Development and Immunoprotection of Bacterium-like Particle Vaccine against Infectious Bronchitis in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Recombinant Baculovirus
2.2. Preparation of GEM
2.3. Production of BLP Displaying the S1 Protein
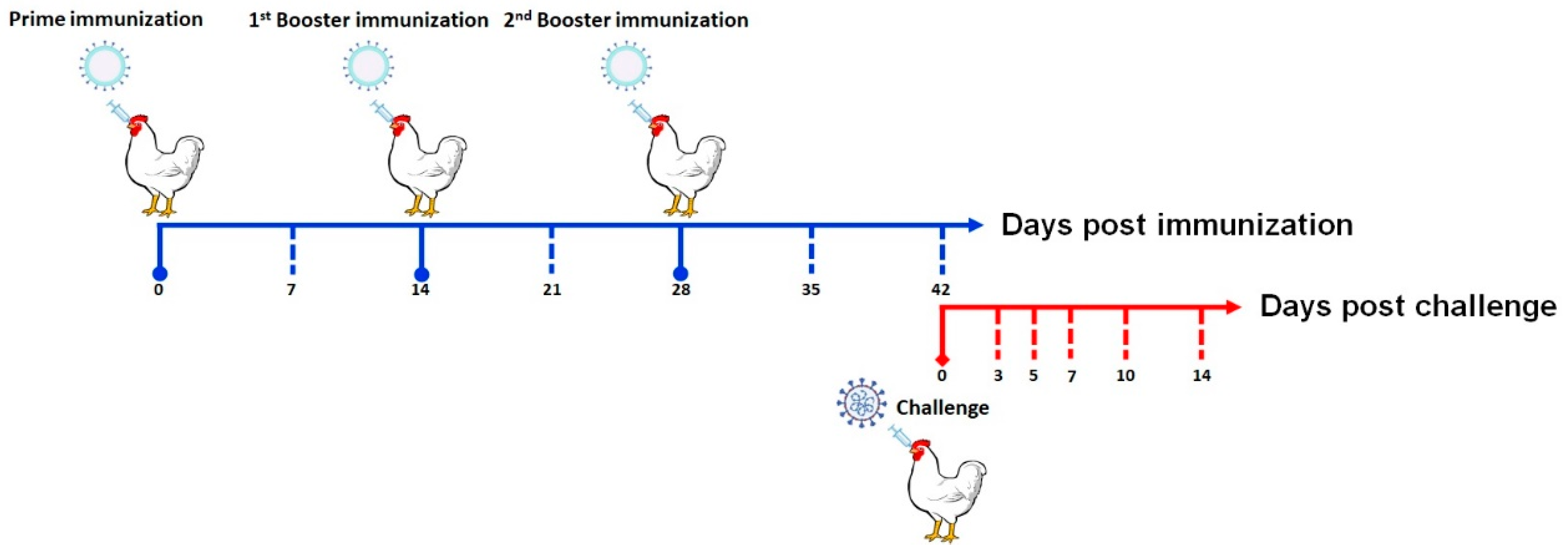
2.4. Animal Immunization and Challenge
2.5. ELISA
2.6. qRT-PCR
2.7. EID50
2.8. Statistical Analysis
2.9. Ethics Statement
3. Results
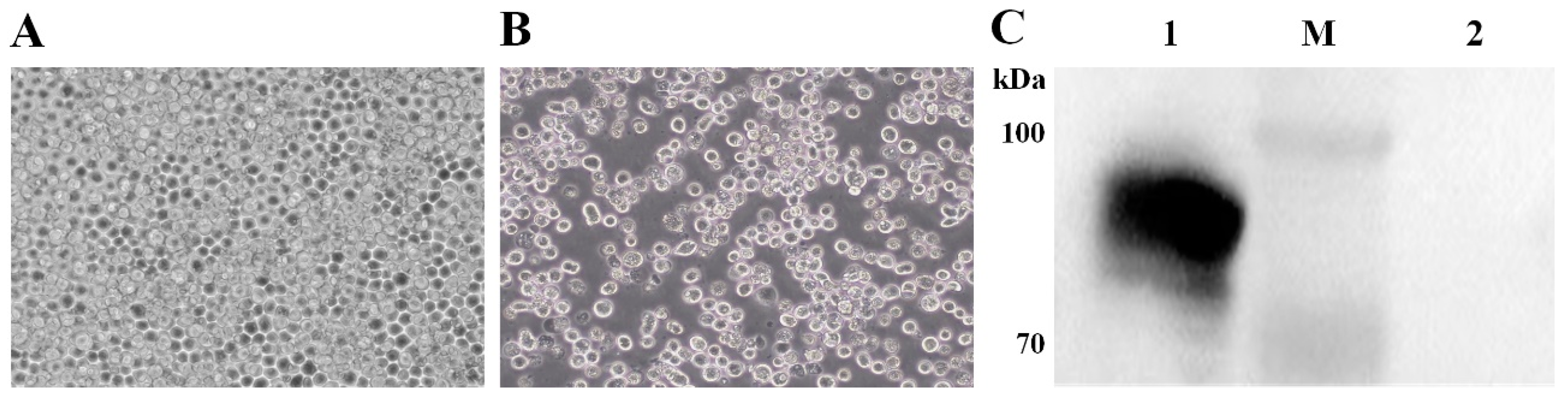
3.1. Expression of the IBV S1 Protein in the Baculovirus–Insect Cell System
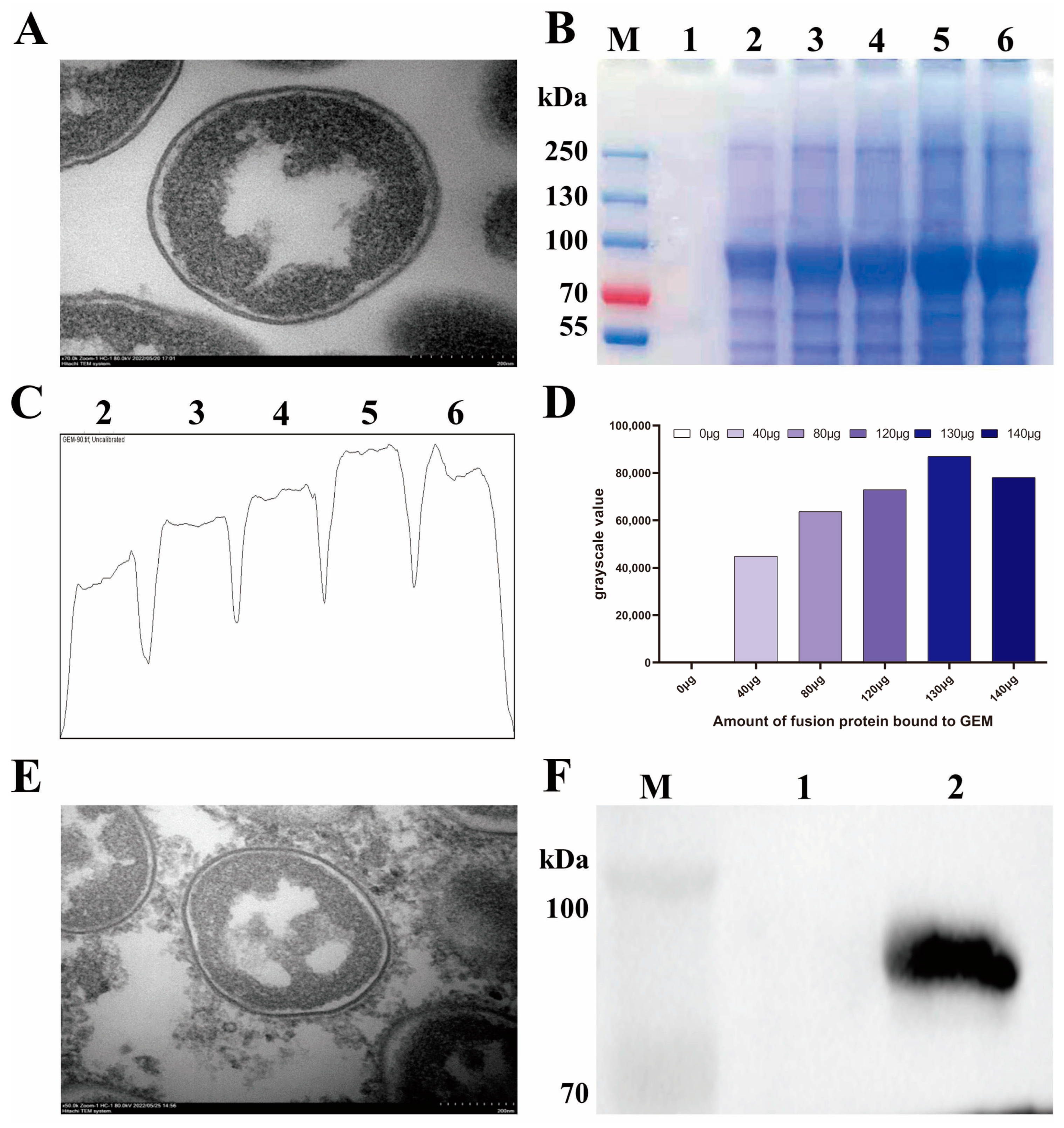
3.2. Identification of BLP Displaying the IBV S1 Protein on the Surface
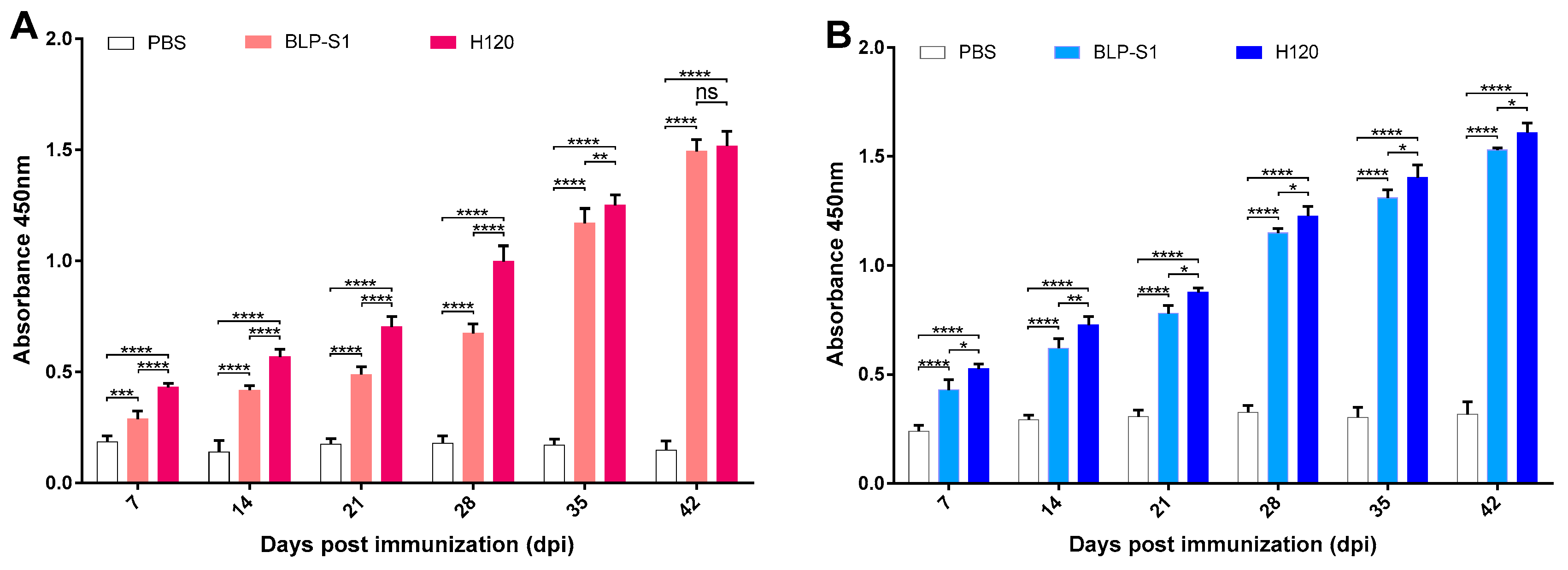
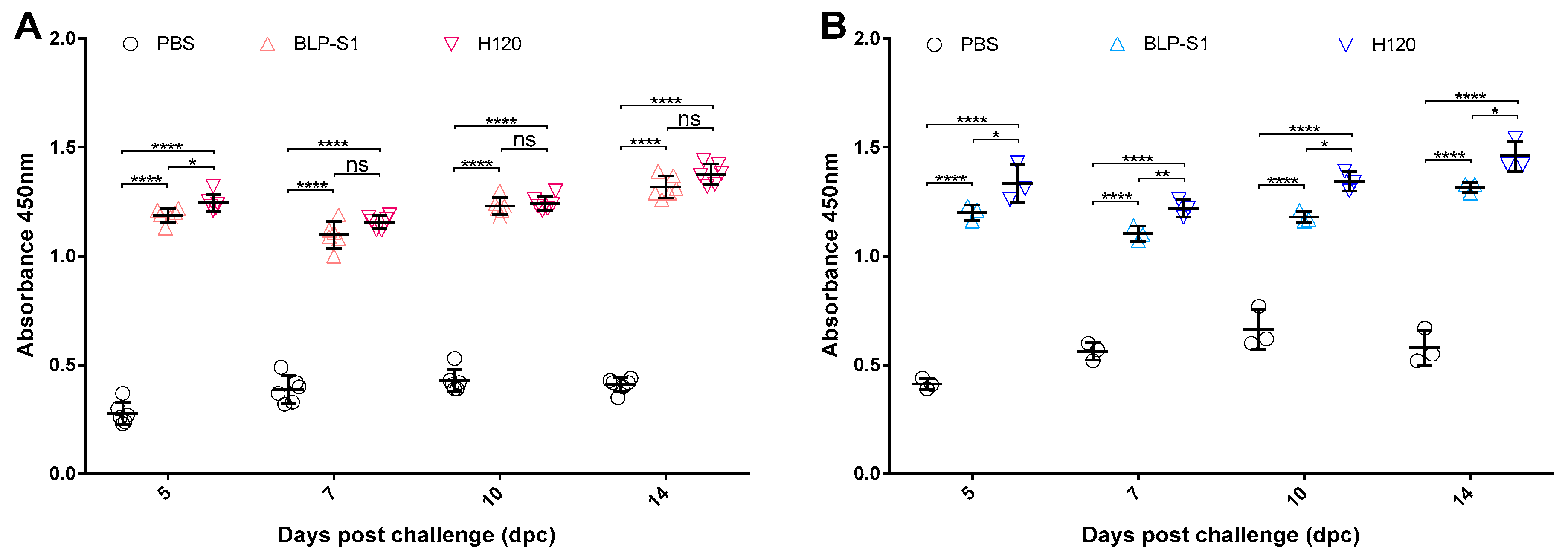
3.3. Determination of Antibody Levels in Sera and Tracheal Lavage Fluids after Immunization with BLP-S1
3.4. Detection of Antibody Levels in Sera and Tracheal Lavage Fluids after IBV Challenge
3.5. Oropharyngeal and Cloacal Shedding after IBV Challenge
3.6. Virus Load in Tissues after IBV Challenge
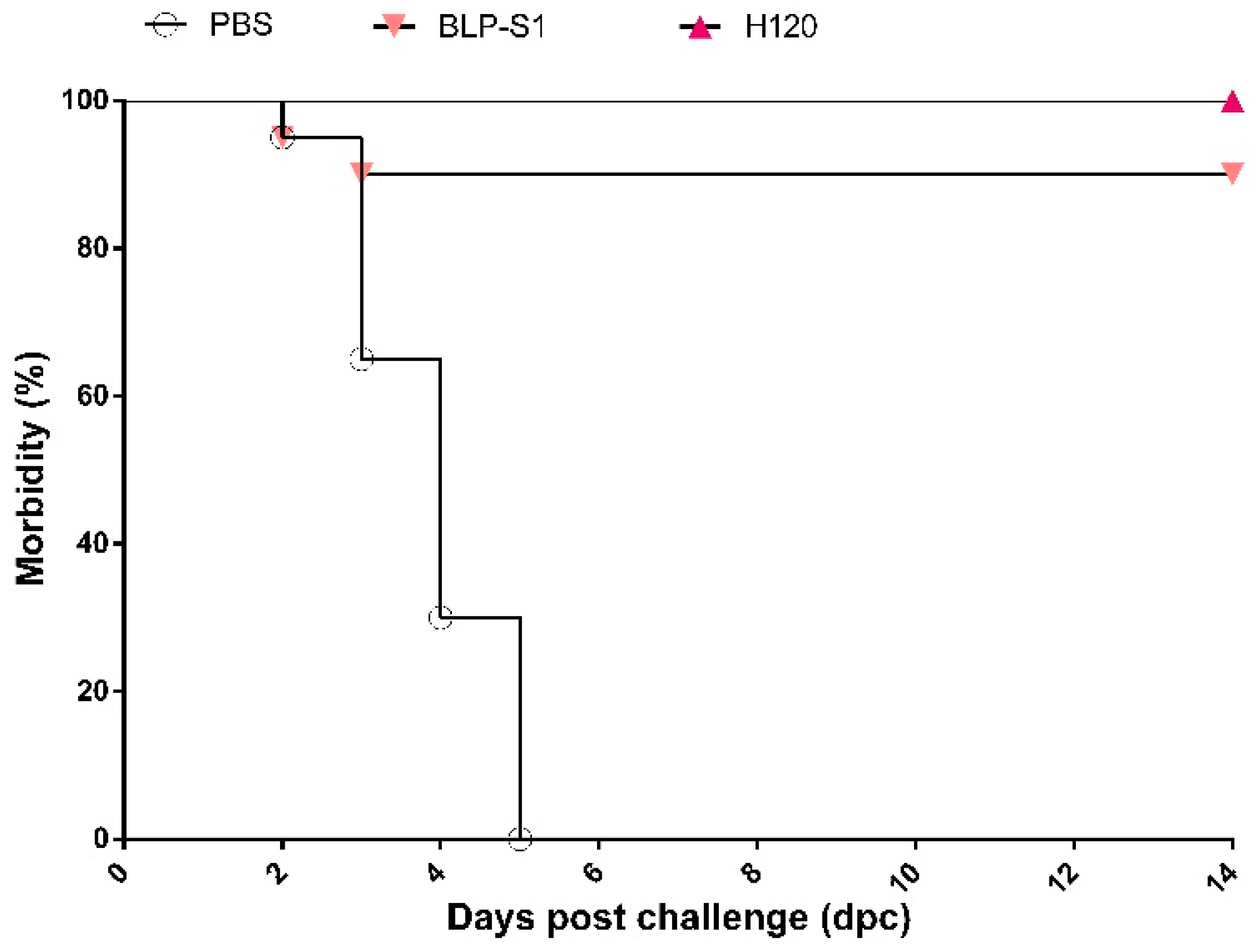
3.7. Clinical Symptoms of Chickens after IBV Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ting, X.; Xiang, C.; Liu, D.X.; Chen, R. Establishment and Cross-Protection Efficacy of a Recombinant Avian Gammacoronavirus Infectious Bronchitis Virus Harboring a Chimeric S1 Subunit. Front Microbiol. 2022, 13, 897560. [Google Scholar] [CrossRef]
- Cook, J.K.; Jackwood, M.; Jones, R.C. The long view: 40 years of infectious bronchitis research. Avian. Pathol. 2012, 41, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Mork, A.K.; Hesse, M.; Abd El Rahman, S.; Rautenschlein, S.; Herrler, G.; Winter, C. Differences in the tissue tropism to chicken oviduct epithelial cells between avian coronavirus IBV strains QX and B1648 are not related to the sialic acid binding properties of their spike proteins. Vet. Res. 2014, 45, 67. [Google Scholar] [CrossRef]
- Bande, F.; Arshad, S.S.; Omar, A.R.; Hair-Bejo, M.; Mahmuda, A.; Nair, V. Global distributions and strain diversity of avian infectious bronchitis virus: A review. Anim. Health Res. Rev. 2017, 18, 70–83. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, T.; Lu, J.; Zhao, P.; Chen, L.; Qian, M.; Guo, Y.; Qiao, H.; Xu, Y.; Wang, Y.; et al. Molecular characterization of variant infectious bronchitis virus in China, 2019: Implications for control programmes. Transbound. Emerg. Dis. 2020, 67, 1349–1355. [Google Scholar] [CrossRef]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, L.; Zhao, W.; Liu, L.; Zhao, Y.; Shao, Y.; Li, H.; Han, Z.; Liu, S. Identification and molecular characterization of a novel serotype infectious bronchitis virus (GI-28) in China. Vet. Microbiol. 2017, 198, 108–115. [Google Scholar] [CrossRef]
- Jiang, L.; Han, Z.; Chen, Y.; Zhao, W.; Sun, J.; Zhao, Y.; Liu, S. Characterization of the complete genome, antigenicity, pathogenicity, tissue tropism, and shedding of a recombinant avian infectious bronchitis virus with a ck/CH/LJL/140901-like backbone and an S2 fragment from a 4/91-like virus. Virus Res. 2018, 244, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Xu, L.; Ren, M.; Shen, J.; Han, Z.; Sun, J.; Zhao, Y.; Liu, S. Novel genotype of infectious bronchitis virus isolated in China. Vet. Microbiol. 2019, 230, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Legnardi, M.; Tucciarone, C.M.; Franzo, G.; Cecchinato, M. Infectious Bronchitis Virus Evolution, Diagnosis and Control. Vet. Sci. 2020, 7, 79. [Google Scholar] [CrossRef]
- Jordan, B. Vaccination against infectious bronchitis virus: A continuous challenge. Vet. Microbiol. 2017, 206, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Gao, Y.; Chen, Q.; Wu, Q.; Xu, X.; Kan, Y.; Yao, L.; Bi, Y.; Xie, Q. Epidemiological investigation of avian infectious bronchitis and locally determined genotype diversity in central China: A 2016–2018 study. Poult. Sci. 2020, 99, 3001–3008. [Google Scholar] [CrossRef]
- Fan, W.S.; Li, H.M.; He, Y.N.; Tang, N.; Zhang, L.H.; Wang, H.Y.; Zhong, L.; Chen, J.C.; Wei, T.C.; Huang, T.; et al. Immune protection conferred by three commonly used commercial live attenuated vaccines against the prevalent local strains of avian infectious bronchitis virus in southern China. J. Vet. Med. Sci. 2018, 80, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Smialek, M.; Tykalowski, B.; Dziewulska, D.; Stenzel, T.; Koncicki, A. Immunological aspects of the efficiency of protectotype vaccination strategy against chicken infectious bronchitis. BMC Vet. Res. 2017, 13, 44. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, T.; Xu, Q.; Gao, M.; Chen, Y.; Wang, Q.; Zhao, Y.; Shao, Y.; Li, H.; Kong, X.; et al. Altered pathogenicity of a tl/CH/LDT3/03 genotype infectious bronchitis coronavirus due to natural recombination in the 5′- 17kb region of the genome. Virus Res. 2016, 213, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, Q.; Zhao, W.; Chen, Y.; Zhang, T.; Han, Z.; Xu, Q.; Kong, X.; Liu, S. Serotype, antigenicity, and pathogenicity of a naturally recombinant TW I genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2016, 191, 1–8. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, M.; Tian, X.; Shao, H.; Qian, K.; Ye, J.; Qin, A. Identification of a novel recombinant virulent avian infectious bronchitis virus. Vet. Microbiol. 2017, 199, 120–127. [Google Scholar] [CrossRef]
- Thor, S.W.; Hilt, D.A.; Kissinger, J.C.; Paterson, A.H.; Jackwood, M.W. Recombination in avian gamma-coronavirus infectious bronchitis virus. Viruses 2011, 3, 1777–1799. [Google Scholar] [CrossRef]
- Moreno, A.; Franzo, G.; Massi, P.; Tosi, G.; Blanco, A.; Antilles, N.; Biarnes, M.; Majo, N.; Nofrarias, M.; Dolz, R.; et al. A novel variant of the infectious bronchitis virus resulting from recombination events in Italy and Spain. Avian. Pathol. 2017, 46, 28–35. [Google Scholar] [CrossRef]
- Hassan, M.S.H.; Ojkic, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Abdul-Careem, M.F. Delmarva (DMV/1639) Infectious Bronchitis Virus (IBV) Variants Isolated in Eastern Canada Show Evidence of Recombination. Viruses 2019, 11, 1054. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Shen, Y.; Xia, J.; Fan, S.; Li, N.; Luo, Y.; Han, X.; Cui, M.; Zhao, Y.; et al. Molecular characterization of infectious bronchitis virus in Southwestern China for the protective efficacy evaluation of four live vaccine strains. Vaccine 2022, 40, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Bande, F.; Arshad, S.S.; Bejo, M.H.; Moeini, H.; Omar, A.R. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J. Immunol. Res. 2015, 2015, 424860. [Google Scholar] [CrossRef]
- Smith, J.; Sadeyen, J.R.; Cavanagh, D.; Kaiser, P.; Burt, D.W. The early immune response to infection of chickens with Infectious Bronchitis Virus (IBV) in susceptible and resistant birds. BMC Vet. Res. 2015, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.A.; Amin, Z.; Rodrigues, K.F.; Saallah, S.; Shaarani, S.M.; Sarker, S.; Siddiquee, S. Infectious Bronchitis Virus (Gammacoronavirus) in Poultry Farming: Vaccination, Immune Response and Measures for Mitigation. Vet. Sci. 2021, 8, 273. [Google Scholar] [CrossRef]
- Li, E.; Chi, H.; Huang, P.; Yan, F.; Zhang, Y.; Liu, C.; Wang, Z.; Li, G.; Zhang, S.; Mo, R.; et al. A Novel Bacterium-Like Particle Vaccine Displaying the MERS-CoV Receptor-Binding Domain Induces Specific Mucosal and Systemic Immune Responses in Mice. Viruses 2019, 11, 799. [Google Scholar] [CrossRef]
- Bosma, T.; Kanninga, R.; Neef, J.; Audouy, S.A.; van Roosmalen, M.L.; Steen, A.; Buist, G.; Kok, J.; Kuipers, O.P.; Robillard, G.; et al. Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl. Environ. Microbiol. 2006, 72, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Keijzer, C.; Haijema, B.J.; Meijerhof, T.; Voorn, P.; de Haan, A.; Leenhouts, K.; van Roosmalen, M.L.; van Eden, W.; Broere, F. Inactivated influenza vaccine adjuvanted with bacterium-like particles induce systemic and mucosal influenza A virus specific T-cell and B-cell responses after nasal administration in a TLR2 dependent fashion. Vaccine 2014, 32, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Sun, Y.Q.; Du, P.; Meng, X.C.; Guo, L.; Li, S.; Zhang, C. The Effect of Lactobacillus actobacillus Peptidoglycan on Bovine β-Lactoglobulin-Sensitized Mice via TLR2/NF-κB Pathway. Iran J. Allergy Asthma Immunol. 2017, 16, 147–158. [Google Scholar] [PubMed]
- Takada, A.; Kida, H. Protective immune response of chickens against Newcastle disease, induced by the intranasal vaccination with inactivated virus. Vet. Microbiol. 1996, 50, 17–25. [Google Scholar] [CrossRef]
- Azevedo-Nogueira, F.; Gomes, S.; Lino, A.; Carvalho, T.; Martins-Lopes, P. Real-time PCR assay for Colletotrichum acutatum sensu stricto quantification in olive fruit samples. Food Chem. 2021, 339, 127858. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hygiene 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Xia, J.; He, X.; Yao, K.C.; Du, L.J.; Liu, P.; Yan, Q.G.; Wen, Y.P.; Cao, S.J.; Han, X.F.; Huang, Y. Phylogenetic and antigenic analysis of avian infectious bronchitis virus in southwestern China, 2012–2016. Infect. Genet. Evol. 2016, 45, 11–19. [Google Scholar] [CrossRef]
- De Geus, E.D.; Degen, W.G.; van Haarlem, D.A.; Schrier, C.; Broere, F.; Vervelde, L. Distribution patterns of mucosally applied particles and characterization of the antigen presenting cells. Avian. Pathol. 2015, 44, 222–229. [Google Scholar] [CrossRef]
- Meir, R.; Krispel, S.; Simanov, L.; Eliahu, D.; Maharat, O.; Pitcovski, J. Immune responses to mucosal vaccination by the recombinant A1 and N proteins of infectious bronchitis virus. Viral. Immunol. 2012, 25, 55–62. [Google Scholar] [CrossRef]
- Lopes, P.D.; Okino, C.H.; Fernando, F.S.; Pavani, C.; Casagrande, V.M.; Lopez, R.F.V.; Montassier, M.F.S.; Montassier, H.J. Inactivated infectious bronchitis virus vaccine encapsulated in chitosan nanoparticles induces mucosal immune responses and effective protection against challenge. Vaccine 2018, 36, 2630–2636. [Google Scholar] [CrossRef]
- Saluja, V.; Amorij, J.P.; van Roosmalen, M.L.; Leenhouts, K.; Huckriede, A.; Hinrichs, W.L.; Frijlink, H.W. Intranasal delivery of influenza subunit vaccine formulated with GEM particles as an adjuvant. AAPS J. 2010, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- De Haan, A.; Haijema, B.J.; Voorn, P.; Meijerhof, T.; van Roosmalen, M.L.; Leenhouts, K. Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration. Vaccine 2012, 30, 4884–4891. [Google Scholar] [CrossRef]
- Van Braeckel-Budimir, N.; Haijema, B.J.; Leenhouts, K. Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications. Front Immunol. 2013, 4, 282. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, J.; Wang, D.; Yu, J.; Gu, T.; Jiang, C.; Kong, W.; Wu, Y. Broad protective immune responses elicited by bacterium-like particle-based intranasal pneumococcal particle vaccine displaying PspA2 and PspA4 fragments. Hum. Vaccin Immunother. 2019, 15, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Ditamo, Y.; Rodriguez, L.; Picking, W.L.; van Roosmalen, M.L.; Leenhouts, K.; Pasetti, M.F. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal. Immunol. 2010, 3, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Bai, Y.; Wang, J.; Jiao, C.; Liu, D.; Zhang, M.; Li, E.; Huang, P.; Gong, Z.; Song, Y.; et al. A bacterium-like particle vaccine displaying Zika virus prM-E induces systemic immune responses in mice. Transbound. Emerg. Dis. 2022, 69, e2516–e2529. [Google Scholar] [CrossRef] [PubMed]
- Nganou-Makamdop, K.; van Roosmalen, M.L.; Audouy, S.A.; van Gemert, G.J.; Leenhouts, K.; Hermsen, C.C.; Sauerwein, R.W. Bacterium-like particles as multi-epitope delivery platform for Plasmodium berghei circumsporozoite protein induce complete protection against malaria in mice. Malar. J. 2012, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Z.P.; He, Y.N.; Fan, W.S.; Dong, Z.H.; Zhang, L.H.; Sun, X.K.; Song, L.L.; Wei, T.C.; Mo, M.L.; et al. Protection against Virulent Infectious Bronchitis Virus Challenge Conferred by a Recombinant Baculovirus Co-Expressing S1 and N Proteins. Viruses 2018, 10, 347. [Google Scholar] [CrossRef] [PubMed]

| Groups | Oropharyngeal Swabs | Cloacal Swabs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 5 d | 7 d | 10 d | 14 d | 3 d | 5 d | 7 d | 10 d | 14 d | |
| BLP-S1 | 3/8 | 5/8 | 2/8 | 0/8 | 0/8 | 1/8 | 2/8 | 0/8 | 0/8 | 0/8 |
| H120 | 1/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| PBS | 8/8 | 8/8 | 8/8 | 8/8 | 7/8 | 5/8 | 5/8 | 7/8 | 6/8 | 7/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Yang, T.; Sun, Y.; Qiao, H.; Hu, N.; Li, X.; Wang, W.; Zhang, L.; Cong, Y. Development and Immunoprotection of Bacterium-like Particle Vaccine against Infectious Bronchitis in Chickens. Vaccines 2023, 11, 1292. https://doi.org/10.3390/vaccines11081292
Zhang P, Yang T, Sun Y, Qiao H, Hu N, Li X, Wang W, Zhang L, Cong Y. Development and Immunoprotection of Bacterium-like Particle Vaccine against Infectious Bronchitis in Chickens. Vaccines. 2023; 11(8):1292. https://doi.org/10.3390/vaccines11081292
Chicago/Turabian StyleZhang, Pengju, Tiantian Yang, Yixue Sun, Haiying Qiao, Nianzhi Hu, Xintao Li, Weixia Wang, Lichun Zhang, and Yanlong Cong. 2023. "Development and Immunoprotection of Bacterium-like Particle Vaccine against Infectious Bronchitis in Chickens" Vaccines 11, no. 8: 1292. https://doi.org/10.3390/vaccines11081292
APA StyleZhang, P., Yang, T., Sun, Y., Qiao, H., Hu, N., Li, X., Wang, W., Zhang, L., & Cong, Y. (2023). Development and Immunoprotection of Bacterium-like Particle Vaccine against Infectious Bronchitis in Chickens. Vaccines, 11(8), 1292. https://doi.org/10.3390/vaccines11081292





