Associations between COVID-19 Vaccination Status and Self-Reported SARS-CoV-2 Infection among 8538 Children Aged 3–17 Years during a Massive COVID-19 Outbreak after China Changed Its Zero-COVID-19 Policy: A Cross-Sectional Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Data Collection
2.3. Sample Size Planning
2.4. Measurements
2.4.1. Questionnaire Development
2.4.2. Background Characteristics of the Children and Adolescents
2.4.3. Children and Adolescents’ History of SARS-CoV-2 Infection
2.4.4. Children and Adolescents’ COVID-19 Vaccination Status
2.5. Statistical Analysis
3. Results
3.1. Background Characteristics of the Participants
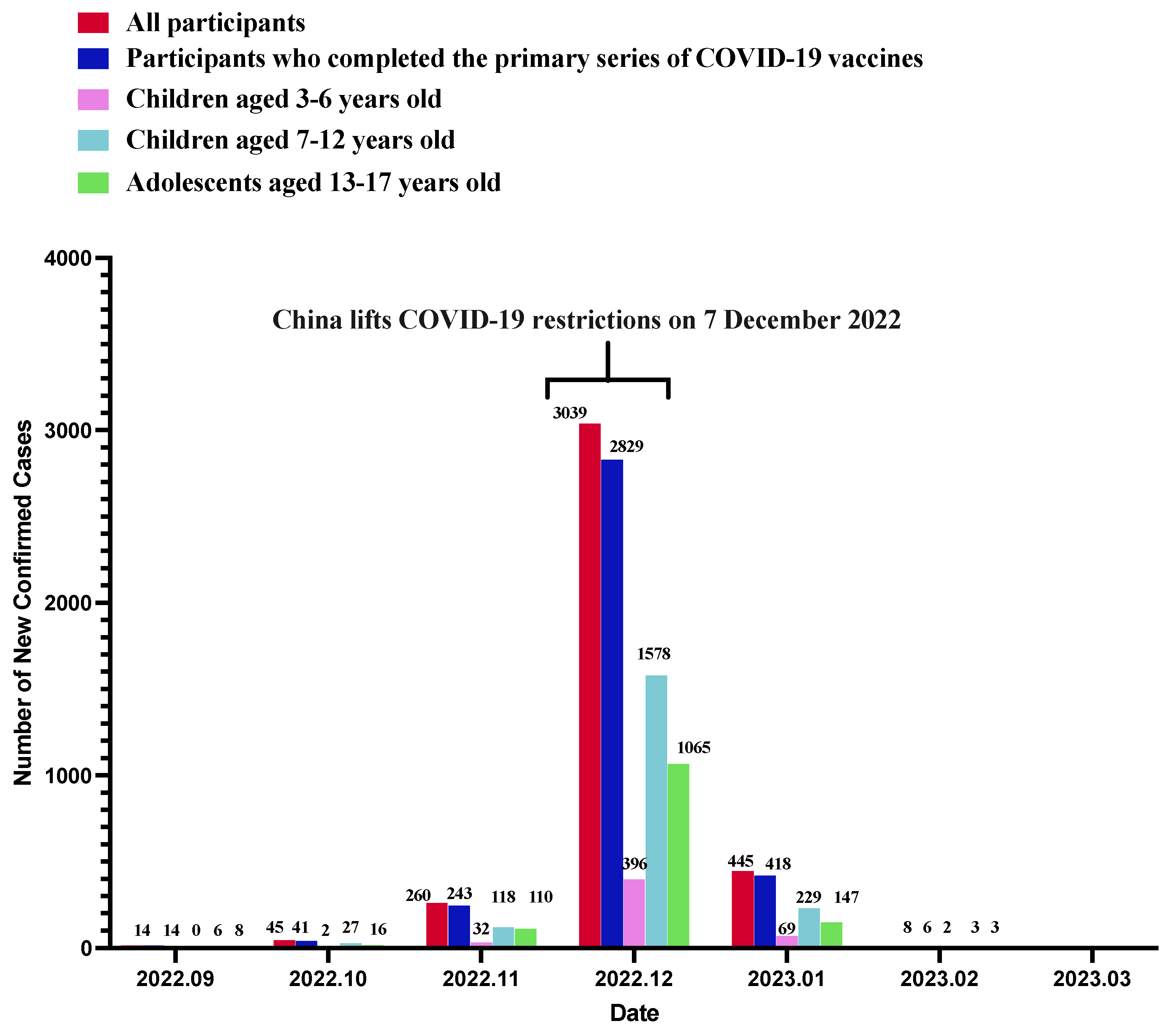
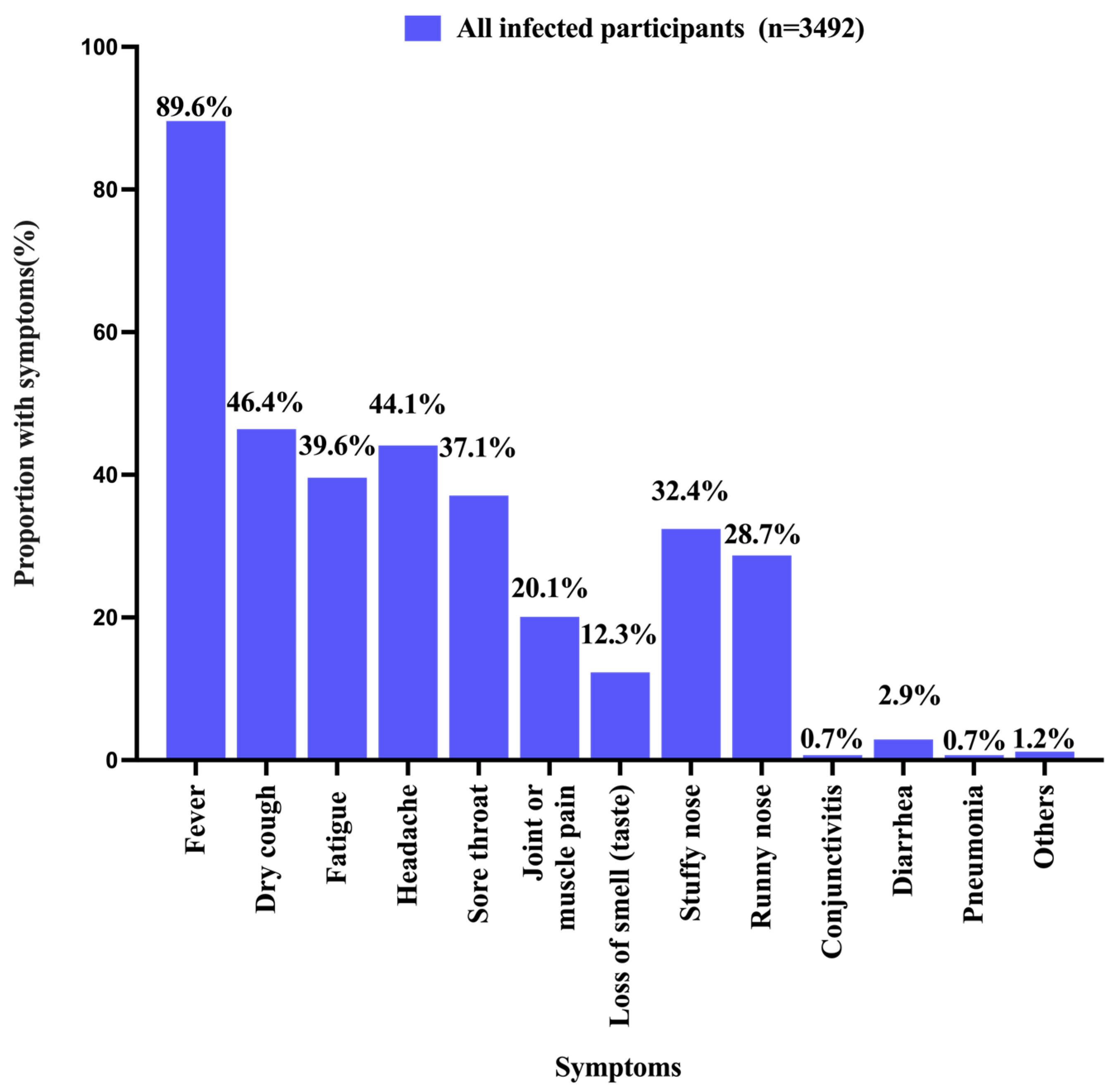
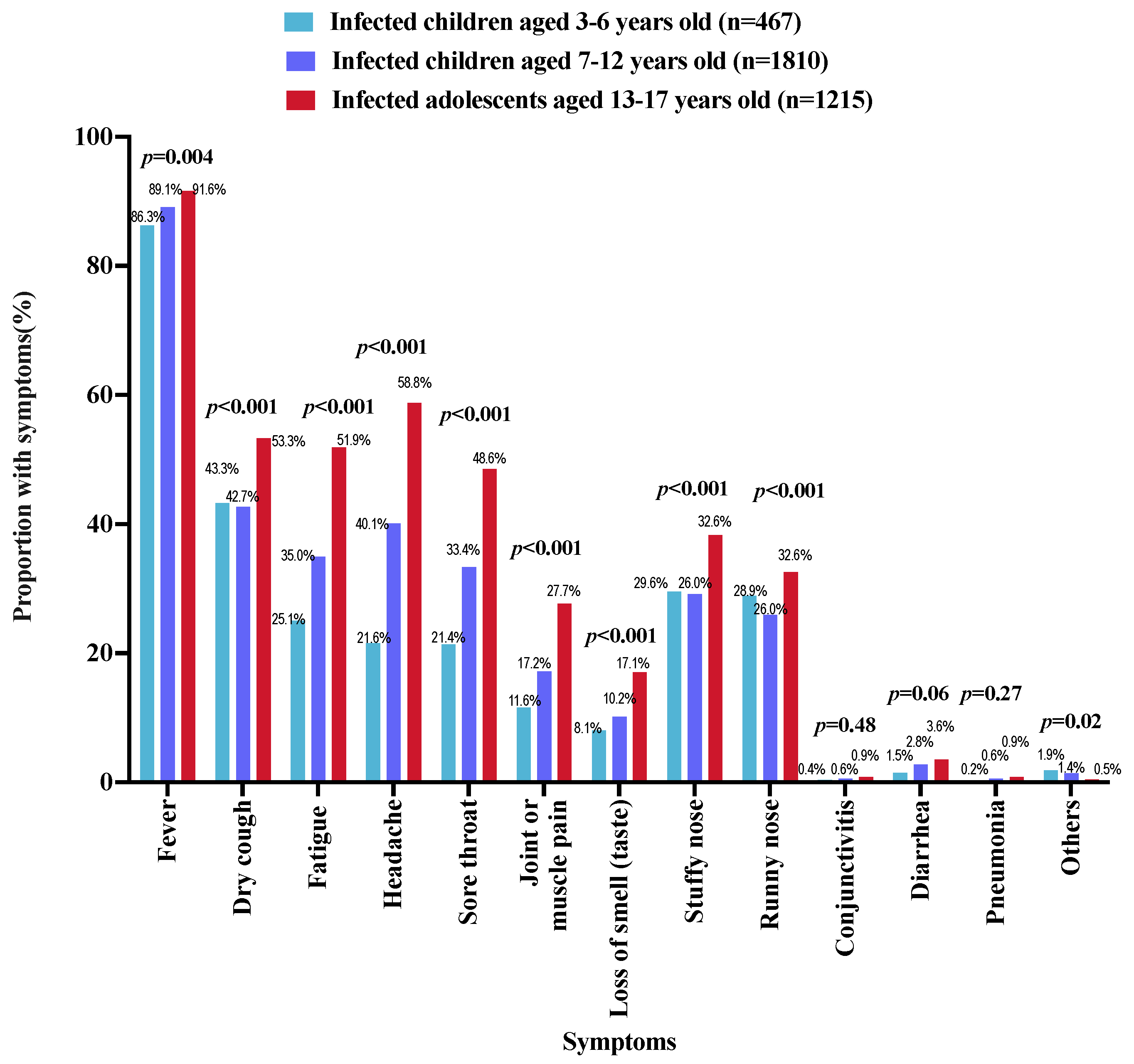
3.2. History of SARS-CoV-2 Infection
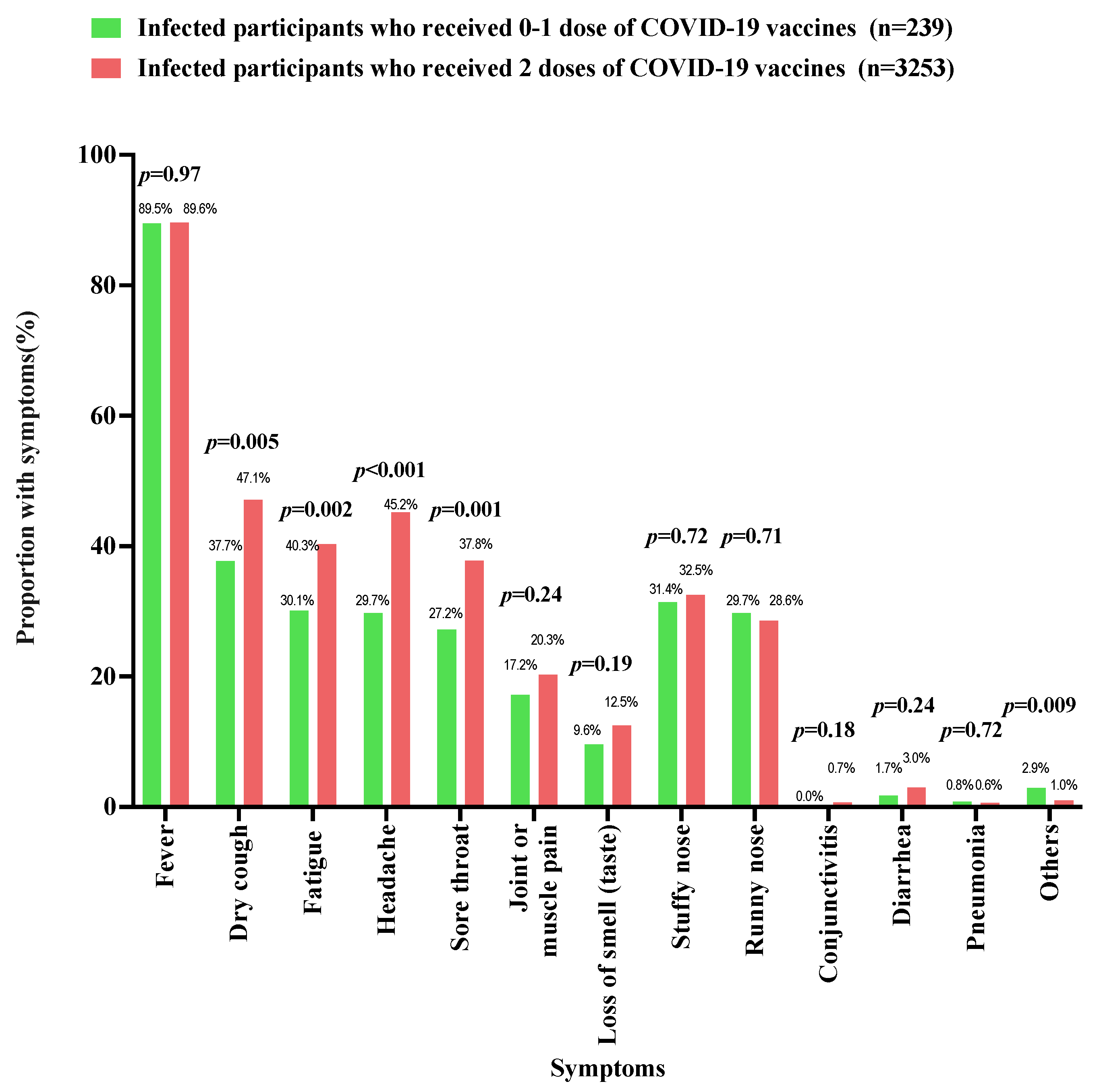
3.3. History of COVID-19 Vaccination
3.4. Association between COVID-19 Vaccination Status and SARS-CoV-2 Infection among All Participants
3.5. Association between COVID-19 Vaccination Status and SARS-CoV-2 Infection among Different Sub-Groups of Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ioannidis, J.P.A.; Zonta, F.; Levitt, M. What Really Happened during the Massive SARS-CoV-2 Omicron Wave in China? JAMA Intern. Med. 2023, 183, 633–634. [Google Scholar] [CrossRef] [PubMed]
- The State Council of the People’s Republic of China. Notice on Further Optimizing and Implementing the Prevention and Control Measures of the COVID-19. 2022. Available online: https://www.gov.cn/xinwen/2022-12/07/content_5730475.htm (accessed on 5 June 2023).
- Leung, K.; Lau, E.H.Y.; Wong, C.K.H.; Leung, G.M.; Wu, J.T. Estimating the transmission dynamics of SARS-CoV-2 Omicron BF.7 in Beijing after adjustment of the zero-COVID policy in November–December 2022. Nat. Med. 2023, 29, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Peng, Z.; Wei, F.; Jin, Z.; Wang, J.; Xu, X.; Zhang, X.; Xu, J.; Ren, Z.; Lu, B.; et al. Study on the COVID-19 epidemic in mainland China between November 2022 and January 2023, with prediction of its tendency. J. Biosaf. Biosecur. 2023, 5, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Press Conference of the Joint Prevention and Control Mechanism of the State Council. Introduction of the Relevant Situation of Medical and Health Services and Drug Production and Supply, and Answer Questions from the Media. 2022. Available online: https://www.gov.cn/xinwen/gwylflkjz221/index.htm (accessed on 7 June 2023).
- Zhou, X.; Wang, S.; Zhang, K.; Chen, S.; Chan, P.S.; Fang, Y.; Cao, H.; Chen, H.; Hu, T.; Chen, Y.; et al. Changes in Parents’ COVID-19 Vaccine Hesitancy for Children Aged 3–17 Years before and after the Rollout of the National Childhood COVID-19 Vaccination Program in China: Repeated Cross-Sectional Surveys. Vaccines 2022, 10, 1478. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Tam, K.L.W.; Kawuki, J.; Chan, P.S.; Chen, S.; Fang, Y.; Cao, H.; Zhou, X.; Chen, Y.; et al. Changes in COVID-19 Vaccine Acceptability among Parents with Children Aged 6–35 Months in China-Repeated Cross-Sectional Surveys in 2020 and 2021. Vaccines 2023, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Demographic Trends of COVID-19 Cases and Deaths in the US Reported to CDC. 2022. Available online: https://covid.cdc.gov/covid-data-tracker/#demographics (accessed on 27 June 2022).
- Statista. Number of Coronavirus (COVID-19) Cases in Germany in 2022, by Age Group and Gender. 2022. Available online: https://www.statista.com/statistics/1105465/coronavirus-covid-19-cases-age-group-germany/ (accessed on 27 June 2022).
- The Government of the Hong Kong SAR. Press Releases: Education Bureau Annouces Continouation of Daily Rapid Antigen Test Arrangements for Schools. Available online: https://www.info.gov.hk/gia/general/202206/17/P2022061700740.htm (accessed on 16 February 2023).
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA 2022, 327, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; LeClair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged 5–11 Years and Adolescents Aged 12–15 Years—PROTECT Cohort, July 2021–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; González, C.; Acevedo, J.; Pizarro, A.; Vergara, V.; Soto-Marchant, M.; Gilabert, R.; Flores, J.C.; et al. Effectiveness of CoronaVac in children 3–5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat. Med. 2022, 28, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.A.; Oliveira, M.C.L.; Silva, A.; Colosimo, E.A.; Mak, R.H.; Vasconcelos, M.A.; Silva, L.R.; Martelli, D.B.; Pinhati, C.C.; Martelli-Junior, H. Effectiveness of BNT162b2 and CoronaVac vaccines against omicron in children aged 5 to 11 years. World J. Pediatr. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chinese Government. COVID-19 Vaccines for Children. 2021. Available online: http://www.nhc.gov.cn/xcs/s7847/202111/79103c66c2de404b8e50583816f5e31e.shtml (accessed on 19 June 2023).
- The People’s Government of Beijing Municipality. COVID-19 Vaccination Situation in Beijing. 2022. Available online: https://www.beijing.gov.cn/ywdt/gzdt/202204/t20220418_2680504.html (accessed on 9 June 2023).
- Mei, B.; Brown, G.T.L. Conducting online surveys in China. Soc. Sci. Comput. Rev. 2018, 36, 721–734. [Google Scholar] [CrossRef]
- Stamm, T.A.; Ritschl, V.; Omara, M.; Andrews, M.R.; Mevenkamp, N.; Rzepka, A.; Schirmer, M.; Walch, S.; Salzberger, T.; Mosor, E. Rasch Model of the COVID-19 Symptom Checklist-A Psychometric Validation Study. Viruses 2021, 13, 1762. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Kubota, M.; Ogimi, C. Clinical characteristics of pediatric patients with COVID-19 between Omicron era vs. pre-Omicron era. J. Infect. Chemother. 2022, 28, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.W.; Xie, J.; Zemek, R.; Winston, K.; Freire, G.; Burstein, B.; Kam, A.; Emsley, J.; Gravel, J.; Porter, R.; et al. Comparison of Symptoms Associated With SARS-CoV-2 Variants Among Children in Canada. JAMA Netw. Open 2023, 6, e232328. [Google Scholar] [CrossRef] [PubMed]
- Atchison, C.J.; Whitaker, M.; Donnelly, C.A.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Ashby, D.; Barclay, W.; Cooke, G.S.; Elliott, P.; et al. Characteristics and predictors of persistent symptoms post-COVID-19 in children and young people: A large community cross-sectional study in England. Arch. Dis. Child. 2023, 108, e12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int/data (accessed on 14 June 2023).
- Toh, Z.Q.; Higgins, R.A.; Do, L.A.H.; Rautenbacher, K.; Mordant, F.L.; Subbarao, K.; Dohle, K.; Nguyen, J.; Steer, A.C.; Tosif, S.; et al. Persistence of SARS-CoV-2-Specific IgG in Children 6 Months After Infection, Australia. Emerg. Infect. Dis. 2021, 27, 2233–2235. [Google Scholar] [CrossRef] [PubMed]
- Chinese Government. COVID-19 Prevention Report in October 2022. 2022. Available online: https://www.gov.cn/xinwen/gwylflkjz211/index.htm (accessed on 19 June 2023).
- Office for National Statistics in the UK. Coronavirus (COVID-19) Latest Insights: Vaccines. 2023. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/vaccines (accessed on 14 June 2023).
- Centers for Disease Control and Prevention. COVID-19 Vaccinations in the United States. 2023. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-booster-percent-pop5 (accessed on 14 June 2023).
- Centers for Disease Control and Prevention. Vaccination Distribution & Coverage. 2023. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccine-delivery-coverage (accessed on 14 June 2023).
- The Government of Hong Kong. Children and Adolescents Should Receive COVID-19 Vaccination as Early as Possible. 2023. Available online: https://www.covidvaccine.gov.hk/en/ChildrenAdolescents (accessed on 19 June 2023).
- Zhang, K.C.; Fang, Y.; Cao, H.; Chen, H.; Hu, T.; Chen, Y.Q.; Zhou, X.; Wang, Z. Parental acceptability of COVID-19 vaccination for children under the age of 18 years: Cross-sectional online survey. JMIR Pediatr. Parent. 2020, 2, e24827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiu, S.; Yang, L.; Han, Y.; Cui, T.; Shi, N.; Liu, M.; Yi, Y.; Liu, C.; Wang, X.; et al. Changes in parental attitudes toward COVID-19 vaccination and routine childhood vaccination during the COVID-19 pandemic: Repeated cross-sectional survey study. JMIR Public Health Surveill. 2022, 8, e33235. [Google Scholar] [PubMed]
- Wu, M.J.; Zhao, K.; Fils-Aime, F. Response rates of online surveys in published research: A meta-analysis. Comput. Hum. Behav. Rep. 2022, 7, 100206. [Google Scholar] [CrossRef]
- Fu, L.; Wang, B.; Chan, P.S.F.; Luo, D.; Zheng, W.; Ju, N.; Hu, Y.; Xiao, X.; Xu, H.; Yang, X.; et al. Associations between COVID-19 related stigma and sleep quality among COVID-19 survivors six months after hospital discharge. Sleep Med. 2022, 91, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Fu, L.; Hu, Y.; Luo, D.; Xiao, X.; Ju, N.; Zheng, W.; Xu, H.; Fang, Y.; et al. Post-traumatic stress disorder symptoms in COVID-19 survivors six months after hospital discharge: An application of the conservation of resource theory. Front. Psychiatry 2022, 12, 773106. [Google Scholar] [PubMed]
- World Health Organization. Advice for the Public: Coronavirus Disease (COVID-19). 2023. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 19 June 2023).
| All Participants (n = 8538) | Participants Aged 3–6 Years Old (n = 1302) | Participants Aged 7–12 Years Old (n = 4666) | Participants Aged 13–17 Years Old (n = 2570) | p Values | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Background characteristics | |||||
| Sex assigned at birth | |||||
| Male | 4748 (55.6) | 713 (54.8) | 2585 (55.4) | 1450 (56.4) | 0.56 |
| Female | 3790 (44.4) | 589 (45.2) | 2081 (44.6) | 1120 (43.6) | |
| Number of other household members | |||||
| 1 | 203 (2.4) | 19 (1.5) | 98 (2.1) | 86 (3.4) | <0.001 |
| 2 | 1361 (15.9) | 185 (14.2) | 723 (15.5) | 453 (17.6) | |
| 3–5 | 6220 (72.9) | 992 (76.2) | 3431 (73.5) | 1797 (69.9) | |
| >5 | 754 (8.8) | 106 (8.1) | 414 (8.9) | 234 (9.1) | |
| Presence of any chronic conditions | |||||
| No | 8444 (98.9) | 1285 (98.7) | 4621 (99.0) | 2538 (98.8) | 0.41 |
| Yes | 94 (1.1) | 17 (1.3) | 45 (1.0) | 32 (1.2) | |
| Perform physical activity regularly (≥3 days/week of physical activity of at least 30 min/day in the past six months) | |||||
| No | 2284 (26.8) | 470 (36.1) | 1239 (26.6) | 575 (22.4) | <0.001 |
| Yes | 6254 (73.2) | 832 (63.9) | 3427 (73.4) | 1995 (77.6) | |
| History of SARS-CoV-2 infection | |||||
| Self-reported SARS-CoV-2 infection on or after 7 December 2022 | |||||
| No | 5046 (59.1) | 835 (64.1) | 2856 (61.2) | 1355 (52.7) | <0.001 |
| Yes | 3492 (40.9) | 467 (35.9) | 1810 (38.8) | 1215 (47.3) | |
| Self-reported SARS-CoV-2 infection before 7 December 2022 | |||||
| No | 8159 (95.6) | 1263 (97.0) | 4485 (96.1) | 2411 (93.8) | <0.001 |
| Yes | 379 (4.4) | 39 (3.0) | 181 (3.9) | 159 (6.2) | |
| COVID-19 vaccination status | |||||
| Number of doses of COVID-19 vaccine received by the children (interval between the second dose of COVID-19 vaccine and 7 December 2022) | |||||
| 0 dose | 358 (4.2) | 184 (14.1) | 124 (2.7) | 50 (1.9) | <0.001 |
| 1 dose | 246 (2.9) | 107 (8.2) | 100 (2.1) | 39 (1.5) | |
| 2 doses (<1 month) | 51 (0.6) | 8 (0.6) | 28 (0.6) | 15 (0.6) | |
| 2 doses (1–3 months) | 436 (5.1) | 76 (5.8) | 260 (5.6) | 100 (3.9) | |
| 2 doses (4–6 months) | 1493 (17.5) | 168 (12.9) | 827 (17.7) | 498 (19.4) | |
| 2 doses (7–9 months) | 1486 (17.4) | 195 (15.0) | 800 (17.1) | 491 (19.1) | |
| 2 doses (10–12 months) | 1040 (12.2) | 191 (14.7) | 589 (12.6) | 260 (10.1) | |
| 2 doses (>12 months) | 3428 (40.1) | 373 (28.7) | 1938 (41.6) | 1117 (43.5) |
| All Participants (n = 8538) | Participants Aged 3–6 Years Old (n = 1302) | Participants Aged 7–12 Years Old (n = 4666) | Participants Aged 13–17 Years Old (n = 2570) | |||||
|---|---|---|---|---|---|---|---|---|
| AOR (95% CI) | p Values | AOR (95% CI) | p Values | AOR (95% CI) | p Values | AOR (95% CI) | p Values | |
| Background characteristics | ||||||||
| Age group, years | ||||||||
| 3–6 | Reference | |||||||
| 7–12 | 1.14 (1.00, 1.31) | 0.049 | ||||||
| 13–17 | 1.63 (1.41, 1.89) | <0.001 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| Sex assigned at birth | ||||||||
| Male | --- | --- | Reference | --- | ||||
| Female | --- | --- | --- | --- | 0.86 (0.76, 0.97) | 0.01 | --- | --- |
| Number of other household members | ||||||||
| 1 | Reference | Reference | --- | Reference | ||||
| 2 | 1.53 (1.12, 2.10) | 0.008 | 4.29 (0.95, 19.25) | 0.06 | --- | --- | 1.65 (1.02, 2.67) | 0.04 |
| 3–5 | 1.51 (1.12, 2.04) | 0.007 | 4.66 (1.06, 20.38) | 0.04 | --- | --- | 1.47 (0.94, 2.31) | 0.09 |
| >5 | 1.33 (0.95, 1.85) | 0.09 | 4.84 (1.06, 22.22) | 0.04 | --- | --- | 1.09 (0.65, 1.83) | 0.74 |
| Presence of any chronic conditions | ||||||||
| No | --- | --- | --- | --- | ||||
| Yes | --- | --- | --- | --- | --- | --- | --- | --- |
| Perform physical activity regularly (≥3 days/week of physical activity of at least 30 min/day in the past six months) | ||||||||
| No | Reference | Reference | Reference | --- | --- | |||
| Yes | 0.80 (0.73, 0.88) | <0.001 | 0.79 (0.62, 0.99) | 0.047 | 0.79 (0.69, 0.90) | <0.001 | --- | --- |
| Self-reported SARS-CoV-2 infection before 7 December 2022 | ||||||||
| No | --- | --- | --- | --- | --- | --- | ||
| Yes | --- | --- | --- | --- | --- | --- | --- | --- |
| COVID-19 vaccination status | ||||||||
| Number of doses of COVID-19 vaccine received by the children (interval between the second dose of COVID-19 vaccine and 7 December 2022) | ||||||||
| 0 dose | Reference | Reference | Reference | Reference | ||||
| 1 dose | 1.19 (0.86, 1.67) | 0.30 | 1.26 (0.78, 2.04) | 0.35 | 1.09 (0.63, 1.89) | 0.77 | 1.36 (0.58, 3.19) | 0.47 |
| 2 doses (>12 months) | 1.33 (1.05, 1.67) | 0.02 | 0.86 (0.60, 1.24) | 0.42 | 1.59 (1.08, 2.33) | 0.02 | 1.73 (0.97, 3.08) | 0.07 |
| 2 doses (10–12 months) | 0.98 (0.76, 1.26) | 0.85 | 0.92 (0.60, 1.40) | 0.69 | 1.10 (0.73, 1.66) | 0.64 | 1.24 (0.67, 2.31) | 0.49 |
| 2 doses (7–9 months) | 1.02 (0.80, 1.30) | 0.86 | 0.97 (0.64, 1.48) | 0.90 | 1.14 (0.77, 1.70) | 0.52 | 1.30 (0.72, 2.36) | 0.39 |
| 2 doses (4–6 months) | 0.80 (0.63, 1.02) | 0.07 | 0.60 (0.38, 0.95) | 0.03 | 1.04 (0.70, 1.55) | 0.84 | 0.88 (0.48, 1.59) | 0.67 |
| 2 doses (1–3 months) | 0.54 (0.41, 0.75) | <0.001 | 0.53 (0.29, 0.90) | 0.04 | 0.71 (0.45, 1.13) | 0.15 | 0.50 (0.25, 1.04) | 0.06 |
| 2 doses (<1 month) | 0.17 (0.07, 0.44) | <0.001 | N.A. | N.A. | 0.33 (0.11, 1.02) | 0.05 | 0.11 (0.01, 0.88) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.; Chen, S.; Chen, H.; Fang, Y.; Peng, W.; Zhou, X.; Luo, J.; Liang, X.; Zhang, K.; Wang, Z. Associations between COVID-19 Vaccination Status and Self-Reported SARS-CoV-2 Infection among 8538 Children Aged 3–17 Years during a Massive COVID-19 Outbreak after China Changed Its Zero-COVID-19 Policy: A Cross-Sectional Survey. Vaccines 2023, 11, 1401. https://doi.org/10.3390/vaccines11091401
Su L, Chen S, Chen H, Fang Y, Peng W, Zhou X, Luo J, Liang X, Zhang K, Wang Z. Associations between COVID-19 Vaccination Status and Self-Reported SARS-CoV-2 Infection among 8538 Children Aged 3–17 Years during a Massive COVID-19 Outbreak after China Changed Its Zero-COVID-19 Policy: A Cross-Sectional Survey. Vaccines. 2023; 11(9):1401. https://doi.org/10.3390/vaccines11091401
Chicago/Turabian StyleSu, Lixian, Siyu Chen, Hongbiao Chen, Yuan Fang, Weijun Peng, Xiaofeng Zhou, Jingwei Luo, Xue Liang, Kechun Zhang, and Zixin Wang. 2023. "Associations between COVID-19 Vaccination Status and Self-Reported SARS-CoV-2 Infection among 8538 Children Aged 3–17 Years during a Massive COVID-19 Outbreak after China Changed Its Zero-COVID-19 Policy: A Cross-Sectional Survey" Vaccines 11, no. 9: 1401. https://doi.org/10.3390/vaccines11091401
APA StyleSu, L., Chen, S., Chen, H., Fang, Y., Peng, W., Zhou, X., Luo, J., Liang, X., Zhang, K., & Wang, Z. (2023). Associations between COVID-19 Vaccination Status and Self-Reported SARS-CoV-2 Infection among 8538 Children Aged 3–17 Years during a Massive COVID-19 Outbreak after China Changed Its Zero-COVID-19 Policy: A Cross-Sectional Survey. Vaccines, 11(9), 1401. https://doi.org/10.3390/vaccines11091401








