Safety and Protective Efficacy of a Candidate Vector-Based Vaccine for Bovine Tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine
- -
- Three FLU NS_ESAT 6 and TB10.4 pilot batches containing 15% Montanide Gel adjuvant (Seppic, France);
- -
- Three FLU NS_ESAT 6 and TB10.4 pilot batches without adjuvant.
2.2. Bacterial Strain
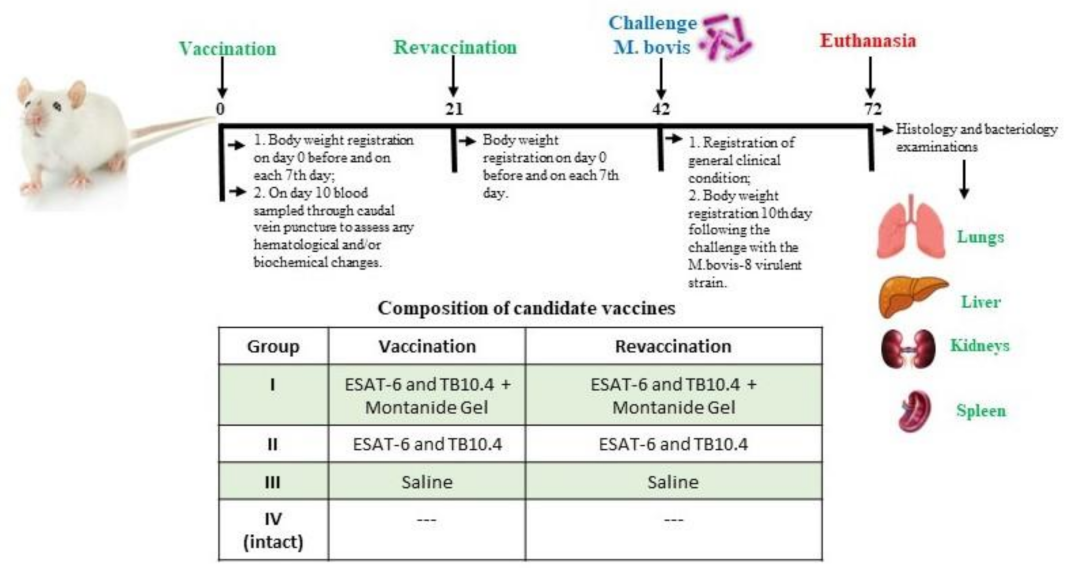
2.3. Animal Studies
2.4. Safety Studies
2.5. Protection Studies
2.6. Blood Test
2.7. Bacteriology
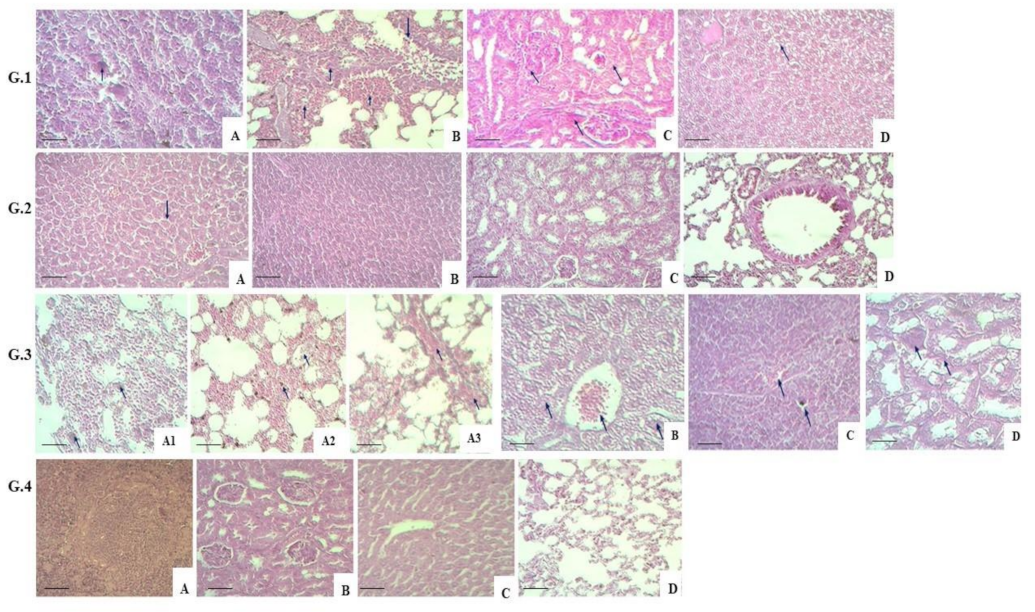
2.8. Histology Tests
2.9. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Refaya, A.K.; Bhargavi, G.; Mathew, N.C.; Rajendran, A.; Krishnamoorthy, R.; Swaminathan, S.; Palaniyandi, K. A review on bovine tuberculosis in India. Tuberculosis 2020, 122, 101923. [Google Scholar] [CrossRef]
- Barandiaran, S.; Martínez Vivot, M.; Pérez, A.M.; Cataldi, A.A.; Zumárraga, M.J. Bovine tuberculosis in domestic pigs: Genotyping and distribution of isolates in Argentina. Res. Vet. Sci. 2015, 103, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fernando Cardoso-Toset, F.; Inmaculada, L.; Librado, C.; Jurado-Martos, F.; Risalde, M.A.; Venteo, A.; Infantes-Lorenzo, J.A.; Bezos, J.; Rueda, P.; Tapia, I.; et al. Evaluation of five serologic assays for bovine tuberculosis surveillance in domestic free-range pigs from southern Spain. Prev. Vet. Med. 2017, 137, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Buddle, B.M.; Livingstone, P.G.; de Lisle, G.W. Advances in ante-mortem diagnosis of tuberculosis in cattle. N. Z. Vet. J. 2009, 57, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Smolonogina, T.A.; Isakova-Sivak, I.N.; Kotomina, T.S.; Evsina, A.S.; Stepanova, E.A.; Prokopenko, P.I.; Leontieva, G.F.; Suvorov, A.N.; Rudenko, L.G. Generation of a vector vaccine against group B streptococcal infection on the base of a cold-adapted influenza A virus. Mol. Gen. Microbiol. Virol. 2019, 37, 25–34. [Google Scholar] [CrossRef]
- Sergeeva, M.V.; Pulkina, A.A.; Vasiliev, K.A.; Romanovskaya-Romanko, E.A.; Komissarov, A.B.; Kuchur, O.A.; Egorov, A.Y.; Tsybalova, L.M.; Stukova, M.A. Safety and immunogenicity of cold-adapted recombinant influenza vector expressing ESAT-6 and Ag85A antigens of M. tuberculosis. Probl. Virol. 2017, 62, 266–272. [Google Scholar] [CrossRef]
- Hoffmann, E.; Neumann, G.; Kawaoka, Y.; Hobom, G.; Webster, R.G. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 2000, 97, 6108–6113. [Google Scholar] [CrossRef]
- Ware, J.H.; Sanzari, J.; Avery, S.; Sayers, C.; Krigsfeld, G.; Nuth, M.; Wan, X.S.; Rusek, A.; Kennedy, A.R. Effects of proton radiation dose, dose rate and dose fractionation on hematopoietic cells in mice. Radiat. Res. 2010, 174, 325–330. [Google Scholar] [CrossRef]
- Maks, C.J.; Wan, X.S.; Ware, J.H.; Romero-Weaver, A.L.; Sanzari, J.K.; Wilson, J.M.; Rightnar, S.; Wroe, A.J.; Koss, P.; Gridley, D.S.; et al. Analysis of white blood cell counts in mice after gamma- or proton-radiation exposure. Radiat Res. 2011, 176, 170–176. [Google Scholar] [CrossRef]
- Veloso, A.G.B.; Lima, N.E.A.; de Marco Ornelas, E.; Cardoso, C.G.; Marques, M.R.; da Costa Aguiar Alves Reis, B.; Fonseca, F.L.A.; Maifrino, L.B.M. Effects of moderate exercise on biochemical, morphological, and physiological parameters of the pancreas of female mice with estrogen deprivation and dyslipidemia. Med. Mol. Morphol. 2018, 51, 118–127. [Google Scholar] [CrossRef]
- Lyubina, A.Y.; Ilyicheva, L.P.; Katasonova, S.A.; Petrosova, S.A. Clinical Laboratory Studies; Medicine: Moscow, Russia, 1984; pp. 63–69. [Google Scholar]
- Skinner, M.A.; Wedlock, D.N.; de Lisle, G.W.; Cooke, M.M.; Tascon, R.E.; Ferraz, J.C.; Lowrie, D.B.; Vordermeier, H.M.; Hewinson, R.G.; Buddle, B.M. The order of prime-boost vaccination of neonatal calves with Mycobacterium bovis BCG and a DNA vaccine encoding mycobacterial proteins Hsp65, Hsp70, and Apa is not critical for enhancing protection against bovine tuberculosis. Infect. Immun. 2005, 73, 41–44. [Google Scholar] [CrossRef]
- Wedlock, D.N.; Denis, M.; Painter, G.F.; Ainge, G.D.; Vordermeier, H.M.; Hewinson, R.G.; Buddle, B.M. Enhanced protection against bovine tuberculosis after coadministration of Mycobacterium bovis BCG with a mycobacterial protein vaccine-adjuvant combination but not after coadministration of adjuvant alone. Clin. Vaccine Immunol. 2008, 15, 65–72. [Google Scholar] [CrossRef]
- Wang, J.; Thorson, L.; Stokes, R.W.; Santosuosso, M.; Huygen, K.; Zganiacz, A.; Hitt, M.; Xing, Z. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 2004, 173, 6357–6365. [Google Scholar] [CrossRef]
- Müller, B.; Dürr, S.; Alonso, S.; Hattendorf, J.; Laisse, C.J.M.; Parsons, S.D.C.; van Helden, P.D.; Zinsstag, J. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg. Infect. Dis. 2013, 19, 899–908. [Google Scholar] [CrossRef]
- Sereinig, S.; Stukova, M.; Zabolotnyh, N.; Ferko, B.; Kittel, C.; Romanova, J.; Vinogradova, T.; Katinger, H.; Kiselev, O.; Egorov, A. Influenza virus NS vectors expressing the Mycobacterium tuberculosis ESAT-6 protein induce CD4+ Th1 immune response and protect animals against tuberculosis challenge. Clin. Vaccine Immunol. 2006, 13, 898–904. [Google Scholar] [CrossRef]
- Wedlock, D.N.; Aldwell, F.E.; Vordermeier, H.M.; Hewinson, R.G.; Buddle, B.M. Protection against bovine tuberculosis induced by oral vaccination of cattle with Mycobacterium bovis BCG is not enhanced by co-administration of mycobacterial protein vaccines. Vet. Immunol. Immunopathol. 2011, 144, 20–27. [Google Scholar] [CrossRef]
- Skinner, M.A.; Buddle, B.M.; Wedlock, D.N.; Keen, D.; de Lisle, G.W. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 2003, 71, 4901–4907. [Google Scholar] [CrossRef]
- Blanco, F.C.; Bianco, M.V.; Garbaccio, S.; Meikle, V.; Gravisaco, M.J. Mycobacterium bovis ∆mce2 double deletion mutant protects cattle against challenge with virulent M. bovis. Tuberculosis 2013, 93, 63–72. [Google Scholar] [CrossRef] [PubMed]
- de Val, B.P.; Vidal, E.; Villarreal-Ramos, B.; Gilbert, S.C.; Andaluz, A. A multi-antigenic adenoviral-vectored vaccine improves BCG-induced protection of goats against pulmonary tuberculosis infection and prevents disease progression. PLoS ONE 2013, 8, 13–17. [Google Scholar]
- Buddle, B.M.; Skinner, M.A.; Wedlock, D.N.; Collins, D.M.; de Lisle, G.W. New generation vaccines and delivery systems for control of bovine tuberculosis in cattle and wildlife. Vet. Immunol. Immunopathol. 2002, 87, 77–85. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, X. Immunogenicity and therapeutic effects of Ag85A/B chimeric DNA vaccine in mice infected with Mycobacterium tuberculosis. FEMS Immunol. Med. 2012, 66, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Stukova, M.A.; Sereinig, S. Vaccine potential of influenza vectors expressing Mycobacterium tuberculosis ESAT-6 protein. Tuberculosis 2006, 86, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Buzitskaya, Z.; Stosman, K.; Khairullin, B.; Kassenov, M.; Nurpeisova, A.; Sansyzbay, A.; Shurygina, A.-P.; Aleksandrov, A.; Sivak, K.; Stukova, M. A new intranasal influenza vector-based vaccine TB/FLU-04L against tuberculosis: Preclinical safety studies. Drug Res. 2022, 72, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Pleschka, S.; Jaskunas, R.; Engelhardt, O.G.; Zürcher, T.; Palese, P.; García-Sastre, A. A plasmid-based reverse genetics system for influenza A virus. J. Virol. 1996, 70, 4188–4192. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Mondal, M.; Zhou, D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum. Vaccines Immunother. 2018, 14, 1679–1685. [Google Scholar] [CrossRef]
- Draper, S.J.; Heeney, J.L. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010, 8, 62–73. [Google Scholar] [CrossRef]
- Chambers, M.A.; Vordermeier, H.; Whelan, A.; Commander, N.; Tascon, R.; Lowrie, D.; Hewinson, R.G. Vaccination of mice and cattle with plasmid DNA encoding the Mycobacterium bovis antigen MPB83. Clin. Infect. Dis. 2000, 30, 283–287. [Google Scholar] [CrossRef]
- Kamal, R.P.; Katz, J.M.; York, I.A. Molecular determinants of influenza virus pathogenesis in mice. Curr. Top Microbiol. Immunol. 2014, 385, 243–274. [Google Scholar]
- Shurygina, A.P.; Zabolotnykh, N.; Vinogradova, T.; Khairullin, B.; Kassenov, M.; Nurpeisova, A.; Sarsenbayeva, G.; Sansyzbay, A.; Vasilyev, K.; Buzitskaya, J.; et al. Preclinical Evaluation of TB/FLU-04L-An Intranasal Influenza Vector-Based Boost Vaccine against Tuberculosis. Int. J. Mol. Sci. 2023, 24, 7439. [Google Scholar] [CrossRef]
- Shurygina, A.-P.; Buzitskaya, Z.; Stukova, M.; Khairullin, B.; Kassenov, M.; Nurpeysova, A.; Zabolotnyh, N.V.; Vinogradova, T. Pre-clinical evaluation of a replication-deficient intranasal influenza vector vaccine expressing two Mycobacterium antigens. In Proceedings of the 45th Union World Conference on Lung Health, Barcelona, Spain, 28 October–1 November 2014. [Google Scholar]
- Garnier, T.; Eiglmeier, K.; Camus, J.-C.; Medina, N.; Mansoor, H.; Pryor, M.; Duthoy, S.; Grondin, S.; Lacroix, C.; Monsempe, C.; et al. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. USA 2003, 100, 7877–7882. [Google Scholar] [CrossRef]
- Whelan, C.; Shuralev, E.; O’Keeffe, G.; Hyland, P.; Kwok, H.F.; Snoddy, P.; O’Brien, A.; Connolly, M.; Quinn, P.; Groll, M.; et al. Multiplex immunoassay for serological diagnosis of Mycobacterium bovis infection in cattle. Clin. Vaccines Immunol. 2008, 15, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Skjot, R.L.V.; Brock, I.; Arend, S.M.; Munk, M.E.; Theisen, M.; Ottenhoff, T.H.M.; Andersen, P. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 2002, 70, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jia, H.; Hou, S.; Zhang, G.; Xin, T.; Li, H.; Yuan, W.; Guo, X.; Gao, X.; Li, M.; et al. Recombinant TB10.4 of Mycobacterium bovis induces cytokine production in RAW264.7 macrophages through activation of the MAPK and NF-κB pathways via TLR2. Mol. Immunol. 2014, 62, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.L.; Nagai, S.; Houen, G.; Andersen, P.; Andersen, A.B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995, 63, 1710–1717. [Google Scholar] [CrossRef]

| Blood Parameters Studied | Study Groups | |||
|---|---|---|---|---|
| I | II | III | IV (Intact Group) | |
| Hemoglobin, g/dL | 40.00 ± 1.27 | 40.00 ± 6.66 | 40.00 ± 0.68 | 41.57 ± 1.23 |
| Hematocrit, % | 0.32 ± 0.03 | 0.38 ± 0.02 | 0.39 ± 0.02 | 0.34 ± 0.04 |
| Red blood cells, ×107 L | 4.0 ± 0.3 | 4.1 ±0.5 | 3.1 ± 0.2 | 3.4 ± 0.3 |
| Eosinophils, % | 1.2 ± 0.0 | 1.4 ± 0.1 | 1.5 ± 0.2 | 1.5 ± 0.3 |
| Monocytes, % | 7.7 ± 1.2 | 5.9 ± 1.2 | 5.3 ± 1.2 | 6.7 ± 1.2 |
| Lymphocytes, % | 47.3 ± 3.3 | 45.7 ± 1.3 | 42.7 ± 3.7 | 45.0 ± 4.0 |
| Blood Parameters Studied | Study Groups | |||
|---|---|---|---|---|
| I | II | III | IV (Intact Group) | |
| Total protein, g/L | 58.9 ± 4.4 | 48.0 ± 1.4 | 40.1 ± 5.7 | 52.1 ± 1.2 |
| Urea, mmol/L | 3.8 ± 0.1 | 3.6 ± 0.8 | 4.2 ± 1.4 | 5.6 ± 1.1 |
| Creatinine, umol/L | 52.3 ± 3.5 | 48.4 ± 4.1 | 39.9 ± 5.1 | 48.5 ± 2.3 |
| Glucose, mol/L | 5.0 ± 1.7 | 5.0 ± 1.9 | 5.5 ± 1.4 | 8.2 ± 2.2 |
| Cholesterol, total, mmol/L | 1.0 ± 0.8 | 1.0 ± 0.4 | 1.4 ± 0.3 | 0.7 ± 0.2 |
| Bilirubin, total, mmol/L | 0.004 ± 0.001 | 0.008 ± 0.001 | 0.008 ± 0.002 | 0.010 ± 0.004 |
| Bilirubin, conjugated, mmol/L | 0.003 ± 0.000 | 0.004 ± 0.000 | 0.003 ± 0.000 | 0.006 ± 0.001 |
| AST, mmol/L-s | 1.56 ± 0.13 | 1.25 ± 0.06 | 1.45 ± 0.32 | 1.92 ± 0.05 |
| ALT, mmol/L-s | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.31 ± 0.03 |
| Groups | Experiment Conditions | Log10 of the Number of Viable Bacteria in the Lungs | Protection Index (Log10) |
|---|---|---|---|
| I | ESAT-6 and TB10.4 + Montanide Gel | 2.39 ± 0.182 | +0.2 |
| II | ESAT-6 and TB10.4 | 1.97 ± 0.447 | +0.62 |
| III | Saline | 4.89 ± 0.044 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abay, Z.; Nurpeisova, A.; Shorayeva, K.; Sadikaliyeva, S.; Yespembetov, B.; Syrym, N.; Sarmykova, M.; Jekebekov, K.; Abitayev, R.; Tokkarina, G.; et al. Safety and Protective Efficacy of a Candidate Vector-Based Vaccine for Bovine Tuberculosis. Vaccines 2023, 11, 1199. https://doi.org/10.3390/vaccines11071199
Abay Z, Nurpeisova A, Shorayeva K, Sadikaliyeva S, Yespembetov B, Syrym N, Sarmykova M, Jekebekov K, Abitayev R, Tokkarina G, et al. Safety and Protective Efficacy of a Candidate Vector-Based Vaccine for Bovine Tuberculosis. Vaccines. 2023; 11(7):1199. https://doi.org/10.3390/vaccines11071199
Chicago/Turabian StyleAbay, Zhandos, Ainur Nurpeisova, Kamshat Shorayeva, Sandugash Sadikaliyeva, Bolat Yespembetov, Nazym Syrym, Makhpal Sarmykova, Kuanysh Jekebekov, Ruslan Abitayev, Gaukhar Tokkarina, and et al. 2023. "Safety and Protective Efficacy of a Candidate Vector-Based Vaccine for Bovine Tuberculosis" Vaccines 11, no. 7: 1199. https://doi.org/10.3390/vaccines11071199
APA StyleAbay, Z., Nurpeisova, A., Shorayeva, K., Sadikaliyeva, S., Yespembetov, B., Syrym, N., Sarmykova, M., Jekebekov, K., Abitayev, R., Tokkarina, G., Kalimolda, E., Absatova, Z., Moldagulova, S., Yoo, H. S., Kassenov, M., Zakarya, K., & Abduraimov, Y. (2023). Safety and Protective Efficacy of a Candidate Vector-Based Vaccine for Bovine Tuberculosis. Vaccines, 11(7), 1199. https://doi.org/10.3390/vaccines11071199








