Intramuscular Immunization with a Liposomal Multi-Epitope Chimeric Protein Induces Strong Cellular Immune Responses against Visceral Leishmaniasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Parasites
2.2. Preparation and Characterization of Liposomes Containing LiChimera
2.3. Animal Immunization and Challenge Protocol
2.4. Determination of Parasite Burden Using Limited Dilution Analysis (LDA)
2.5. Evaluation of Parasite-Specific Immune Responses
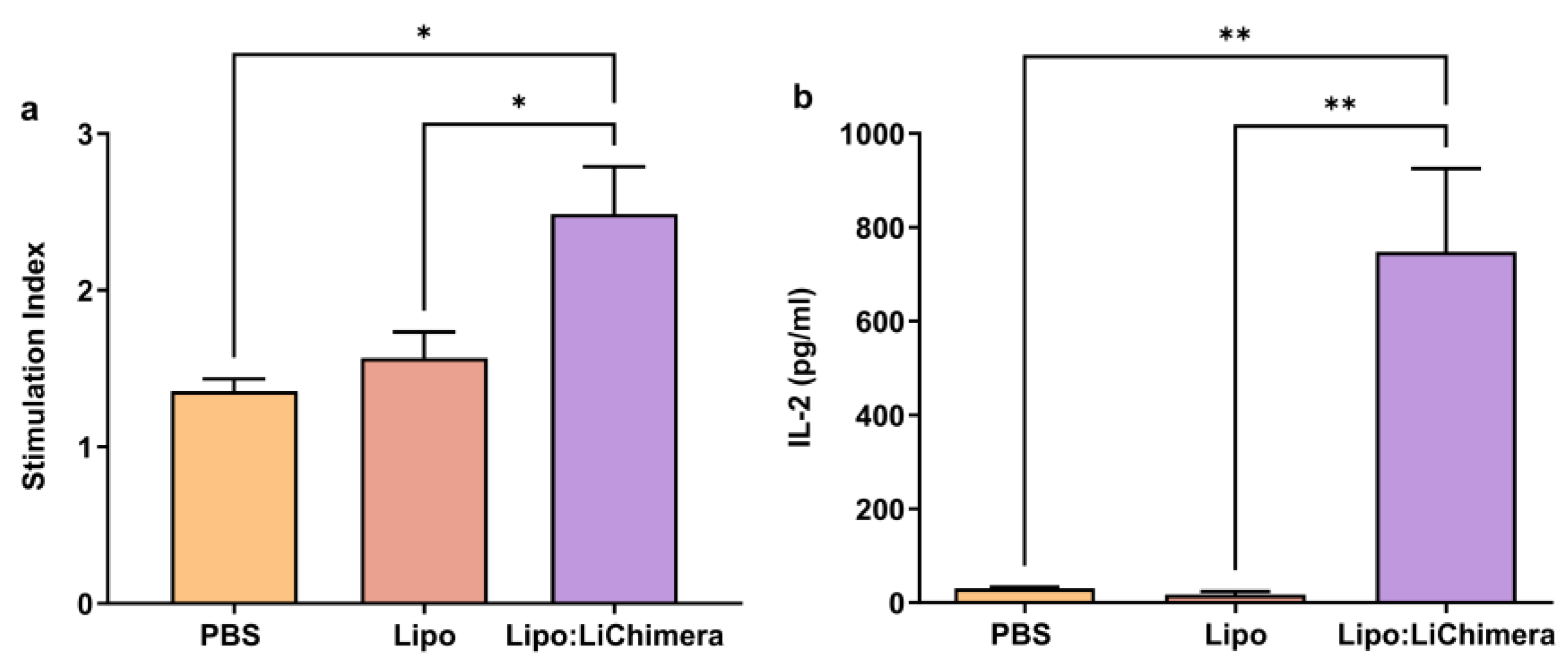
2.6. Antigen-Specific Proliferation Assay
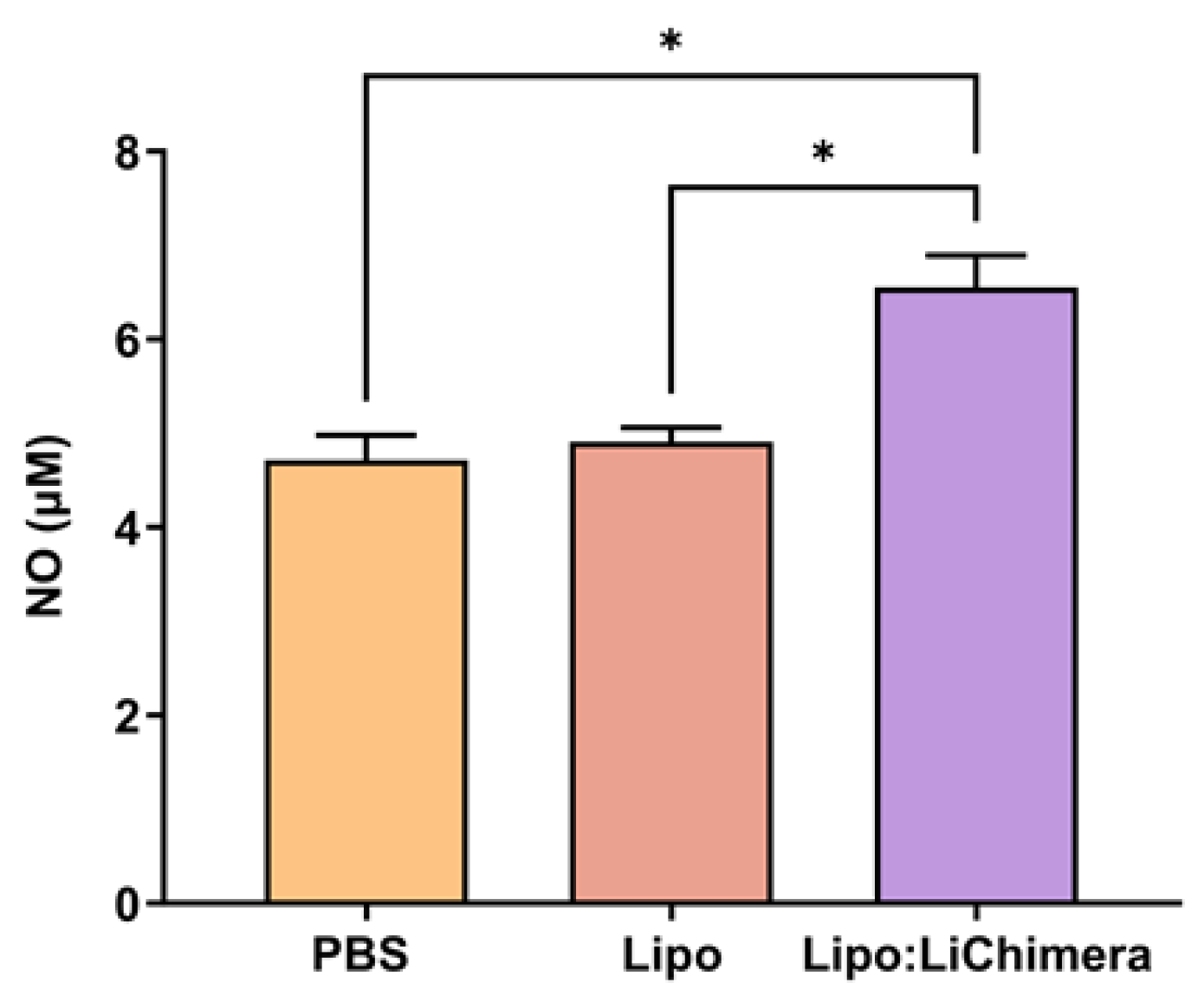
2.7. Evaluation of Antigen-Specific Cytokine and NO Production
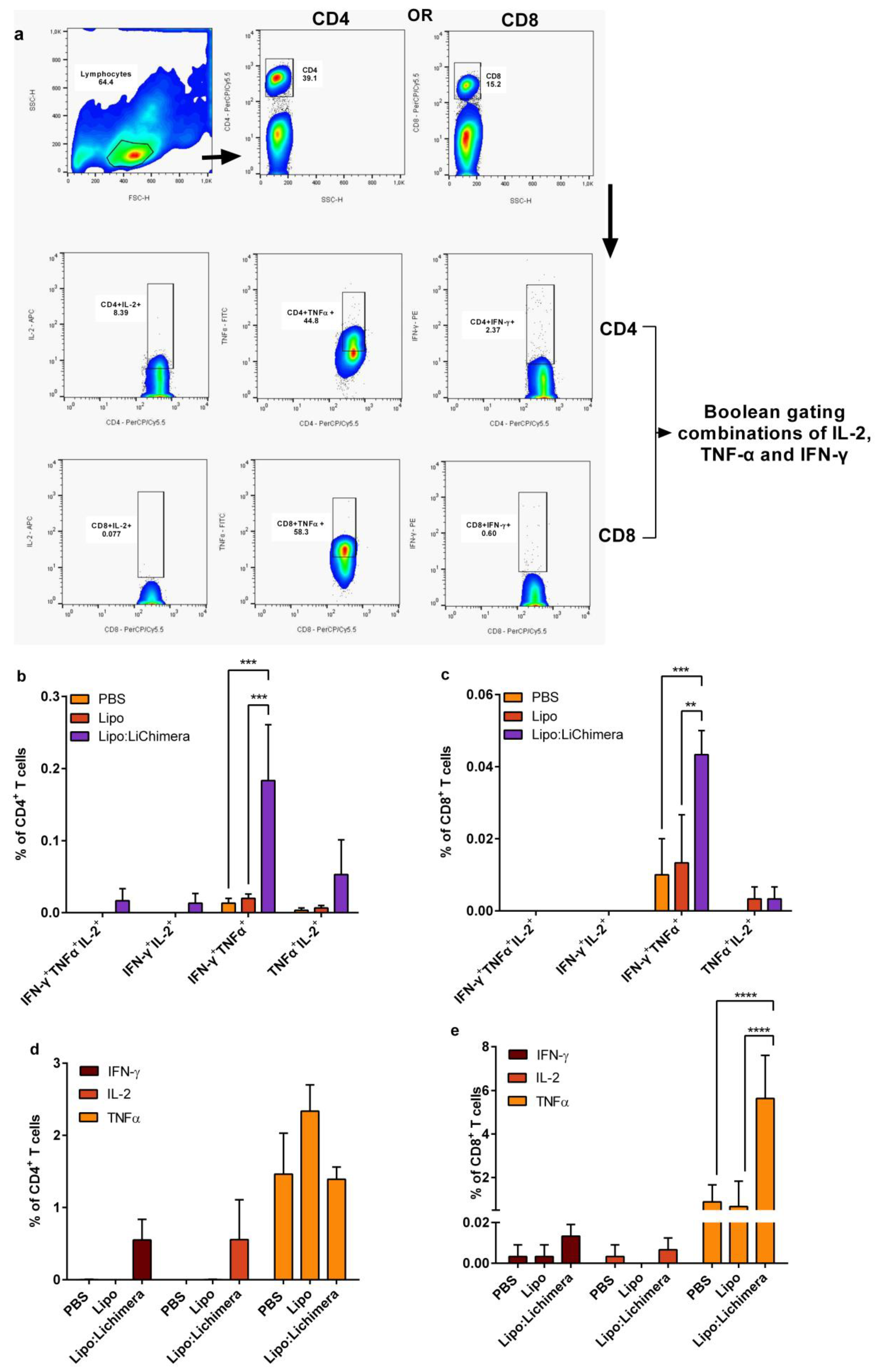
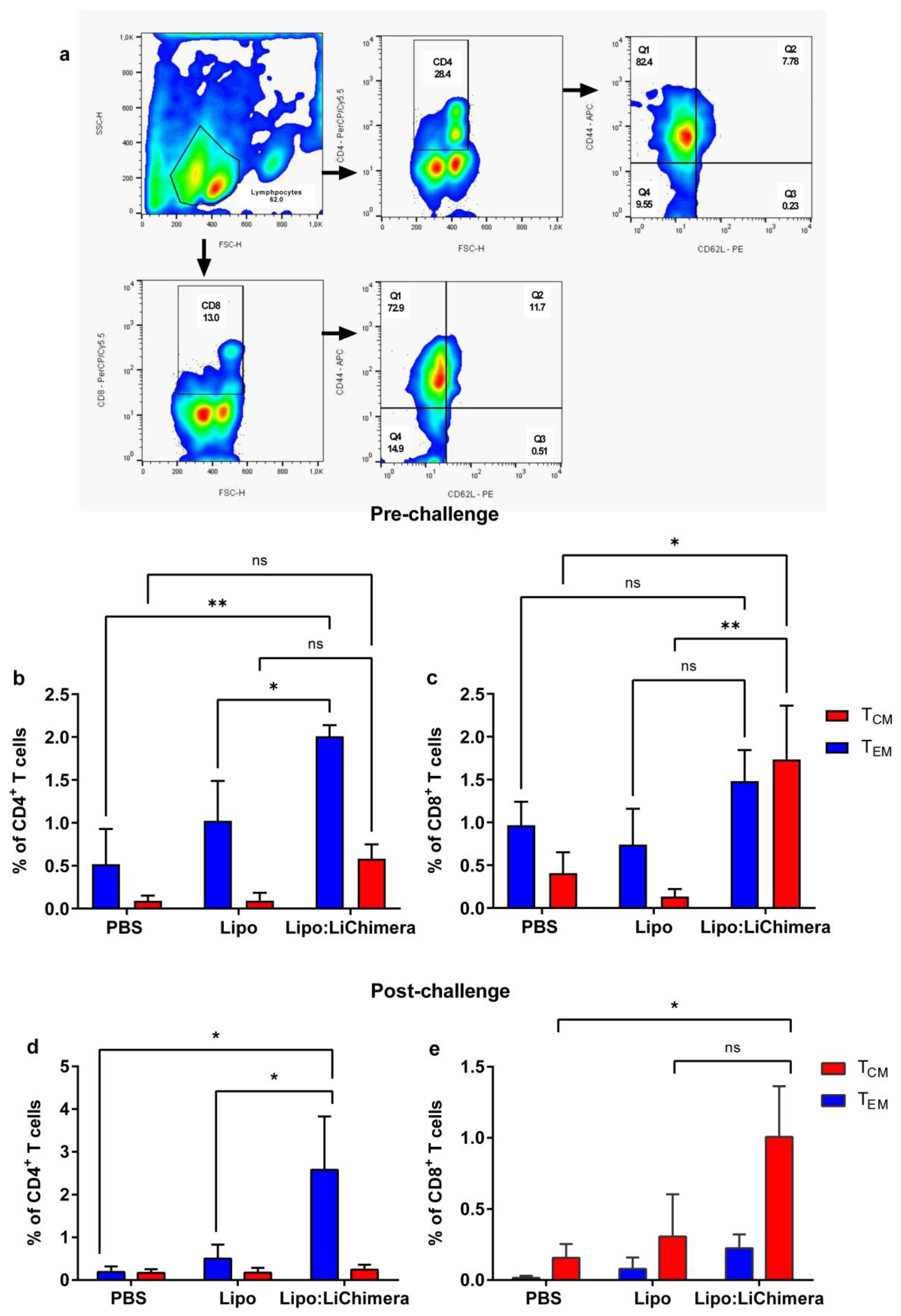
2.8. Detection of Antigen-Specific Memory and Multifunctional T cells
2.9. Statistical Analysis
3. Results
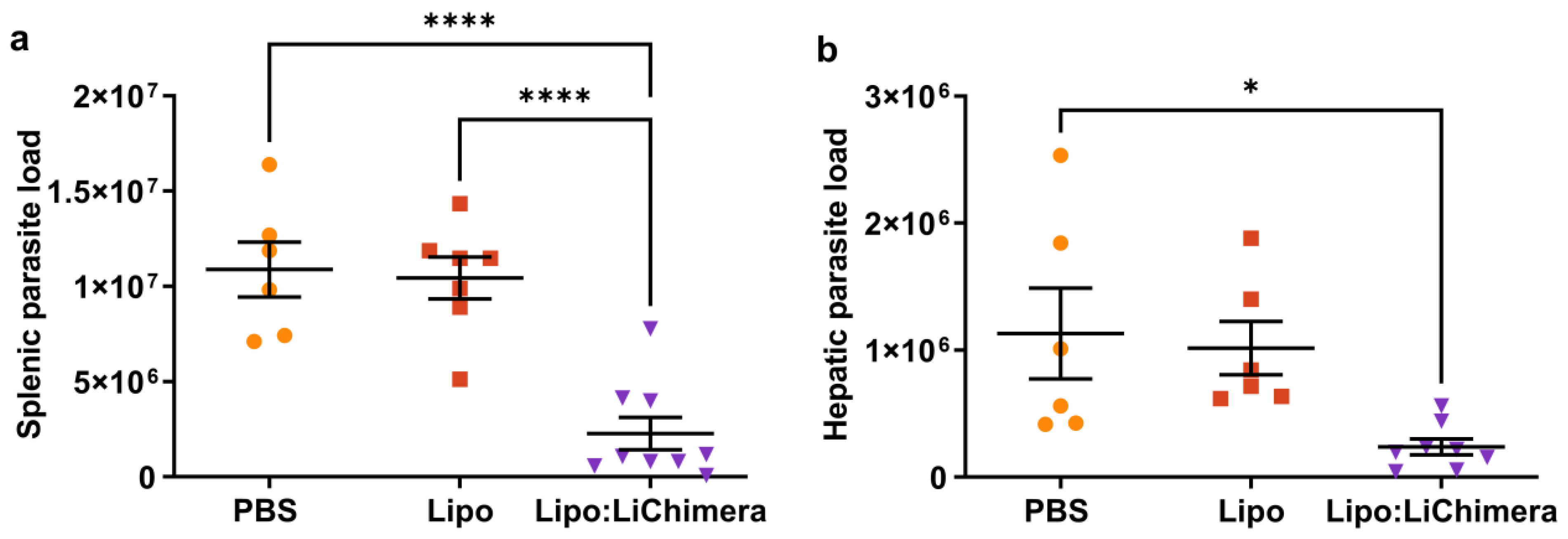
3.1. LiChimera Encapsulated in Liposomes Protects against L. infantum Challenge
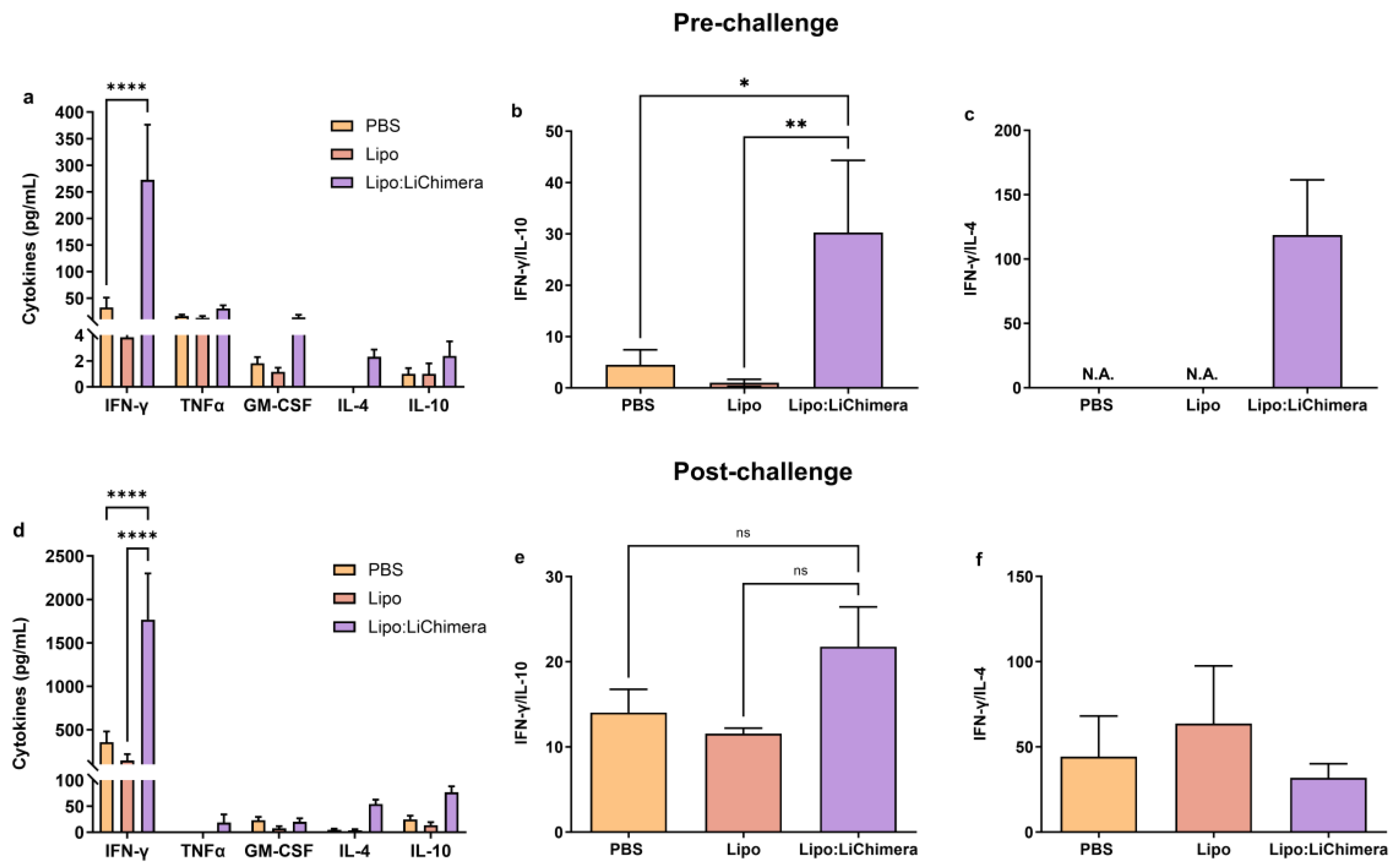
3.2. Lipo:LiChimera Immunization Protects against L. infantum Challenge via Maintenance of Antigen-Specific TH1-Biased Immune Response
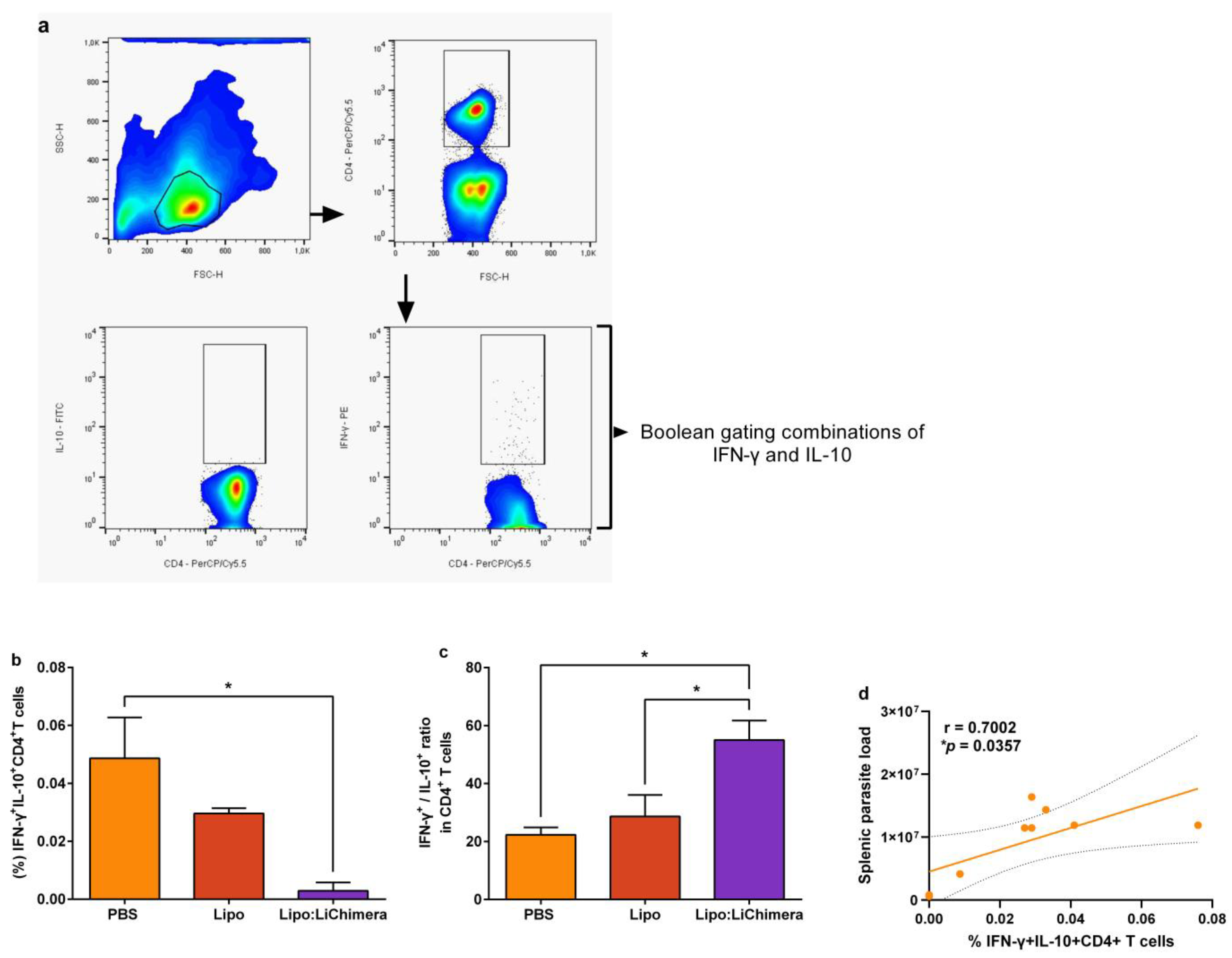
3.3. Lipo:LiChimera-Induced Protection Correlates with Decreased Numbers of Regulatory T Cells in the Spleen
3.4. Lipo:LiChimera Immunization Induces the Differentiation of Multifunctional CD4+ and CD8+ T Cells Immunization
3.5. Lipo:LiChimera Immunization Induces the Differentiation of Long-Lasting Memory CD4+ and CD8+ T Cell Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martinez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Nogueira, F.D.S.; Menz, I.; Tabanez, P.; da Silva, S.M.; Ribeiro, V.M.; Miro, G.; Cardoso, L.; Petersen, C.; Baneth, G.; et al. Vaccination against canine leishmaniasis in Brazil. Int. J. Parasitol. 2020, 50, 171–176. [Google Scholar] [CrossRef]
- Fernandez Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Maranon, F.; Fabra, M.; Gomez-Nieto, L.C.; Alonso, C. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend(R) against canine leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Khamesipour, A.; Dowlati, Y.; Asilian, A.; Hashemi-Fesharki, R.; Javadi, A.; Noazin, S.; Modabber, F. Leishmanization: Use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine 2005, 23, 3642–3648. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Bacellar, O.; Barral, A.; Badaro, R.; Johnson, W.D. Antigen-Specific Immunosuppression in Visceral Leishmaniasis Is Cell-Mediated. J. Clin. Investig. 1989, 83, 860–864. [Google Scholar] [CrossRef]
- Bacellar, O.; D’Oliveira, A., Jr.; Jeronimo, S.; Carvalho, E.M. IL-10 and IL-12 are the main regulatory cytokines in visceral leishmaniasis. Cytokine 2000, 12, 1228–1231. [Google Scholar] [CrossRef]
- Samant, M.; Sahu, U.; Pandey, S.C.; Khare, P. Role of Cytokines in Experimental and Human Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2021, 11, 624009. [Google Scholar] [CrossRef]
- Serafim, T.D.; Coutinho-Abreu, I.V.; Dey, R.; Kissinger, R.; Valenzuela, J.G.; Oliveira, F.; Kamhawi, S. Leishmaniasis: The act of transmission. Trends Parasitol. 2021, 37, 976–987. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef]
- Maroof, A.; Brown, N.; Smith, B.; Hodgkinson, M.R.; Maxwell, A.; Losch, F.O.; Fritz, U.; Walden, P.; Lacey, C.N.; Smith, D.F.; et al. Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J. Infect. Dis. 2012, 205, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, E.K.; Alkhaldi, A.A.M.; Saleh, A.A. Host immune response against leishmaniasis and parasite persistence strategies: A review and assessment of recent research. Biomed. Pharmacother. 2021, 139, 111671. [Google Scholar] [CrossRef] [PubMed]
- De Brito, R.C.F.; Cardoso, J.M.O.; Reis, L.E.S.; Vieira, J.F.; Mathias, F.A.S.; Roatt, B.M.; Aguiar-Soares, R.; Ruiz, J.C.; Resende, D.M.; Reis, A.B. Peptide Vaccines for Leishmaniasis. Front. Immunol. 2018, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Athanasiou, E.; Koutsoni, O.; Dotsika, E.; Karagouni, E. Experimental Validation of Multi-Epitope Peptides Including Promising MHC Class I- and II-Restricted Epitopes of Four Known Leishmania infantum Proteins. Front. Immunol. 2014, 5, 268. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, E.; Agallou, M.; Tastsoglou, S.; Kammona, O.; Hatzigeorgiou, A.; Kiparissides, C.; Karagouni, E. A Poly(Lactic-co-Glycolic) Acid Nanovaccine Based on Chimeric Peptides from Different Leishmania infantum Proteins Induces Dendritic Cells Maturation and Promotes Peptide-Specific IFNgamma-Producing CD8(+) T Cells Essential for the Protection against Experimental Visceral Leishmaniasis. Front. Immunol. 2017, 8, 684. [Google Scholar] [CrossRef]
- Alves-Silva, M.V.; Nico, D.; Morrot, A.; Palatnik, M.; Palatnik-de-Sousa, C.B. A Chimera Containing CD4+ and CD8+ T-Cell Epitopes of the Leishmania donovani Nucleoside Hydrolase (NH36) Optimizes Cross-Protection against Leishmania amazonesis Infection. Front. Immunol. 2017, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.T.; Duarte, M.C.; Lage, D.P.; Costa, L.E.; Carvalho, A.M.; Mendes, T.A.; Roatt, B.M.; Menezes-Souza, D.; Soto, M.; Coelho, E.A. A recombinant chimeric protein composed of human and mice-specific CD4(+) and CD8(+) T-cell epitopes protects against visceral leishmaniasis. Parasite Immunol. 2017, 39, e12359. [Google Scholar] [CrossRef]
- Lage, D.P.; Ribeiro, P.A.F.; Dias, D.S.; Mendonca, D.V.C.; Ramos, F.F.; Carvalho, L.M.; de Oliveira, D.; Steiner, B.T.; Martins, V.T.; Perin, L.; et al. A candidate vaccine for human visceral leishmaniasis based on a specific T cell epitope-containing chimeric protein protects mice against Leishmania infantum infection. NPJ Vaccines 2020, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Badiee, A.; Heravi Shargh, V.; Khamesipour, A.; Jaafari, M.R. Micro/nanoparticle adjuvants for antileishmanial vaccines: Present and future trends. Vaccine 2013, 31, 735–749. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Jaafari, M.R.; Khamesipour, A.; Badiee, A. Liposomal adjuvant development for leishmaniasis vaccines. Ther. Adv. Vaccines 2017, 5, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, L.; Doherty, T.M.; Korsholm, K.S.; Andersen, P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect. Immun. 2004, 72, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.; Elhay, M.; Rosenkrands, I.; Lindblad, E.B.; Andersen, P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 2000, 68, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, K.A.; Marasini, N.; Giddam, A.K.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. Liposome-based intranasal delivery of lipopeptide vaccine candidates against group A streptococcus. Acta Biomater. 2016, 41, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Aibani, N.; Patel, P.; Buchanan, R.; Strom, S.; Wasan, K.M.; Hancock, R.E.W.; Gerdts, V.; Wasan, E.K. Assessing the In Vivo Effectiveness of Cationic Lipid Nanoparticles with a Triple Adjuvant for Intranasal Vaccination against the Respiratory Pathogen Bordetella pertussis. Mol. Pharm. 2022, 19, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.Z.; Bartlett, S.; Hussein, W.M.; Lu, L.; Wright, Q.; Huang, W.; Nahar, U.J.; Yang, J.; Khongkow, M.; Veitch, M.; et al. Liposomal Formulations of a Polyleucine-Antigen Conjugate as Therapeutic Vaccines against Cervical Cancer. Pharmaceutics 2023, 15, 602. [Google Scholar] [CrossRef]
- Korsholm, K.S. One does not fit all: New adjuvants are needed and vaccine formulation is critical. Expert Rev. Vaccines 2011, 10, 45–48. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Maji, M.; Mazumder, S.; Bhattacharya, S.; Choudhury, S.T.; Sabur, A.; Shadab, M.; Bhattacharya, P.; Ali, N. A Lipid Based Antigen Delivery System Efficiently Facilitates MHC Class-I Antigen Presentation in Dendritic Cells to Stimulate CD8(+) T Cells. Sci. Rep. 2016, 6, 27206. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Ravindran, R.; Banerjee, A.; Ali, N. Non-coding pDNA bearing immunostimulatory sequences co-entrapped with leishmanial antigens in cationic liposomes elicits almost complete protection against experimental visceral leishmaniasis in BALB/c mice. Vaccine 2007, 25, 8771–8781. [Google Scholar] [CrossRef]
- Bhowmick, S.; Ravindran, R.; Ali, N. Leishmanial antigens in liposomes promote protective immunity and provide immunotherapy against visceral leishmaniasis via polarized Th1 response. Vaccine 2007, 25, 6544–6556. [Google Scholar] [CrossRef]
- Bhowmick, S.; Ravindran, R.; Ali, N. gp63 in stable cationic liposomes confers sustained vaccine immunity to susceptible BALB/c mice infected with Leishmania donovani. Infect. Immun. 2008, 76, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Sabur, A.; Bhowmick, S.; Chhajer, R.; Ejazi, S.A.; Didwania, N.; Asad, M.; Bhattacharyya, A.; Sinha, U.; Ali, N. Liposomal Elongation Factor-1alpha Triggers Effector CD4 and CD8 T Cells for Induction of Long-Lasting Protective Immunity against Visceral Leishmaniasis. Front. Immunol. 2018, 9, 18. [Google Scholar] [CrossRef]
- Agallou, M.; Athanasiou, E.; Samiotaki, M.; Panayotou, G.; Karagouni, E. Identification of Immunoreactive Leishmania infantum Protein Antigens to Asymptomatic Dog Sera through Combined Immunoproteomics and Bioinformatics Analysis. PLoS ONE 2016, 11, e0149894. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sisodia, B.; Misra, P.; Sundar, S.; Shasany, A.K.; Dube, A. Proteome mapping of overexpressed membrane-enriched and cytosolic proteins in sodium antimony gluconate (SAG) resistant clinical isolate of Leishmania donovani. Br. J. Clin. Pharmacol. 2010, 70, 609–617. [Google Scholar] [CrossRef]
- Matrangolo, F.S.; Liarte, D.B.; Andrade, L.C.; de Melo, M.F.; Andrade, J.M.; Ferreira, R.F.; Santiago, A.S.; Pirovani, C.P.; Silva-Pereira, R.A.; Murta, S.M. Comparative proteomic analysis of antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum chagasi lines. Mol. Biochem. Parasitol. 2013, 190, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.L.; Pescher, P.; MacDonald, A.; Hem, S.; Zander, D.; Retzlaff, S.; Blisnick, T.; Rotureau, B.; Rosenqvist, H.; Wiese, M.; et al. The Leishmania donovani chaperone cyclophilin 40 is essential for intracellular infection independent of its stage-specific phosphorylation status. Mol. Microbiol. 2014, 93, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, M.C.; Bourassa, S.; Legare, D.; Poirier, G.G.; Droit, A.; Ouellette, M. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Roberts, S.; Ullman, B.; Madhubala, R. A quantitative proteomic screen to identify potential drug resistance mechanism in alpha-difluoromethylornithine (DFMO) resistant Leishmania donovani. J. Proteom. 2014, 102, 44–59. [Google Scholar] [CrossRef]
- Kumar, A.; Samant, M.; Misra, P.; Khare, P.; Sundar, S.; Garg, R.; Dube, A. Immunostimulatory potential and proteome profiling of Leishmania donovani soluble exogenous antigens. Parasite Immunol. 2015, 37, 368–375. [Google Scholar] [CrossRef]
- Braga, M.S.; Neves, L.X.; Campos, J.M.; Roatt, B.M.; de Oliveira Aguiar Soares, R.D.; Braga, S.L.; de Melo Resende, D.; Reis, A.B.; Castro-Borges, W. Shotgun proteomics to unravel the complexity of the Leishmania infantum exoproteome and the relative abundance of its constituents. Mol. Biochem. Parasitol. 2014, 195, 43–53. [Google Scholar] [CrossRef]
- Cuervo, P.; De Jesus, J.B.; Saboia-Vahia, L.; Mendonca-Lima, L.; Domont, G.B.; Cupolillo, E. Proteomic characterization of the released/secreted proteins of Leishmania (Viannia) braziliensis promastigotes. J. Proteom. 2009, 73, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Lemesre, J.L.; Holzmuller, P.; Goncalves, R.B.; Bourdoiseau, G.; Hugnet, C.; Cavaleyra, M.; Papierok, G. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: Double-blind randomised efficacy field trial. Vaccine 2007, 25, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, V.; Kushawaha, P.K.; Tripathi, C.P.; Joshi, S.; Sahasrabuddhe, A.A.; Mitra, K.; Sundar, S.; Siddiqi, M.I.; Dube, A. Characterization of glycolytic enzymes—rAldolase and rEnolase of Leishmania donovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis. PLoS ONE 2014, 9, e86073. [Google Scholar] [CrossRef] [PubMed]
- Keerti; Yadav, N.K.; Joshi, S.; Ratnapriya, S.; Sahasrabuddhe, A.A.; Dube, A. Immunotherapeutic potential of Leishmania (Leishmania) donovani Th1 stimulatory proteins against experimental visceral leishmaniasis. Vaccine 2018, 36, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.T.O.; Martins, V.T.; Lage, D.P.; Costa, L.E.; Salles, B.C.S.; Carvalho, A.; Dias, D.S.; Ribeiro, P.A.F.; Chavez-Fumagalli, M.A.; Machado-de-Avila, R.A.; et al. Probing the efficacy of a heterologous Leishmania/L. Viannia braziliensis recombinant enolase as a candidate vaccine to restrict the development of L. infantum in BALB/c mice. Acta Trop. 2017, 171, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gomes, G.M.; Rodrigues, A.; Teixeira, F.; Carreira, J.; Alexandre-Pires, G.; Carvalho, S.; Santos-Mateus, D.; Martins, C.; Vale-Gato, I.; Marques, C.; et al. Immunization with the Leishmania infantum recombinant cyclophilin protein 1 confers partial protection to subsequent parasite infection and generates specific memory T cells. Vaccine 2014, 32, 1247–1253. [Google Scholar] [CrossRef]
- Agallou, M.; Margaroni, M.; Kotsakis, S.D.; Karagouni, E. A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania infantum. Vaccines 2020, 8, 350. [Google Scholar] [CrossRef]
- Agallou, M.; Margaroni, M.; Tsanaktsidou, E.; Badounas, F.; Kammona, O.; Kiparissides, C.; Karagouni, E. A liposomal vaccine promotes strong adaptive immune responses via dendritic cell activation in draining lymph nodes. J. Control. Release Off. J. Control. Release Soc. 2023, 356, 386–401. [Google Scholar] [CrossRef]
- Agallou, M.; Margaroni, M.; Athanasiou, E.; Toubanaki, D.K.; Kontonikola, K.; Karidi, K.; Kammona, O.; Kiparissides, C.; Karagouni, E. Identification of BALB/c Immune Markers Correlated with a Partial Protection to Leishmania infantum after Vaccination with a Rationally Designed Multi-epitope Cysteine Protease A Peptide-Based Nanovaccine. PLoS Negl. Trop. Dis. 2017, 11, e0005311. [Google Scholar] [CrossRef]
- Nylen, S.; Maurya, R.; Eidsmo, L.; Manandhar, K.D.; Sundar, S.; Sacks, D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J. Exp. Med. 2007, 204, 805–817. [Google Scholar] [CrossRef]
- Silvestre, R.; Cordeiro-Da-Silva, A.; Santarem, N.; Vergnes, B.; Sereno, D.; Ouaissi, A. SIR2-deficient Leishmania infantum induces a defined IFN-gamma/IL-10 pattern that correlates with protection. J. Immunol. 2007, 179, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Flores-Garcia, Y.; Rosales-Encina, J.L.; Satoskar, A.R.; Talamas-Rohana, P. IL-10-IFN-gamma double producers CD4+ T cells are induced by immunization with an amastigote stage specific derived recombinant protein of Trypanosoma cruzi. Int. J. Biol. Sci. 2011, 7, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Mege, J.L.; Meghari, S.; Honstettre, A.; Capo, C.; Raoult, D. The two faces of interleukin 10 in human infectious diseases. Lancet. Infect. Dis. 2006, 6, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, C.M.; Shen, H.; Pearce, E.J. Memory CD4 T cells enhance primary CD8 T-cell responses. Infect. Immun. 2007, 75, 3556–3560. [Google Scholar] [CrossRef]
- Shedlock, D.J.; Shen, H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003, 300, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Ramsburg, E.A.; Publicover, J.M.; Coppock, D.; Rose, J.K. Requirement for CD4 T cell help in maintenance of memory CD8 T cell responses is epitope dependent. J. Immunol. 2007, 178, 6350–6358. [Google Scholar] [CrossRef] [PubMed]
- De Brito, R.C.F.; Cardoso, J.M.O.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.O.; Teixeira-Carvalho, A.; Roatt, B.M.; Correa-Oliveira, R.; Ruiz, J.C.; Resende, D.M.; et al. Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice. Vaccines 2019, 7, 162. [Google Scholar] [CrossRef]
- Hofmeyer, K.A.; Duthie, M.S.; Laurance, J.D.; Favila, M.A.; Van Hoeven, N.; Coler, R.N.; Reed, S.G. Optimizing Immunization Strategies for the Induction of Antigen-Specific CD4 and CD8 T Cell Responses for Protection against Intracellular Parasites. Clin. Vaccine Immunol. CVI 2016, 23, 785–794. [Google Scholar] [CrossRef]
- Provine, N.M.; Larocca, R.A.; Penaloza-MacMaster, P.; Borducchi, E.N.; McNally, A.; Parenteau, L.R.; Kaufman, D.R.; Barouch, D.H. Longitudinal requirement for CD4+ T cell help for adenovirus vector-elicited CD8+ T cell responses. J. Immunol. 2014, 192, 5214–5225. [Google Scholar] [CrossRef]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Bhatia, A.; Raman, V.S.; Vidal, S.E.; Bertholet, S.; Coler, R.N.; Howard, R.F.; Reed, S.G. Leishmania infantum sterol 24-c-methyltransferase formulated with MPL-SE induces cross-protection against L. major infection. Vaccine 2009, 27, 2884–2890. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Kirman, J.R.; Rotte, M.J.; Davey, D.F.; Perfetto, S.P.; Rhee, E.G.; Freidag, B.L.; Hill, B.J.; Douek, D.C.; Seder, R.A. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 2002, 3, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Zaph, C.; Uzonna, J.; Beverley, S.M.; Scott, P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 2004, 10, 1104–1110. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Li, Y.; Millott, S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J. Immunol. 1990, 145, 4306–4310. [Google Scholar] [CrossRef]
- Bogdan, C.; Moll, H.; Solbach, W.; Rollinghoff, M. Tumor necrosis factor-alpha in combination with interferon-gamma, but not with interleukin 4 activates murine macrophages for elimination of Leishmania major amastigotes. Eur. J. Immunol. 1990, 20, 1131–1135. [Google Scholar] [CrossRef]
- Anderson, C.F.; Oukka, M.; Kuchroo, V.J.; Sacks, D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 2007, 204, 285–297. [Google Scholar] [CrossRef]
- Stager, S.; Maroof, A.; Zubairi, S.; Sanos, S.L.; Kopf, M.; Kaye, P.M. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur. J. Immunol. 2006, 36, 1764–1771. [Google Scholar] [CrossRef]
- Ranatunga, D.; Hedrich, C.M.; Wang, F.; McVicar, D.W.; Nowak, N.; Joshi, T.; Feigenbaum, L.; Grant, L.R.; Stager, S.; Bream, J.H. A human IL10 BAC transgene reveals tissue-specific control of IL-10 expression and alters disease outcome. Proc. Natl. Acad. Sci. USA 2009, 106, 17123–17128. [Google Scholar] [CrossRef] [PubMed]
- Resende, M.; Moreira, D.; Augusto, J.; Cunha, J.; Neves, B.; Cruz, M.T.; Estaquier, J.; Cordeiro-da-Silva, A.; Silvestre, R. Leishmania-infected MHC class IIhigh dendritic cells polarize CD4+ T cells toward a nonprotective T-bet+ IFN-gamma+ IL-10+ phenotype. J. Immunol. 2013, 191, 262–273. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agallou, M.; Margaroni, M.; Karagouni, E. Intramuscular Immunization with a Liposomal Multi-Epitope Chimeric Protein Induces Strong Cellular Immune Responses against Visceral Leishmaniasis. Vaccines 2023, 11, 1384. https://doi.org/10.3390/vaccines11081384
Agallou M, Margaroni M, Karagouni E. Intramuscular Immunization with a Liposomal Multi-Epitope Chimeric Protein Induces Strong Cellular Immune Responses against Visceral Leishmaniasis. Vaccines. 2023; 11(8):1384. https://doi.org/10.3390/vaccines11081384
Chicago/Turabian StyleAgallou, Maria, Maritsa Margaroni, and Evdokia Karagouni. 2023. "Intramuscular Immunization with a Liposomal Multi-Epitope Chimeric Protein Induces Strong Cellular Immune Responses against Visceral Leishmaniasis" Vaccines 11, no. 8: 1384. https://doi.org/10.3390/vaccines11081384
APA StyleAgallou, M., Margaroni, M., & Karagouni, E. (2023). Intramuscular Immunization with a Liposomal Multi-Epitope Chimeric Protein Induces Strong Cellular Immune Responses against Visceral Leishmaniasis. Vaccines, 11(8), 1384. https://doi.org/10.3390/vaccines11081384






