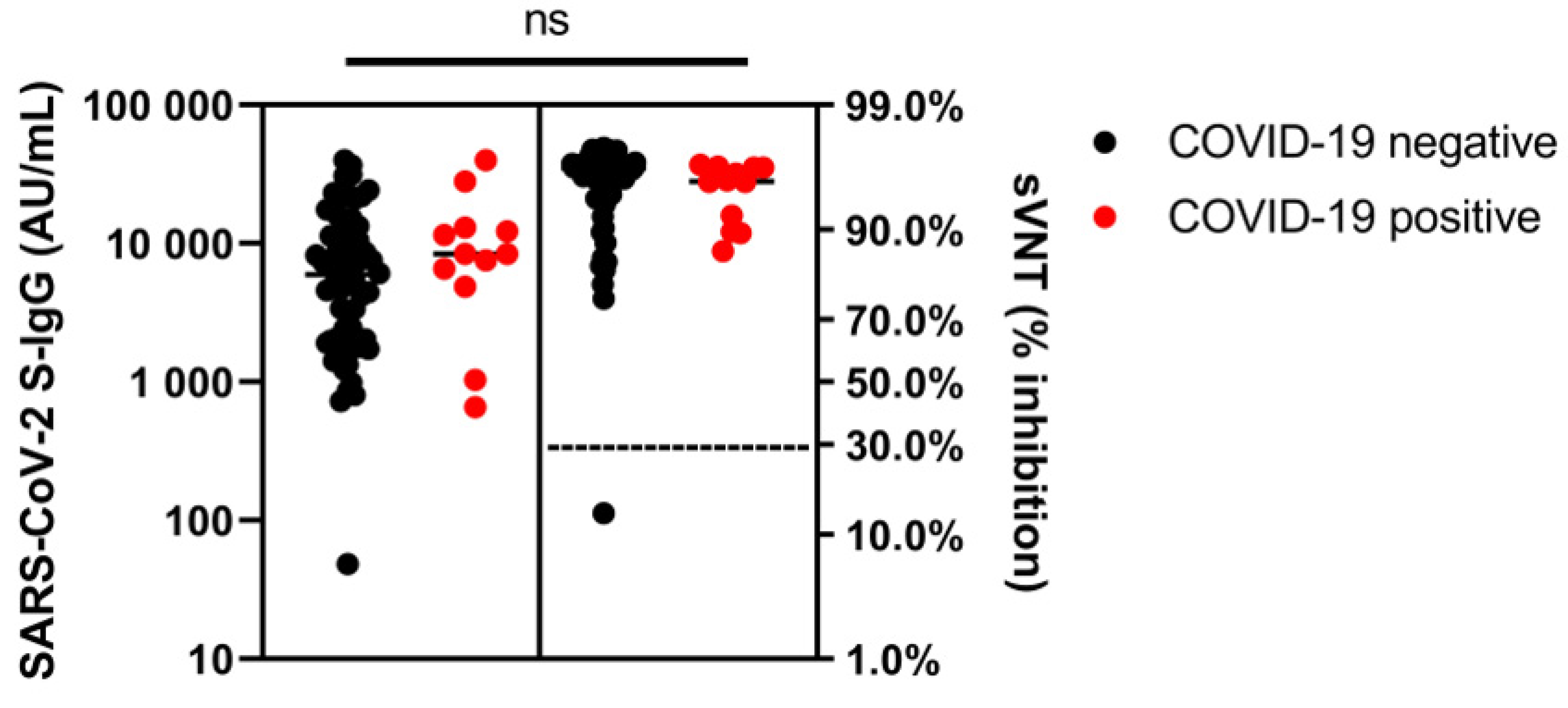
3.2. Humoral Immunity in Immunosuppressed IBD Patients after SARS-CoV-2 Vaccination: Comparison with Healthy Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-Drelated coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Tepasse, P.-R.; Hafezi, W.; Lutz, M.; Kühn, J.; Wilms, C.; Wiewrodt, R.; Sackarnd, J.; Keller, M.; Schmidt, H.H.; Vollenberg, R. Persisting SARS-CoV-2 viraemia after rituximab therapy: Two cases with fatal outcome and a review of the literature. Br. J. Haematol. 2020, 190, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Vollenberg, R.; Matern, P.; Nowacki, T.; Fuhrmann, V.; Padberg, J.-S.; Ochs, K.; Schütte-Nütgen, K.; Strauß, M.; Schmidt, H.; Tepasse, P.-R. Prone Position in Mechanically Ventilated COVID-19 Patients: A Multicenter Study. J. Clin. Med. 2021, 10, 1046. [Google Scholar] [CrossRef]
- Beaugerie, L.; Rahier, J.-F.; Kirchgesner, J. Predicting, Preventing, and Managing Treatment-Related Complications in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1324–1335.e2. [Google Scholar] [CrossRef]
- Planas, D.; Bruel, T.; Staropoli, I.; Guivel-Benhassine, F.; Porrot, F.; Maes, P.; Grzelak, L.; Prot, M.; Mougari, S.; Planchais, C.; et al. Resistance of Omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies. Nat. Commun. 2023, 14, 824. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Vollenberg, R.; Tepasse, P.-R.; Lorentzen, E.; Nowacki, T.M. Impaired Humoral Immunity with Concomitant Preserved T Cell Reactivity in IBD Patients on Treatment with Infliximab 6 Month after Vaccination with the SARS-CoV-2 mRNA Vaccine BNT162b2: A Pilot Study. J. Pers. Med. 2022, 12, 694. [Google Scholar] [CrossRef]
- Vollenberg, R.; Tepasse, P.-R.; Kühn, J.E.; Hennies, M.; Strauss, M.; Rennebaum, F.; Schomacher, T.; Boeckel, G.; Lorentzen, E.; Bokemeyer, A.; et al. Humoral Immune Response in IBD Patients Three and Six Months after Vaccination with the SARS-CoV-2 mRNA Vaccines mRNA-1273 and BNT162b2. Biomedicines 2022, 10, 171. [Google Scholar] [CrossRef]
- Tepasse, P.-R.; Vollenberg, R.; Nowacki, T.M. Vaccination against SARS-CoV-2 in Patients with Inflammatory Bowel Diseases: Where Do We Stand? Life 2021, 11, 1220. [Google Scholar] [CrossRef]
- Von Rhein, C.; Scholz, T.; Henss, L.; Kronstein-Wiedemann, R.; Schwarz, T.; Rodionov, R.N.; Corman, V.M.; Tonn, T.; Schnierle, B.S. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. J. Virol. Methods 2021, 288, 114031. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Graninger, M.; Jani, C.M.; Reuberger, E.; Prüger, K.; Gaspar, P.; Springer, D.N.; Borsodi, C.; Weidner, L.; Rabady, S.; Puchhammer-Stöckl, E.; et al. Comprehensive Comparison of Seven SARS-CoV-2-Specific Surrogate Virus Neutralization and Anti-Spike IgG Antibody Assays Using a Live-Virus Neutralization Assay as a Reference. Microbiol. Spectr. 2023, 11, e0231422. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59, e02438-20. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Kennedy, N.A.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Castro Seoane, R.; Liu, Z.; Nice, R.; Bewshea, C.; D’Mello, A.; et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 342–352. [Google Scholar] [CrossRef]
- Weaver, K.N.; Zhang, X.; Dai, X.; Watkins, R.; Adler, J.; Dubinsky, M.C.; Kastl, A.; Bousvaros, A.; Strople, J.A.; Cross, R.K.; et al. Impact of SARS-CoV-2 Vaccination on Inflammatory Bowel Disease Activity and Development of Vaccine-Related Adverse Events: Results From PREVENT-COVID. Inflamm. Bowel Dis. 2022, 28, 1497–1505. [Google Scholar] [CrossRef]
- Schell, T.L.; Knutson, K.L.; Saha, S.; Wald, A.; Phan, H.S.; Almasry, M.; Chun, K.; Grimes, I.; Lutz, M.; Hayney, M.S.; et al. Humoral Immunogenicity of 3 COVID-19 Messenger RNA Vaccine Doses in Patients With Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1781–1786. [Google Scholar] [CrossRef]
- Long, M.D.; Weaver, K.N.; Zhang, X.; Chun, K.; Kappelman, M.D. Strong Response to SARS-CoV-2 Vaccine Additional Doses Among Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2022, 20, 1881–1883.e1. [Google Scholar] [CrossRef]
- Quan, J.; Ma, C.; Panaccione, R.; Hracs, L.; Sharifi, N.; Herauf, M.; Makovinović, A.; Coward, S.; Windsor, J.W.; Caplan, L.; et al. Serological responses to three doses of SARS-CoV-2 vaccination in inflammatory bowel disease. Gut 2023, 72, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Liu, Z.; Muñoz Sandoval, D.; Reynolds, C.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Castro Seoane, R.; Anand, N.; Nice, R.; et al. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 1005–1015. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Janjua, M.; Chanchlani, N.; Lin, S.; Bewshea, C.; Nice, R.; McDonald, T.J.; Auckland, C.; Harries, L.W.; Davies, M.; et al. Vaccine escape, increased breakthrough and reinfection in infliximab-treated patients with IBD during the Omicron wave of the SARS-CoV-2 pandemic. Gut 2023, 72, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Le, K.; Zhou, X.; Alexander, J.L.; Lin, S.; Bewshea, C.; Chanchlani, N.; Nice, R.; McDonald, T.J.; Lamb, C.A.; et al. Neutralising antibody potency against SARS-CoV-2 wild-type and omicron BA.1 and BA.4/5 variants in patients with inflammatory bowel disease treated with infliximab and vedolizumab after three doses of COVID-19 vaccine (CLARITY IBD): An analysis of a prospective multicentre cohort study. Lancet Gastroenterol. Hepatol. 2023, 8, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Eder, L.; Croxford, R.; Drucker, A.M.; Mendel, A.; Kuriya, B.; Touma, Z.; Johnson, S.R.; Cook, R.; Bernatsky, S.; Haroon, N.; et al. Understanding COVID-19 Risk in Patients With Immune-Mediated Inflammatory Diseases: A Population-Based Analysis of SARS-CoV-2 Testing. Arthritis Care Res. 2023, 75, 317–325. [Google Scholar] [CrossRef]
- Teich, N.; Stallmach, A. COVID-19 und chronisch-entzündliche Darmerkrankungen. Die Gastroenterol. 2023, 18, 100–106. [Google Scholar] [CrossRef]
- Teich, N.; Ludewig, C.; Schmelz, R.; Bästlein, E.C.; Geißler, S.; Nagl, S.; Walldorf, J.; Krause, T.; Maaser, C.; Mohl, W.; et al. Auswirkungen einer SARS-CoV-2-Infektion auf Symptomatik und Therapie chronisch-entzündlicher Darmerkrankungen. Z. Gastroenterol. 2021, 59, 1189–1196. [Google Scholar] [CrossRef]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020, 159, 481–491.e3. [Google Scholar] [CrossRef]
- Lukin, D.J.; Kumar, A.; Hajifathalian, K.; Sharaiha, R.Z.; Scherl, E.J.; Longman, R.S. Baseline Disease Activity and Steroid Therapy Stratify Risk of COVID-19 in Patients With Inflammatory Bowel Disease. Gastroenterology 2020, 159, 1541–1544.e2. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.C.; Brenner, E.J.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Steinwurz, F.; et al. Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut 2021, 70, 725–732. [Google Scholar] [CrossRef]
- Akiyama, S.; Hamdeh, S.; Micic, D.; Sakuraba, A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: A systematic review and meta-analysis. Ann. Rheum. Dis. 2021, 80, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.; Hyrich, K.L.; Al-Adely, S.; Carmona, L.; Danila, M.I.; Gossec, L.; Izadi, Z.; Jacobsohn, L.; Katz, P.; Lawson-Tovey, S.; et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020, 79, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Izadi, Z.; Brenner, E.J.; Mahil, S.K.; Dand, N.; Yiu, Z.Z.N.; Yates, M.; Ungaro, R.C.; Zhang, X.; Agrawal, M.; Colombel, J.-F.; et al. Association Between Tumor Necrosis Factor Inhibitors and the Risk of Hospitalization or Death Among Patients With Immune-Mediated Inflammatory Disease and COVID-19. JAMA Netw. Open 2021, 4, e2129639. [Google Scholar] [CrossRef]
- Chanchlani, N.; Lin, S.; Chee, D.; Hamilton, B.; Nice, R.; Arkir, Z.; Bewshea, C.; Cipriano, B.; Derikx, L.A.A.P.; Dunlop, A.; et al. Adalimumab and Infliximab Impair SARS-CoV-2 Antibody Responses: Results from a Therapeutic Drug Monitoring Study in 11 422 Biologic-Treated Patients. J. Crohns Colitis 2022, 16, 389–397. [Google Scholar] [CrossRef]
- Amanat, F.; Thapa, M.; Lei, T.; Ahmed, S.M.S.; Adelsberg, D.C.; Carreño, J.M.; Strohmeier, S.; Schmitz, A.J.; Zafar, S.; Zhou, J.Q.; et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 2021, 184, 3936–3948.e10. [Google Scholar] [CrossRef]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.; de Marco, A.; Lempp, F.A.; Tortorici, M.A.; Pinto, D.; Walls, A.C.; Beltramello, M.; Chen, A.; Liu, Z.; Zatta, F.; et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 2021, 184, 2332–2347.e16. [Google Scholar] [CrossRef]
- Piccoli, L.; Park, Y.-J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef] [PubMed]
| No Previous SARS-CoV-2 Infection | Previous SARS-CoV-2 Infection | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 12) | IBD (n = 60) | p-Value 1 | Vedolizumab (n = 11) | Anti-TNF (n = 37) | Azathioprine (n = 3) | Ustekinumab (n = 9) | p-Value 2 | Controls (n = 3) | IBD (n = 12) | p-Value 1 | Anti-TNF (n = 9) | Others (n = 3) | p-Value 2 | ||
| Patient characteristics | Age, years median (IQR) | 46 (33–57) | 50 (35–57) | 0.678 | 50 (40–66) | 52 (34–57) | 48 | 48 (37–56) | 0.780 | 36 | 39 (29–47) | 0.734 | 38 (26–44) | 46 | 0.104 |
| Sex, male (%) | 42 | 37 (62) | 0.219 | 10 (91) | 22 (60) | 0 (0) | 5 (56) | 0.027 | 1 (33) | 7 (58) | 0.003 | 4 (44) | 3 (100) | 0.108 | |
| BMI | 22 (21–25) | 24 (23–27) | 0.125 | 26 (23–28) | 24 (23–27) | 23 | 24 (23–26) | 0.454 | 21 | 23 (22–25) | 0.101 | 23 (21–25) | 25 | 0.314 | |
| IBD | Crohn’s disease (%) | 0 (0) | 37 (62) | 4 | 24 | 2 (67) | 7 (78) | 0.255 | 0 (0) | 10 (83) | 8 (89) | 2 (67) | 0.418 | ||
| CDAI score, median (IQR) | 0 (0) | 0 (0–0) | 34 (0–205) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.207 | 0 (0) | 0 (0–30) | 0 (0–0) | 59 | 0.439 | |||
| CDAI score >/=150 (%) | 0 (0) | 2 (5) | 1 (9) | 0 (0) | 0 (0) | 1 (11) | 0.141 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 | |||
| Ulcerative colitis (%) | 0 (0) | 23 (38) | 7 | 13 | 1 (33) | 2 (22) | 0.207 | 0 (0) | 2 (17) | 1 (11) | 1 (33) | 0.418 | |||
| Mayo score, median (IQR) | 0 (0) | 0 (0–4) | 3 (0–4) | 0 (0–2.5) | 0 (0–0) | 2,5 | 0.282 | 0 (0) | 0 (0–0) | 0 (0–0) | 0 | 1.000 | |||
| Mayo score >/=1 (%) | 0 (0) | 11 (48) | 5 (46) | 4 (31) | 0 (0) | 2 (100) | 0.107 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 | |||
| Medication | Prednisolone p.o. (%) | 0 (0) | 4 (6) | 1 (9) | 3 (8) | 0 (0) | 0 (0) | 0.794 | 0 (0) | 1 (8) | 0 (0) | 1 (33) | 0.082 | ||
| Budesonide p.o. (%) | 0 (0) | 1 (1) | 1 (9) | 0 (0) | 0 (0) | 0 (0) | 0.218 | 0 (0) | 1 (8) | 1 (11) | 0 (0) | 0.588 | |||
| Budesonide supp. (%) | 0 (0) | 3 (4) | 1 (9) | 2 (5) | 0 (0) | 0 (0) | 0.806 | 0 (0) | 1 (8) | 0 (0) | 0 (0) | 1.000 | |||
| Mesalazine p.o. (%) | 0 (0) | 25 (34) | 4 (36) | 13 (35) | 2 (67) | 6 (67) | 0.289 | 0 (0) | 4 (33) | 2 (22) | 2 (67) | 0.188 | |||
| Mesalazine supp. (%) | 0 (0) | 5 (7) | 1 (9) | 2 (5) | 1 (33) | 1 (11) | 0.413 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 | |||
| Pre-existing conditions | Cardiovascular disease | 0 (0) | 12 (17) | 4 (36) | 8 (22) | 0 (0) | 0 (0) | 0.186 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 | ||
| Respiratory disease (%) | 0 (0) | 3 (4) | 2 (18) | 1 (3) | 0 (0) | 0 (0) | 0.173 | 0 (0) | 2 (17) | 1 (11) | 1 (33) | 0.418 | |||
| Kidney insufficiency (%) | 0 (0) | 2 (3) | 1 (9) | 1 (3) | 0 (0) | 0 (0) | 0.678 | 0 (0) | 1 (8) | 1 (11) | 0 (0) | 0.588 | |||
| Metastatic neoplasm (%) | 0 (0) | 4 (6) | 2 (18) | 2 (5) | 0 (0) | 0 (0) | 0.363 | 0 (0) | 1 (8) | 0 (0) | 1 (33) | 0.082 | |||
| Diabetes (%) | 0 (0) | 1 (2) | 1 (9) | 0 (0) | 0 (0) | 0 (0) | 0.218 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 | |||
| Death (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 | |||
| COVID-19-Negative * | Controls (n = 12) | IBD (n = 60) | p-Value 1 | Vedolizumab (n = 11) | Anti-TNF (n = 37) | Azathioprine (n = 3) | Ustekinumab (n = 9) | p-Value 2 |
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 S-IgG (AU/mL), median (IQR) | 9627 (7420–25,950) | 5956 (1996–12,461) | 0.034 | 11,388 (7319–22,027) | 3410 (1843–6617) | 5903 (989–5903) | 20,928 (7882–23,558) | <0.001 |
| Seroconversion rate S-IgG (%) | 100 | 98 | 1.000 | 100 | 97 | 100 | 100 | 0.889 |
| sVNT (% inhibition), median (IQR) | 97 (96–97) | 96 (94–97) | 0.012 | 96 (96–97) | 95 (90–96) | 96 (83–96) | 97 (96–97) | <0.001 |
| Seroconversion rate sVNT (%) | 100 | 98 | 1.000 | 100 | 97 | 100 | 100 | 0.889 |
| COVID-19-Positive * | Controls (n = 3) | IBD (n = 12) | p-Value 1 | Others (n = 3) | Anti-TNF (n = 9) | p-Value 2 | ||
| SARS-CoV-2 S-IgG (AU/mL), median (IQR) | 29,365 (1934–29,365) | 8409 (5314–12,846) | 0.365 | 6577 (1032–6577) | 8434 (6227–20,155) | 0.518 | ||
| Seroconversion rate S-IgG (%) | 100 | 100 | 1.000 | 100 | 100 | 1.000 | ||
| sVNT (% inhibition), median (IQR) | 96 (95–96) | 95 (90–96) | 0.840 | 92 (90–92) | 95 (92–96) | 0.926 | ||
| Seroconversion rate sVNT (%) | 100 | 100 | 1.000 | 100 | 100 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollenberg, R.; Lorentzen, E.U.; Kühn, J.; Nowacki, T.M.; Meier, J.A.; Trebicka, J.; Tepasse, P.-R. Humoral Immunity in Immunosuppressed IBD Patients after the Third SARS-CoV-2 Vaccination: A Comparison with Healthy Control Subjects. Vaccines 2023, 11, 1411. https://doi.org/10.3390/vaccines11091411
Vollenberg R, Lorentzen EU, Kühn J, Nowacki TM, Meier JA, Trebicka J, Tepasse P-R. Humoral Immunity in Immunosuppressed IBD Patients after the Third SARS-CoV-2 Vaccination: A Comparison with Healthy Control Subjects. Vaccines. 2023; 11(9):1411. https://doi.org/10.3390/vaccines11091411
Chicago/Turabian StyleVollenberg, Richard, Eva Ulla Lorentzen, Joachim Kühn, Tobias Max Nowacki, Jörn Arne Meier, Jonel Trebicka, and Phil-Robin Tepasse. 2023. "Humoral Immunity in Immunosuppressed IBD Patients after the Third SARS-CoV-2 Vaccination: A Comparison with Healthy Control Subjects" Vaccines 11, no. 9: 1411. https://doi.org/10.3390/vaccines11091411
APA StyleVollenberg, R., Lorentzen, E. U., Kühn, J., Nowacki, T. M., Meier, J. A., Trebicka, J., & Tepasse, P.-R. (2023). Humoral Immunity in Immunosuppressed IBD Patients after the Third SARS-CoV-2 Vaccination: A Comparison with Healthy Control Subjects. Vaccines, 11(9), 1411. https://doi.org/10.3390/vaccines11091411






