A Recombinant Chimera Vaccine Composed of LTB and Mycoplasma hyopneumoniae Antigens P97R1, mhp390 and P46 Elicits Cellular Immunologic Response in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Cultivation Conditions, and Genomic DNA Extraction
2.2. Primers, Amplification of Coding Sequences, and Site-Directed Mutagenesis
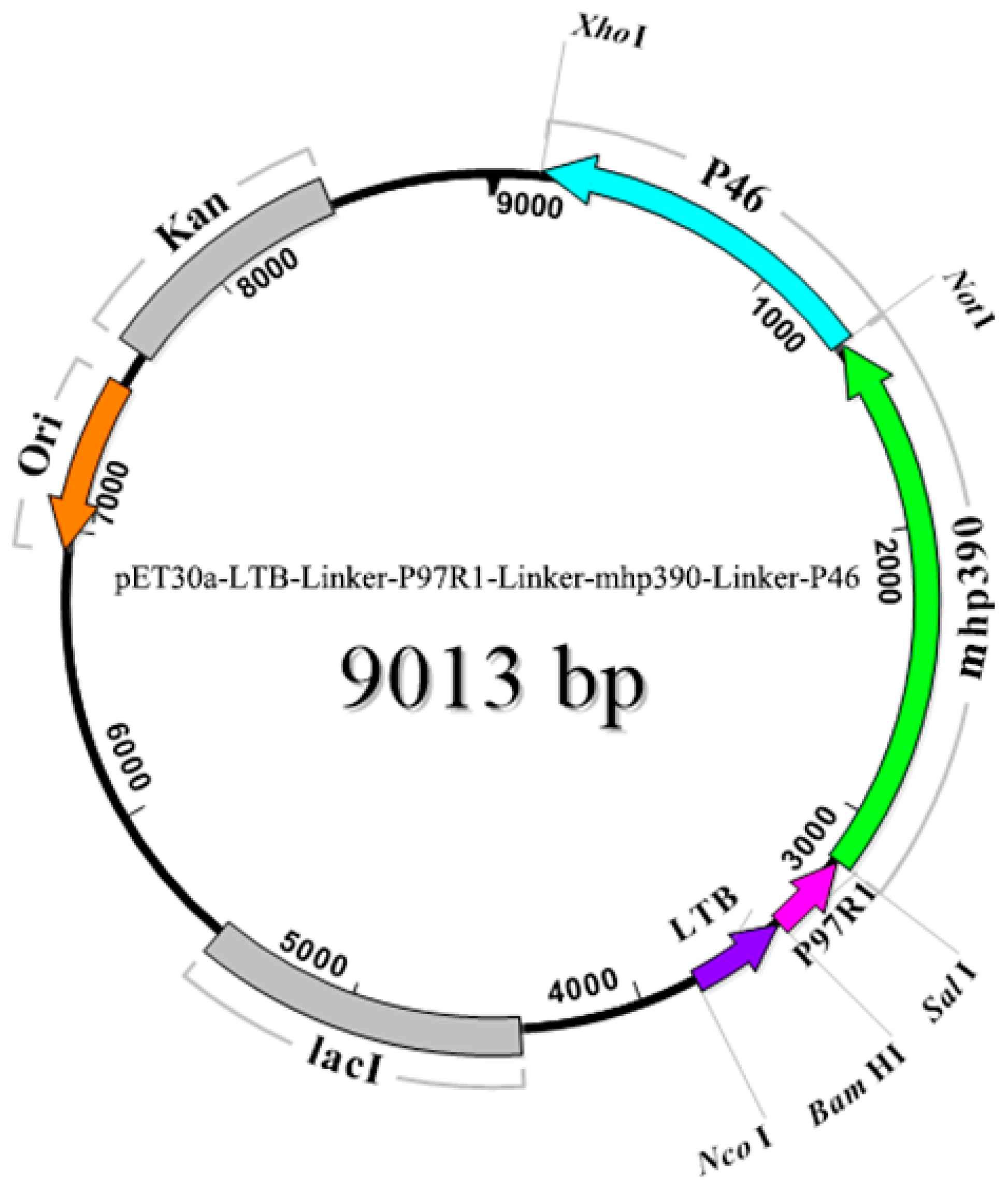
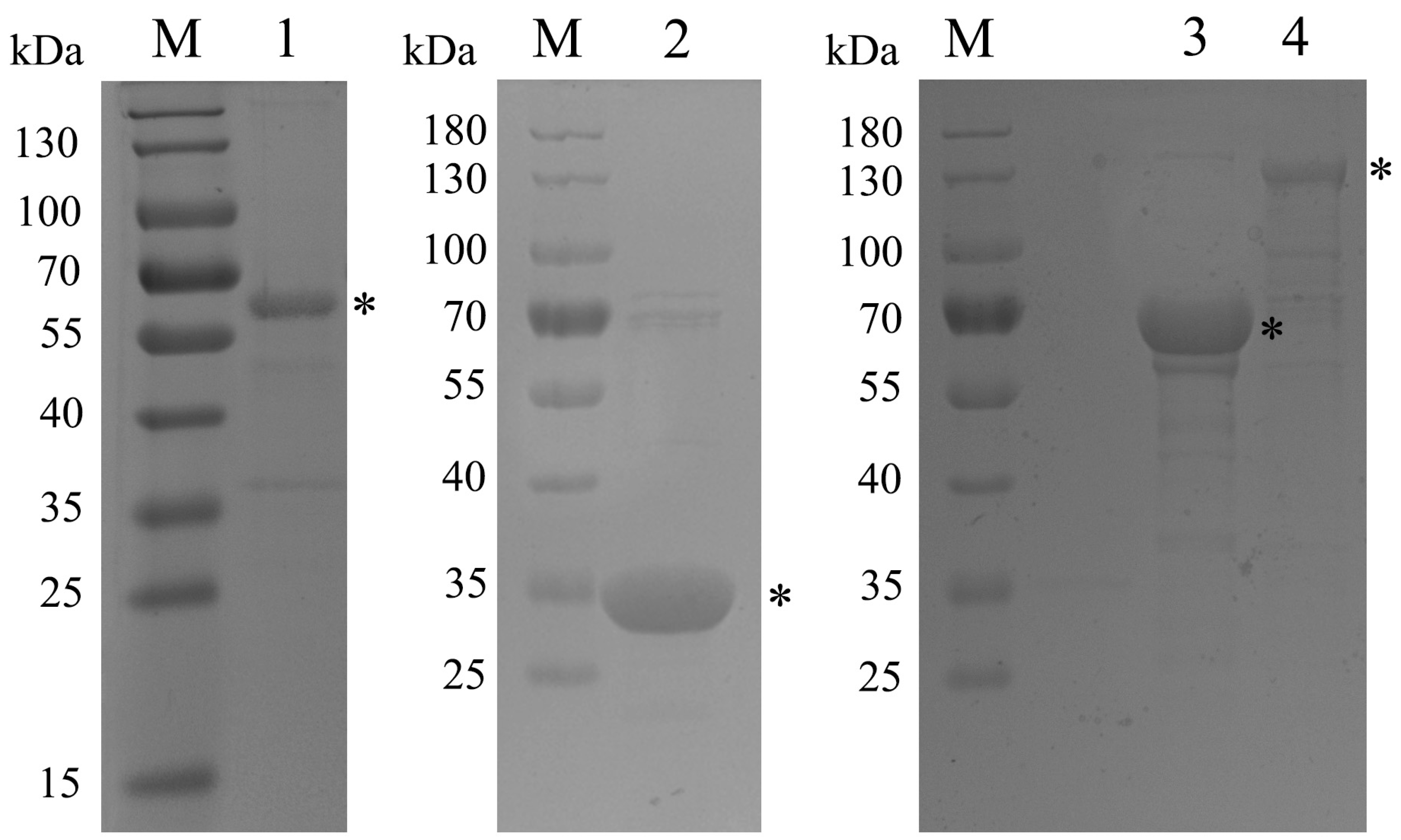
2.3. Expression, Characterization, and Purification of Recombinant Proteins
2.4. Experimental Design and Immunization Routes
2.5. Assessment of the Humoral Immune Response via Indirect ELISA Utilizing M. hyopneumoniae Extract
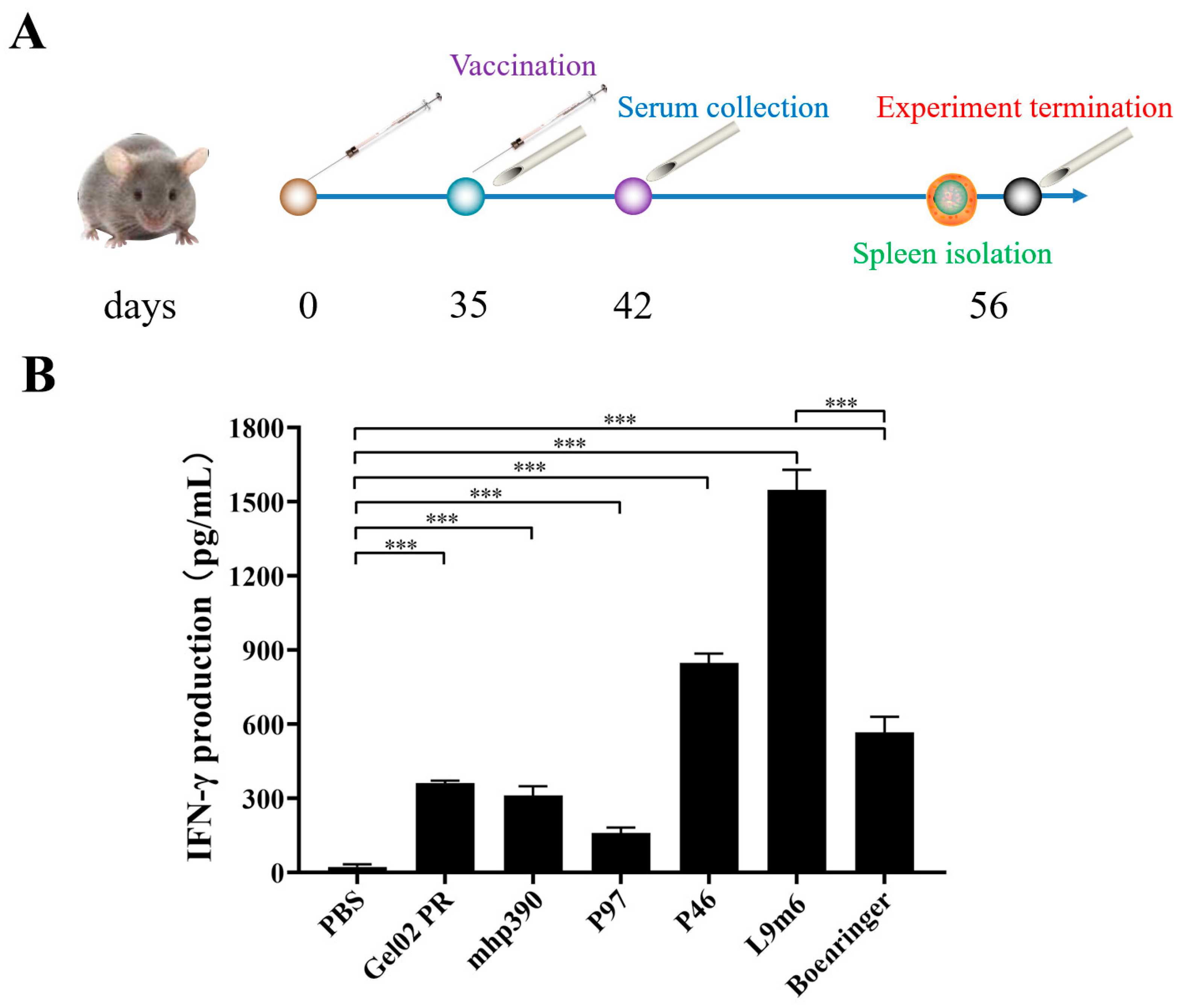
2.6. Quantification of IFN-γ Expression via ELISA
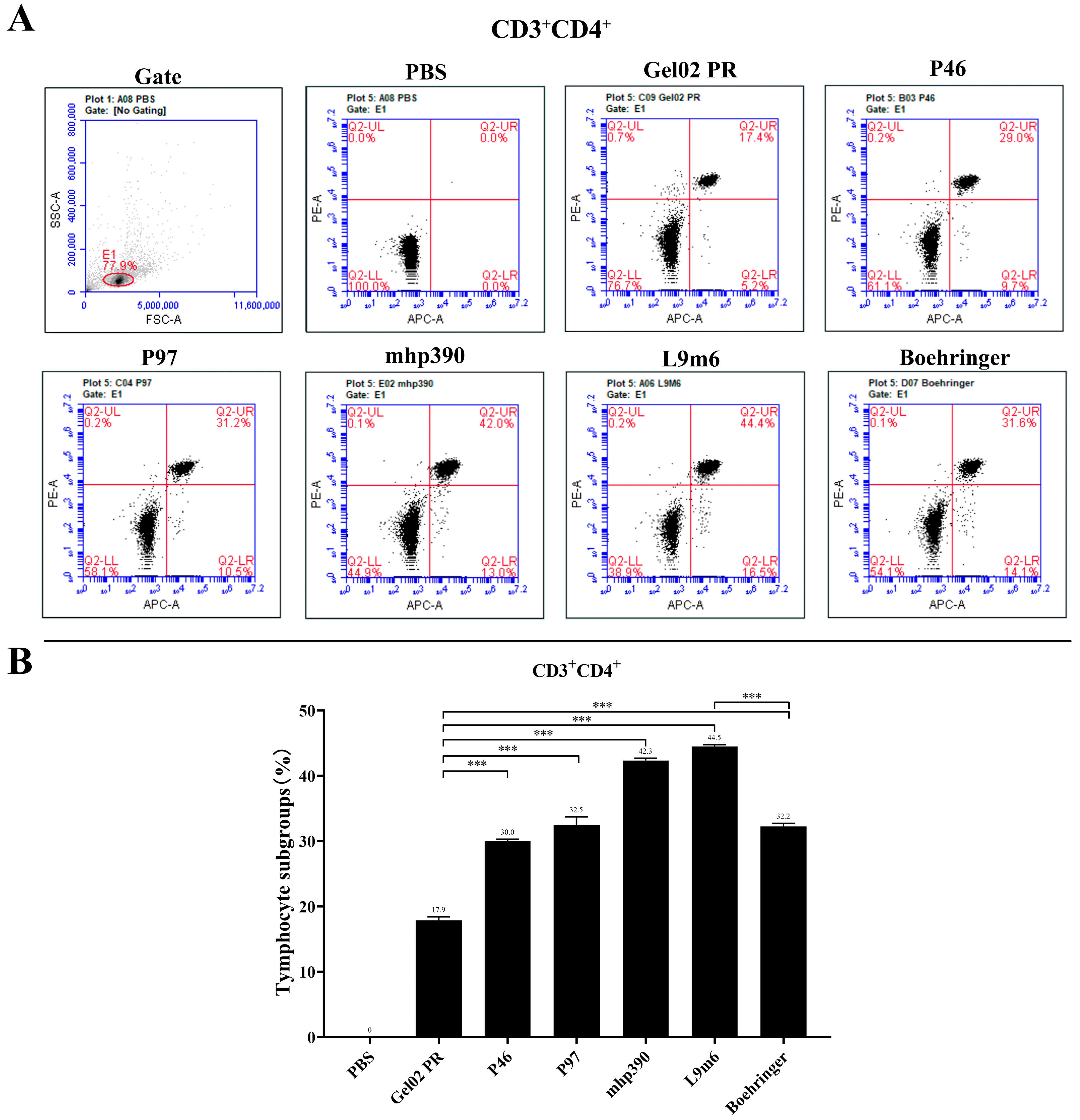
2.7. Intracellular Cytokine Staining and Flow Cytometry Analysis
2.8. Statistical Analysis
3. Results
3.1. Amplification of Target Genes and Purification of Recombinant Proteins
3.2. Cellular Immune Responses to the rL9m6 Fusion Protein Formulated with Gel02 PR Adjuvant
3.3. Humoral Immune Response to the rL9m6 Fusion Protein Formulated with Gel02 PR Adjuvant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minion, F.C.; Lefkowitz, E.J.; Madsen, M.L.; Cleary, B.J.; Swartzell, S.M.; Mahairas, G.G. The genome sequence of Mycoplasma hyopneumoniae strain 232, the agent of swine mycoplasmosis. J. Bacteriol. 2004, 186, 7123–7133. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.M.; Chemale, G.; de Castro, L.A.; Costa, A.P.; Kich, J.D.; Vainstein, M.H.; Zaha, A.; Ferreira, H.B. Proteomic survey of the pathogenic Mycoplasma hyopneumoniae strain 7448 and identification of novel post-translationally modified and antigenic proteins. Vet. Microbiol. 2007, 121, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Fablet, C.; Marois-Crehan, C.; Simon, G.; Grasland, B.; Jestin, A.; Kobisch, M.; Madec, F.; Rose, N. Infectious agents associated with respiratory diseases in 125 farrow-to-finish pig herds: A cross-sectional study. Vet. Microbiol. 2012, 157, 152–163. [Google Scholar] [CrossRef]
- Maes, D.; Segales, J.; Meyns, T.; Sibila, M.; Pieters, M.; Haesebrouck, F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2008, 126, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Saade, G.; Deblanc, C.; Bougon, J.; Marois-Crehan, C.; Fablet, C.; Auray, G.; Belloc, C.; Leblanc-Maridor, M.; Gagnon, C.A.; Zhu, J.; et al. Coinfections and their molecular consequences in the porcine respiratory tract. Vet. Res. 2020, 51, 80. [Google Scholar] [CrossRef]
- Galli, V.; Simionatto, S.; Marchioro, S.B.; Klabunde, G.H.; Conceicao, F.R.; Dellagostin, O.A. Recombinant secreted antigens from Mycoplasma hyopneumoniae delivered as a cocktail vaccine enhance the immune response of mice. Clin. Vaccine Immunol. 2013, 20, 1370–1376. [Google Scholar] [CrossRef]
- Haesebrouck, F.; Pasmans, F.; Chiers, K.; Maes, D.; Ducatelle, R.; Decostere, A. Efficacy of vaccines against bacterial diseases in swine: What can we expect? Vet. Microbiol. 2004, 100, 255–268. [Google Scholar] [CrossRef]
- Kobisch, M.; Friis, N.F. Swine mycoplasmoses. Rev. Sci. Tech. 1996, 15, 1569–1605. [Google Scholar] [CrossRef]
- Marchioro, S.B.; Fisch, A.; Gomes, C.K.; Jorge, S.; Galli, V.; Haesebrouck, F.; Maes, D.; Dellagostin, O.; Conceicao, F.R. Local and systemic immune responses induced by a recombinant chimeric protein containing Mycoplasma hyopneumoniae antigens fused to the B subunit of Escherichia coli heat-labile enterotoxin LTB. Vet. Microbiol. 2014, 173, 166–171. [Google Scholar] [CrossRef]
- Chen, A.Y.; Fry, S.R.; Daggard, G.E.; Mukkur, T.K. Evaluation of immune response to recombinant potential protective antigens of Mycoplasma hyopneumoniae delivered as cocktail DNA and/or recombinant protein vaccines in mice. Vaccine 2008, 26, 4372–4378. [Google Scholar] [CrossRef]
- Okamba, F.R.; Arella, M.; Music, N.; Jia, J.J.; Gottschalk, M.; Gagnon, C.A. Potential use of a recombinant replication-defective adenovirus vector carrying the C-terminal portion of the P97 adhesin protein as a vaccine against Mycoplasma hyopneumoniae in swine. Vaccine 2010, 28, 4802–4809. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Hariri, S.; Lin, C.; Dunne, E.F.; Steinau, M.; McQuillan, G.; Unger, E.R. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J. Infect. Dis. 2013, 208, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Sibila, M.; Nofrarias, M.; Perez-Martin, E.; Olvera, A.; Mateu, E.; Segales, J. Evaluation of cell-mediated immune responses against porcine circovirus type 2 (PCV2) Cap and Rep proteins after vaccination with a commercial PCV2 sub-unit vaccine. Vet. Immunol. Immunopathol. 2012, 150, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Oishi, E.; Muneta, Y.; Sano, A.; Hikono, H.; Shibahara, T.; Yagi, Y.; Shimoji, Y. Oral vaccination against mycoplasmal pneumonia of swine using a live Erysipelothrix rhusiopathiae vaccine strain as a vector. Vaccine 2009, 27, 4543–4550. [Google Scholar] [CrossRef]
- Shimoji, Y.; Oishi, E.; Muneta, Y.; Nosaka, H.; Mori, Y. Vaccine efficacy of the attenuated Erysipelothrix rhusiopathiae YS-19 expressing a recombinant protein of Mycoplasma hyopneumoniae P97 adhesin against mycoplasmal pneumonia of swine. Vaccine 2003, 21, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Lee, J.A.; Park, S.Y.; Song, C.S.; Choi, I.S.; Lee, J.B. A review of vaccine development and research for industry animals in Korea. Clin. Exp. Vaccine Res. 2012, 1, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, D.; Yuan, F.; Liu, Z.; Duan, Z.; Yang, K.; Guo, R.; Li, M.; Li, S.; Fang, L.; et al. Surface proteins mhp390 (P68) contributes to cilium adherence and mediates inflammation and apoptosis in Mycoplasma hyopneumoniae. Microb. Pathog. 2019, 126, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, P.; Yang, K.; Song, Q.; Yuan, F.; Liu, Z.; Gao, T.; Zhou, D.; Guo, R.; Li, C.; et al. Mycoplasma hyopneumoniae Infection Activates the NOD1 Signaling Pathway to Modulate Inflammation. Front. Cell. Infect. Microbiol. 2022, 12, 927840. [Google Scholar] [CrossRef]
- Feng, Z.X.; Wei, Y.N.; Li, G.L.; Lu, X.M.; Wan, X.F.; Pharr, G.T.; Wang, Z.W.; Kong, M.; Gan, Y.; Bai, F.F.; et al. Development and validation of an attenuated Mycoplasma hyopneumoniae aerosol vaccine. Vet. Microbiol. 2013, 167, 417–424. [Google Scholar] [CrossRef]
- Simionatto, S.; Marchioro, S.B.; Galli, V.; Brum, C.B.; Klein, C.S.; Rebelatto, R.; Silva, E.F.; Borsuk, S.; Conceicao, F.R.; Dellagostin, O.A. Immunological characterization of Mycoplasma hyopneumoniae recombinant proteins. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 209–216. [Google Scholar] [CrossRef]
- Simionatto, S.; Marchioro, S.B.; Galli, V.; Luerce, T.D.; Hartwig, D.D.; Moreira, A.N.; Dellagostin, O.A. Efficient site-directed mutagenesis using an overlap extension-PCR method for expressing Mycoplasma hyopneumoniae genes in Escherichia coli. J. Microbiol. Methods 2009, 79, 101–105. [Google Scholar] [CrossRef]
- Simionatto, S.; Marchioro, S.B.; Galli, V.; Hartwig, D.D.; Carlessi, R.M.; Munari, F.M.; Laurino, J.P.; Conceicao, F.R.; Dellagostin, O.A. Cloning and purification of recombinant proteins of Mycoplasma hyopneumoniae expressed in Escherichia coli. Protein Expr. Purif. 2010, 69, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; Simionatto, S.; Marchioro, S.B.; Fisch, A.; Gomes, C.K.; Conceicao, F.R.; Dellagostin, O.A. Immunisation of mice with Mycoplasma hyopneumoniae antigens P37, P42, P46 and P95 delivered as recombinant subunit or DNA vaccines. Vaccine 2012, 31, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Wang, Y. Recent advances in the production of recombinant subunit vaccines in Pichia pastoris. Bioengineered 2016, 7, 155–165. [Google Scholar] [CrossRef]
- Shao, M.; Cui, N.; Tang, Y.; Chen, F.; Cui, Y.; Dang, G.; Liu, S. A candidate subunit vaccine induces protective immunity against Mycobacterium avium subspecies paratuberculosis in mice. NPJ Vaccines 2023, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Dietrich, J.; Tan, E.; Abalos, R.M.; Burgos, J.; Bigbee, C.; Bigbee, M.; Milk, L.; Gideon, H.P.; Rodgers, M.; et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J. Clin. Investig. 2012, 122, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Young, T.F.; Ross, R.F. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect. Immun. 1995, 63, 1013–1019. [Google Scholar] [CrossRef]
- Zhang, Q.; Young, T.F.; Ross, R.F. Glycolipid receptors for attachment of Mycoplasma hyopneumoniae to porcine respiratory ciliated cells. Infect. Immun. 1994, 62, 4367–4373. [Google Scholar] [CrossRef]
- Minion, F.C.; Adams, C.; Hsu, T. R1 region of P97 mediates adherence of Mycoplasma hyopneumoniae to swine cilia. Infect. Immun. 2000, 68, 3056–3060. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Wu, X.M.; Che, Y.L.; Chen, R.J.; Hou, B.; Wang, C.Y.; Wang, L.B.; Zhou, L.J. The Immune Efficacy of Inactivated Pseudorabies Vaccine Prepared from FJ-2012DeltagE/gI Strain. Microorganisms 2022, 10, 1880. [Google Scholar] [CrossRef]
- Ismail, N.M.; El-Deeb, A.H.; Emara, M.M.; Tawfik, H.I.; Wanis, N.A.; Hussein, H.A. Prime-boost vaccination strategy against avian influenza and Newcastle disease viruses reduces shedding of the challenge viruses. Virusdisease 2018, 29, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Wangkaghart, E.; Deville, S.; Wang, B.; Srisapoome, P.; Wang, T.; Secombes, C.J. Immune response and protective efficacy of two new adjuvants, Montanide ISA 763B VG and Montanide GEL02, administered with a Streptococcus agalactiae ghost vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2021, 116, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.J.; Pei, S.X.; Song, J.J.; Zhan, P.F.; Han, Y.N.; Xue, Y.; Ding, K.; Zhao, Z.Q. Screening immune adjuvants for an inactivated vaccine against Erysipelothrix rhusiopathiae. Front. Vet. Sci. 2022, 9, 922867. [Google Scholar] [CrossRef]
- Qi, Y.; Kang, H.; Zheng, X.; Wang, H.; Gao, Y.; Yang, S.; Xia, X. Incorporation of membrane-anchored flagellin or Escherichia coli heat-labile enterotoxin B subunit enhances the immunogenicity of rabies virus-like particles in mice and dogs. Front. Microbiol. 2015, 6, 169. [Google Scholar] [CrossRef]
- Thiam, F.; Charpilienne, A.; Poncet, D.; Kohli, E.; Basset, C. B subunits of cholera toxin and thermolabile enterotoxin of Escherichia coli have similar adjuvant effect as whole molecules on rotavirus 2/6-VLP specific antibody responses and induce a Th17-like response after intrarectal immunization. Microb. Pathog. 2015, 89, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Recent advances in nontoxic Escherichia coli heat-labile toxin and its derivative adjuvants. Expert Rev. Vaccines 2016, 15, 1361–1371. [Google Scholar] [CrossRef]
- Knapp, M.A.; Johnson, T.A.; Ritter, M.K.; Rainer, R.O.; Fiester, S.E.; Grier, J.T.; Connell, T.D.; Arce, S. Immunomodulatory regulation by heat-labile enterotoxins and potential therapeutic applications. Expert Rev. Vaccines 2021, 20, 975–987. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Shi, Y.; Wu, C.; Zhang, W.J.; Mao, X.H.; Guo, G.; Li, H.X.; Zou, Q.M. Therapeutic efficacy of a multi-epitope vaccine against Helicobacter pylori infection in BALB/c mice model. Vaccine 2009, 27, 5013–5019. [Google Scholar] [CrossRef]
- Grassmann, A.A.; Felix, S.R.; dos Santos, C.X.; Amaral, M.G.; Seixas Neto, A.C.; Fagundes, M.Q.; Seixas, F.K.; da Silva, E.F.; Conceicao, F.R.; Dellagostin, O.A. Protection against lethal leptospirosis after vaccination with LipL32 coupled or coadministered with the B subunit of Escherichia coli heat-labile enterotoxin. Clin. Vaccine Immunol. 2012, 19, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Lipsit, S.; Facciuolo, A.; Scruten, E.; Wilkinson, J.; Plastow, G.; Kusalik, A.; Napper, S. Signaling differences in peripheral blood mononuclear cells of high and low vaccine responders prior to, and following, vaccination in piglets. Vaccine X 2022, 11, 100167. [Google Scholar] [CrossRef] [PubMed]
- Beuckelaere, L.; Haspeslagh, M.; Biebaut, E.; Boyen, F.; Haesebrouck, F.; Krejci, R.; Meyer, E.; Gleerup, D.; De Spiegelaere, W.; Devriendt, B.; et al. Different local, innate and adaptive immune responses are induced by two commercial Mycoplasma hyopneumoniae bacterins and an adjuvant alone. Front. Immunol. 2022, 13, 1015525. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Chae, C.; Ryu, D.Y. A recombinant chimera comprising the R1 and R2 repeat regions of M. hyopneumoniae P97 and the N-terminal region of A. pleuropneumoniae ApxIII elicits immune responses. BMC Vet. Res. 2014, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Okamba, F.R.; Moreau, E.; Cheikh Saad Bouh, K.; Gagnon, C.A.; Massie, B.; Arella, M. Immune responses induced by replication-defective adenovirus expressing the C-terminal portion of the Mycoplasma hyopneumoniae P97 adhesin. Clin. Vaccine Immunol. 2007, 14, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; de Oliveira, N.R.; Marchioro, S.B.; Fisch, A.; Gomes, C.K.; Hartleben, C.P.; Conceicao, F.R.; Dellagostin, O.A. The Mycoplasma hyopneumoniae recombinant heat shock protein P42 induces an immune response in pigs under field conditions. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 229–236. [Google Scholar] [CrossRef]
- Vranckx, K.; Maes, D.; Marchioro, S.B.; Villarreal, I.; Chiers, K.; Pasmans, F.; Haesebrouck, F. Vaccination reduces macrophage infiltration in bronchus-associated lymphoid tissue in pigs infected with a highly virulent Mycoplasma hyopneumoniae strain. BMC Vet. Res. 2012, 8, 24. [Google Scholar] [CrossRef]
- Thacker, E.L.; Thacker, B.J.; Kuhn, M.; Hawkins, P.A.; Waters, W.R. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am. J. Vet. Res. 2000, 61, 1384–1389. [Google Scholar] [CrossRef]
- Dobbs, N.A.; Odeh, A.N.; Sun, X.; Simecka, J.W. The Multifaceted Role of T Cell-Mediated Immunity in Pathogenesis and Resistance to Mycoplasma Respiratory Disease. Curr. Trends Immunol. 2009, 10, 1–19. [Google Scholar] [PubMed]
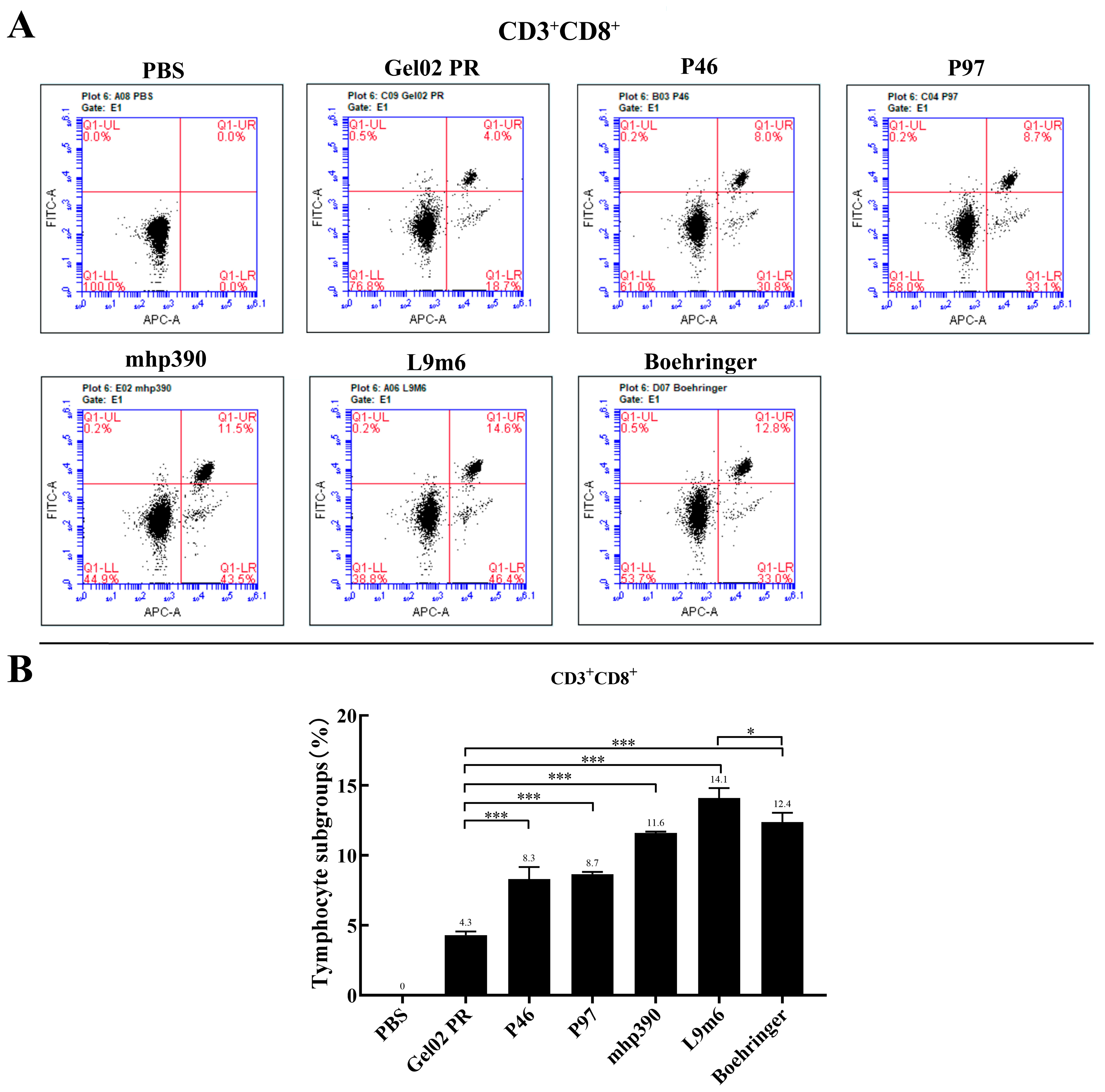
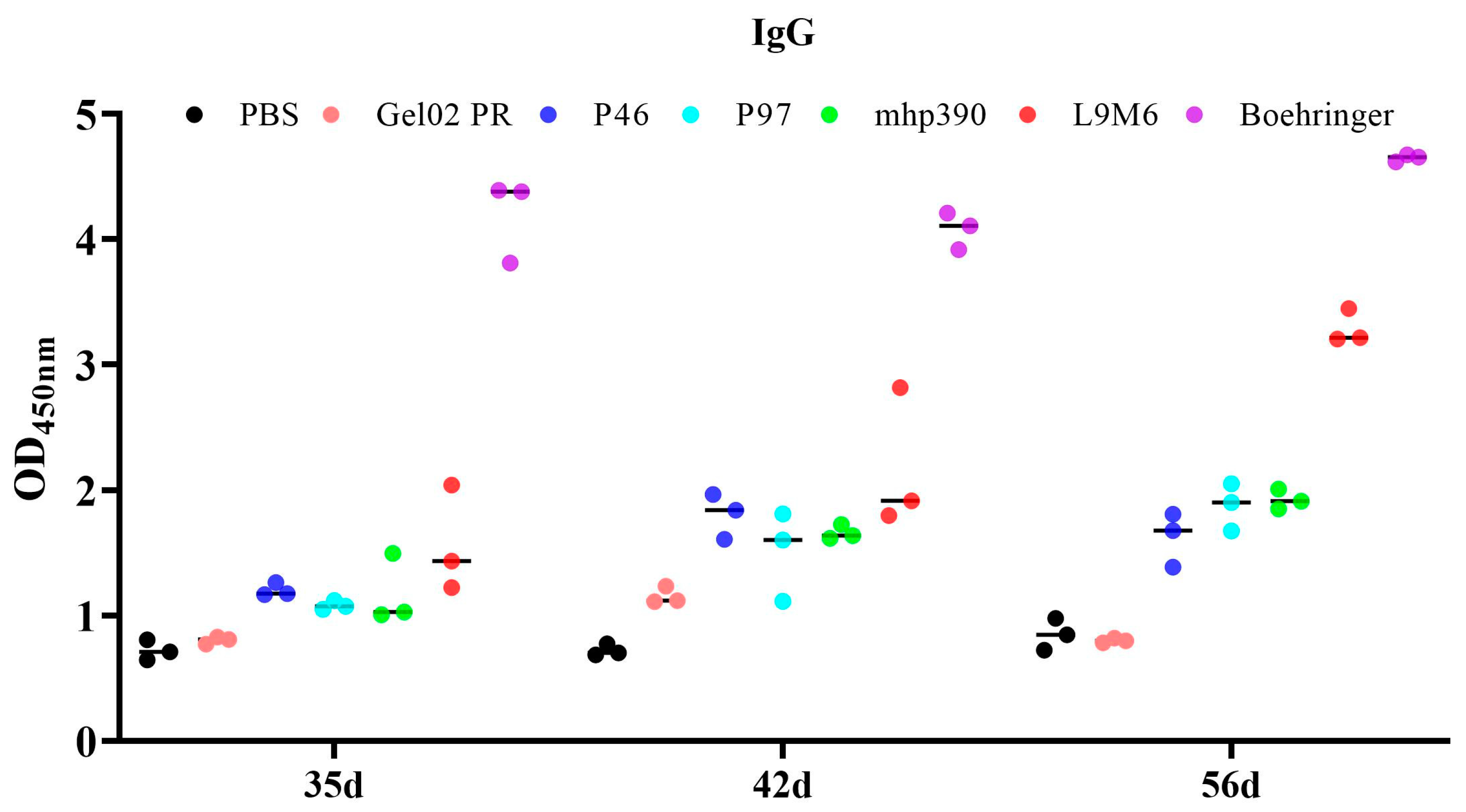
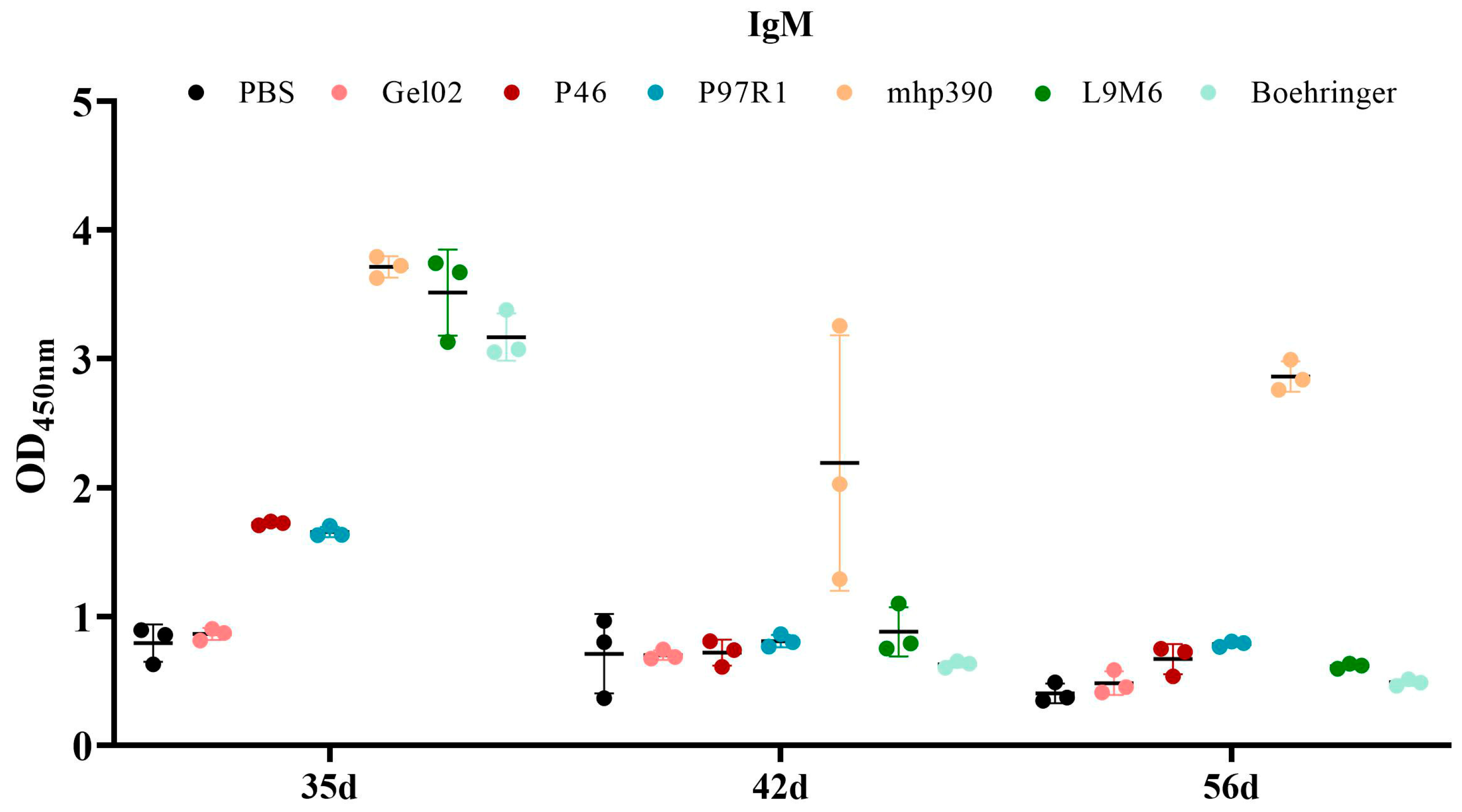
| Gene | Genbank Accession | Primer | Primer Sequence | Restriction Enzyme |
|---|---|---|---|---|
| LTB | MF990202.1 | LTB-F a | TTTCCATGGCTATGGCTCCCCAGACTATTACA | Nco I |
| LTB-R a | TCTGGATCCCATACTGATTGCCG | BamH I | ||
| P97R1 | ADQ90328.1 | P97-F a | TTTGGATCCGGCAGCGGCAGCGGCAGCGGCAGCATGTTACCTCAGCCGCCAGCAGCT | BamH I |
| P97-R a | TTTGTCGACAGCCATTGGGAAATAGTCTTCTTTTGGTTTATTT | Sal I | ||
| mhp390 | AGM22196.1 | mhp390-F a | TTTGTCGACGGCAGCGGCAGCGGCAGCGGCAGCATGAATTTCAAAAAATATTTAAAAT | Sal I |
| mhp390-R a | TCTGCGGCCGCATTTTGTTCATCAATTAGTTTTAGAATTTCTTGC | Not I | ||
| P46 | ADQ90718.1 | P46-F a | TTTGCGGCCGCAGGCAGCGGCAGCGGCAGCGGCAGCATGACTTCTGATTCTAAACCAC | Not I |
| P46-R a | TTTCTCGAGTTAGGCATCAGGATTATCAACATTAGCTTTTGT | Xho I | ||
| P97R1 | ADQ90328.1 | P1 b | TTTGGATCCATGTTACCTCAGCCGCC | BamHI Xho I |
| P2 b | TTTCTCGAGTTAGCCATTGGGAAAT | |||
| mhp390 | AGM22196.1 | P3 b | TCTGGATCCATGAATTTCAAAAAATATTTAAAAT | BamHI Sal I |
| P4 b | TTTGTCGACTTAATTTTGTTCATCAATTAGTTT | |||
| P46 | ADQ90718.1 | P5 b | TTTGGATCCATGAAAAAAATGCTTAG | BamHI Xho I |
| P6 b | TTTCTCGAGTTAGGCATCAGGATTAT |
| Group | Immunogen | Dose | Route |
|---|---|---|---|
| Group 1 | Phosphate-buffered saline | 100 μL | i.m. |
| Group 2 | Gel02 PR | 100 μL PBS with 10% Gel02 PR | i.m. |
| Group 3 | rP46 | 50 μg + 10% Gel02 PR | i.m. |
| Group 4 | rP97R1 | 50 μg + 10% Gel02 PR | i.m. |
| Group 5 | rMhp390 | 50 μg + 10% Gel02 PR | i.m. |
| Group 6 | rL9m6 | 50 μg + 10% Gel02 PR | i.m. |
| Group 7 | Boehringer | 100 μL | i.m. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Jiang, P.; Song, T.; Yang, K.; Yuan, F.; Gao, T.; Liu, Z.; Li, C.; Guo, R.; Xiao, S.; et al. A Recombinant Chimera Vaccine Composed of LTB and Mycoplasma hyopneumoniae Antigens P97R1, mhp390 and P46 Elicits Cellular Immunologic Response in Mice. Vaccines 2023, 11, 1291. https://doi.org/10.3390/vaccines11081291
Liu W, Jiang P, Song T, Yang K, Yuan F, Gao T, Liu Z, Li C, Guo R, Xiao S, et al. A Recombinant Chimera Vaccine Composed of LTB and Mycoplasma hyopneumoniae Antigens P97R1, mhp390 and P46 Elicits Cellular Immunologic Response in Mice. Vaccines. 2023; 11(8):1291. https://doi.org/10.3390/vaccines11081291
Chicago/Turabian StyleLiu, Wei, Peizhao Jiang, Tao Song, Keli Yang, Fangyan Yuan, Ting Gao, Zewen Liu, Chang Li, Rui Guo, Shaobo Xiao, and et al. 2023. "A Recombinant Chimera Vaccine Composed of LTB and Mycoplasma hyopneumoniae Antigens P97R1, mhp390 and P46 Elicits Cellular Immunologic Response in Mice" Vaccines 11, no. 8: 1291. https://doi.org/10.3390/vaccines11081291
APA StyleLiu, W., Jiang, P., Song, T., Yang, K., Yuan, F., Gao, T., Liu, Z., Li, C., Guo, R., Xiao, S., Tian, Y., & Zhou, D. (2023). A Recombinant Chimera Vaccine Composed of LTB and Mycoplasma hyopneumoniae Antigens P97R1, mhp390 and P46 Elicits Cellular Immunologic Response in Mice. Vaccines, 11(8), 1291. https://doi.org/10.3390/vaccines11081291







