SARS-CoV-2 Vaccine Uptake among Patients with Chronic Liver Disease: A Cross-Sectional Analysis in Hebei Province, China
Abstract
:1. Background
2. Methods
2.1. Questionnaire and Participants
2.2. SARS-CoV-2 Vaccination Program
2.3. Statistical Analyses
3. Results
3.1. Participant Characteristics
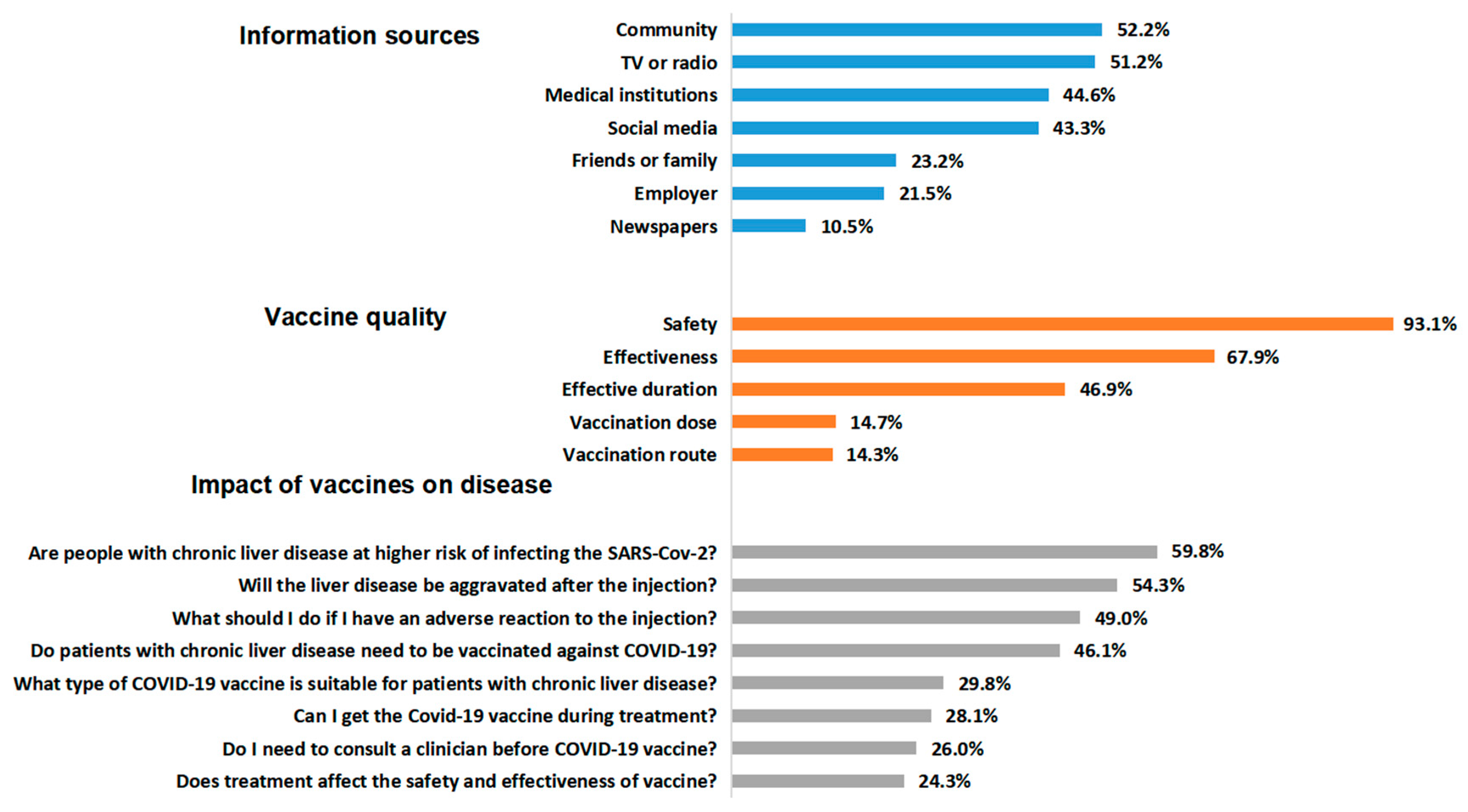
3.2. Issues that CLD Patients Were Concerned about before SARS-CoV-2 Vaccination
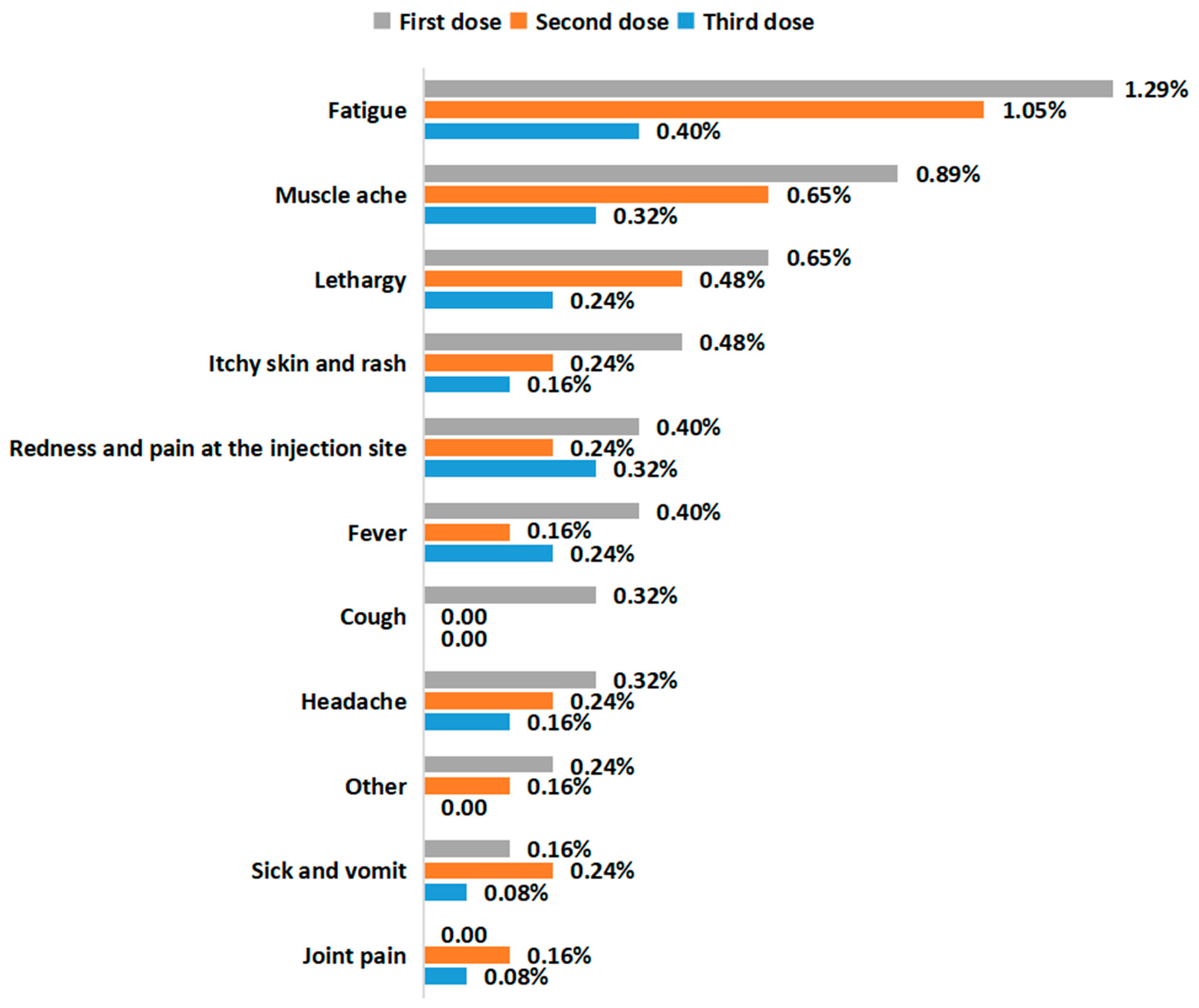
3.3. SARS-CoV-2 Vaccination Status
3.4. Risk Factors Associated with Unvaccinated Status
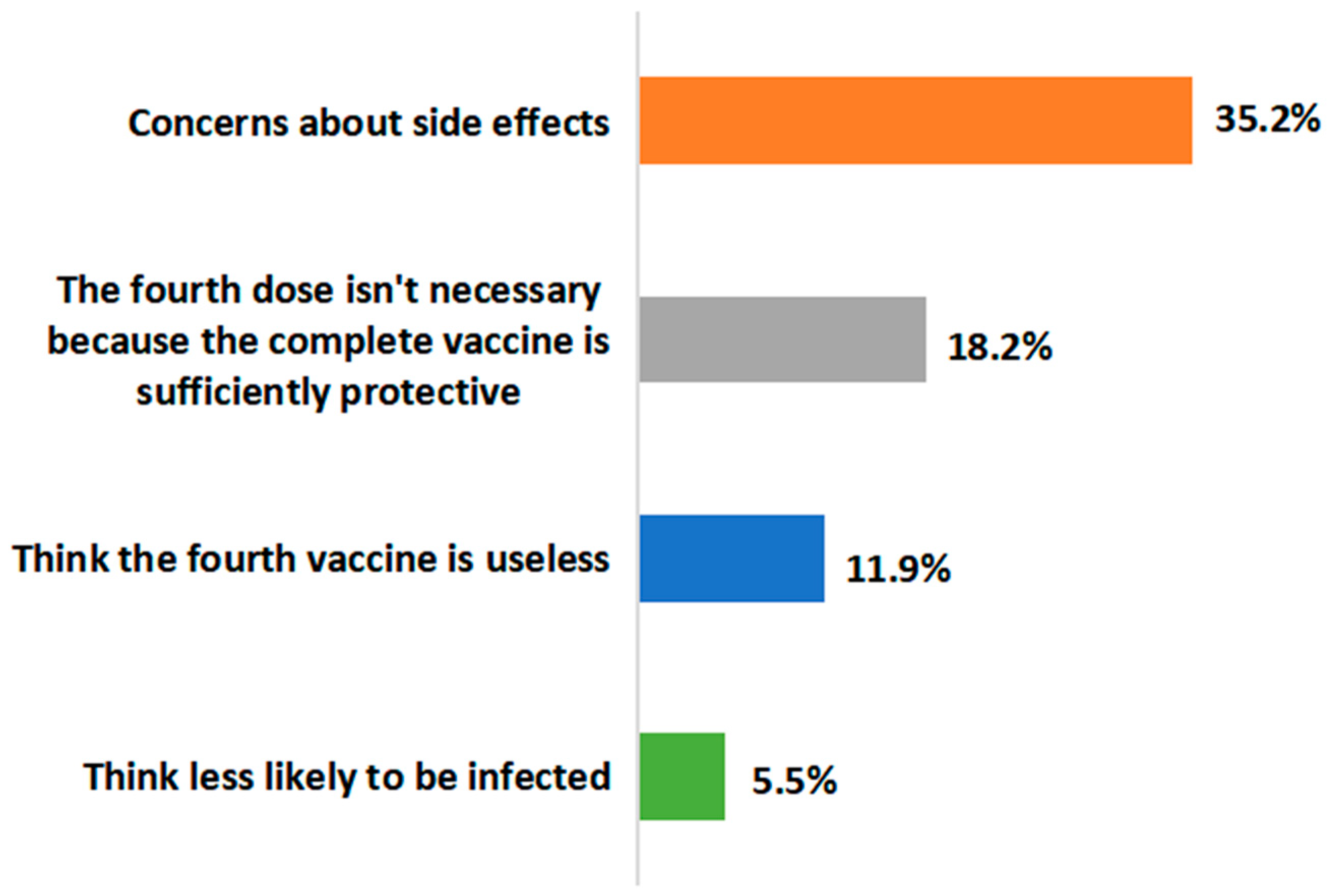
3.5. Attitudes to the Fourth Dose of the SARS-CoV-2 Vaccine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2021, 12, 809244. [Google Scholar] [CrossRef]
- Frederiksen, L.; Zhang, Y.; Foged, C.; Thakur, A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front. Immunol. 2020, 11, 1817. [Google Scholar] [CrossRef]
- Marjot, T.; Webb, G.J.; Barritt, A.S., IV; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and liver disease: Mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Liang, P.S.; Locke, E.; Green, P.; Berry, K.; O’hare, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Fan, V.S.; et al. Cirrhosis and Severe Acute Respiratory Syndrome Coronavirus 2 Infection in US Veterans: Risk of Infection, Hospitalization, Ventilation, and Mortality. Hepatology 2021, 74, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moon, A.M.; Cook, J.A.; Abd-Elsalam, S.; Aloman, C.; Armstrong, M.J.; Pose, E.; Brenner, E.J.; Cargill, T.; Catana, M.-A.; et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J. Hepatol. 2021, 74, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Yan, P.; Chotani, R.A.; Shaikh, O.S. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int. 2021, 41, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Fix, O.K.; Blumberg, E.A.; Chang, K.M.; Chu, J.; Chung, R.T.; Goacher, E.K.; Hameed, B.; Kaul, D.R.; Kulik, L.M.; Kwok, R.M.; et al. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology 2021, 74, 1049–1064. [Google Scholar] [CrossRef]
- Cornberg, M.; Buti, M.; Eberhardt, C.S.; Grossi, P.A.; Shouval, D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J. Hepatol. 2021, 74, 944–951. [Google Scholar] [CrossRef]
- Ai, J.; Wang, J.; Liu, D.; Xiang, H.; Guo, Y.; Lv, J.; Zhang, Q.; Li, J.; Zhang, X.; Li, Q.; et al. Safety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin. Gastroenterol. Hepatol. 2022, 20, 1516–1524. [Google Scholar] [CrossRef]
- John, B.V.; Deng, Y.; Schwartz, K.B.; Taddei, T.H.; Kaplan, D.E.; Martin, P.; Chao, H.; Dahman, B. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology 2022, 76, 126–138. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, C.; Zhao, S.; Sheng, Z.; Xiang, X.; Li, R.; Qian, Z.; Wang, Y.; Chen, B.; Li, Z.; et al. COVID-19 vaccines in patients with decompensated cirrhosis: A retrospective cohort on safety data and risk factors associated with unvaccinated status. Infect. Dis. Poverty 2022, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Serper, M.; Reddy, K.R.; Bewtra, M.; Ahmad, N.; Mehta, S.J. COVID-19 Vaccine Perceptions Among Patients With Chronic Disease in a Large Gastroenterology and Hepatology Practice. Am. J. Gastroenterol. 2021, 116, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Nicholas, S.; Leng, A.; Maitland, E.; Wang, J. COVID-19 Vaccination Willingness among Chinese Adults under the Free Vaccination Policy. Vaccines 2021, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Disease Control Prevention NHCO. Guidelines of vaccination for COVID-19 vaccines in China (First edition). Chin. J. Viral Dis. 2021, 11, 161–162. [Google Scholar]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Liang, B.; Wang, H.; Quan, X.; He, S.; Zhou, H.; He, Y.; Yang, D.; Wang, B.; Zheng, X. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cell. Mol. Immunol. 2021, 18, 2679–2681. [Google Scholar] [CrossRef]
- He, T.; Zhou, Y.; Xu, P.; Ling, N.; Chen, M.; Huang, T.; Zhang, B.; Yang, Z.; Ao, L.; Li, H.; et al. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis B virus infection. Liver Int. 2022, 42, 1287–1296. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Ai, J.; Liu, D.; Liu, C.; Xiang, H.; Gu, Y.; Guo, Y.; Lv, J.; Huang, Y.; et al. Safety and immunogenicity of SARS-CoV-2 vaccines in Chinese patients with cirrhosis: A prospective multicenter study. Hepatol. Int. 2022, 16, 691–701. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, J.; Ma, B.; Chen, G.; Wang, Z.; Wang, S.; Jing, N.; Zhang, J.; Wang, B.; Yan, W.; et al. Characteristics of humoral and cellular responses to coronavirus disease 2019 (COVID-19) inactivated vaccine in central China: A prospective, multicenter, longitudinal study. Front. Immunol. 2023, 14, 1107866. [Google Scholar] [CrossRef]
- Hu, M.; Guo, W.; Liu, L.; Yang, Y.; Xu, Q.; Cheng, F.; Zeng, F.; Zhang, Y. Safety monitoring in inactivated COVID-19 vaccines by clinical pharmacists from a single center in China. Front. Immunol. 2022, 13, 882919. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, L.; Zhu, Y.; Ye, Q.; Yu, X.; Fu, M.; Lu, J.; Li, X.; Huang, Y.; Zhang, J.; et al. Safety survey by clinical pharmacists on COVID-19 vaccination from a single center in China. Hum. Vaccin. Immunother. 2021, 17, 2863–2867. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Al Ketbi, L.M.; Al Kaabi, N.; Al Mansoori, M.; Al Maskari, N.N.; Al Shamsi, M.S.; Alderei, A.S.; El Eissaee, N.; Al Ketbi, R.M.; Al Shamsi, N.S.; et al. Vaccine Side Effects Following COVID-19 Vaccination Among the Residents of the UAE-An Observational Study. Front. Public Health 2022, 10, 876336. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw Open 2021, 4, e2140364. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and mRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef]
- Cao, H.; Huang, Y.; Zhong, C.; Liao, X.; Tan, W.; Zhao, S.; Guo, L.; Fan, R. Antibody response and safety of inactivated SARS-CoV-2 vaccines in chronic hepatitis B patients with and without cirrhosis. Front. Immunol. 2023, 14, 1167533. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Moon, A.M.; Webb, G.J.; Garcia-Juarez, I.; Kulkarni, A.V.; Adali, G.; Wong, D.K.; Lusina, B.; Dalekos, G.N.; Masson, S.; Shore, B.M.; et al. SARS-CoV-2 Infections Among Patients With Liver Disease and Liver Transplantation Who Received COVID-19 Vaccination. Hepatol. Commun. 2022, 6, 889–897. [Google Scholar] [CrossRef]
- Wang, M.W.; Wen, W.; Wang, N.; Zhou, M.Y.; Wang, C.Y.; Ni, J.; Jiang, J.-J.; Zhang, X.-W.; Feng, Z.-H.; Cheng, Y.-R. COVID-19 Vaccination Acceptance Among Healthcare Workers and Non-healthcare Workers in China: A Survey. Front. Public Health 2021, 9, 709056. [Google Scholar] [CrossRef]
- Xiong, L.; Han, M.; Wang, C.; Liu, K.; Liu, T. Frailty is an independent determinant of COVID-19 vaccine hesitancy in the elderly: A cross-sectional study. Cell. Mol. Biol. 2022, 68, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Qunaibi, E.A.; Helmy, M.; Basheti, I.; Sultan, I. A high rate of COVID-19 vaccine hesitancy in a large-scale survey on Arabs. eLife 2021, 10, e68038. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.M.; Mohamed, E.Y.; Elsabagh, H.M.; Ahmad, M.S.; Shaik, R.A.; Mehta, V.; Mathur, A.; Ghatge, S.B. COVID-19 Vaccine Hesitancy among the General Population: A Cross-Sectional Study. Vaccines 2023, 11, 1125. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Wong, E.L.Y.; Cheung, A.W.L.; Huang, J.; Lai, C.K.C.; Yeoh, E.K.; Chan, P.K.S. COVID-19 Vaccine Hesitancy in a City with Free Choice and Sufficient Doses. Vaccines 2021, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Su, X.; Xiao, W.; Wang, H.; Si, M.; Wang, W.; Gu, X.; Ma, L.; Li, L.; Zhang, S.; et al. COVID-19 vaccine hesitancy among different population groups in China: A national multicenter online survey. BMC Infect. Dis. 2022, 22, 153. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.-M.; Su, X.-Y.; Xiao, W.-J.; Si, M.-Y.; Wang, W.-J.; Gu, X.-F.; Ma, L.; Li, L.; Zhang, S.-K.; et al. Acceptance of the COVID-19 vaccine based on the health belief model: A multicenter national survey among medical care workers in China. Hum. Vaccin. Immunother. 2022, 18, 2076523. [Google Scholar] [CrossRef]
- Wynter-Adams, D.; Thomas-Brown, P. COVID-19 vaccine hesitancy in a developing country: Prevalence, explanatory factors and implications for the future. Public Health 2023, 217, 146–154. [Google Scholar] [CrossRef]
- Qin, C.; Du, M.; Wang, Y.; Liu, Q.; Yan, W.; Tao, L.; Liu, M.; Liu, J. Assessing acceptability of the fourth dose against COVID-19 among Chinese adults: A population-based survey. Hum. Vaccin. Immunother. 2023, 19, 2186108. [Google Scholar] [CrossRef]
- Thuluvath, P.J.; Robarts, P.; Chauhan, M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J. Hepatol. 2021, 75, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Technical Working Group. Technical Vaccination Recommendations for COVID-19 Vaccines in China (First Edition). China CDC Wkly. 2021, 3, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Tarimo, C.S.; Wang, M.; Gu, J.; Wei, W.; Ma, M.; Zhao, L.; Mu, Z.; Miao, Y. COVID-19 Vaccine Hesitancy Among Chinese Population: A Large-Scale National Study. Front. Immunol. 2021, 12, 781161. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.; Hervey, J.; Hoffman, K.; Wood, J.; Johnson, J.; Deighton, D.; Clermont, D.; Loew, B.; Goldberg, S.L. COVID-19 Vaccine Hesitancy and Acceptance Among Individuals With Cancer, Autoimmune Diseases, or Other Serious Comorbid Conditions: Cross-sectional, Internet-Based Survey. JMIR Public Health Surveill. 2022, 8, e29872. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 1491) | With COVID-19 Vaccination (n = 1239) | Without COVID-19 Vaccination (n = 252) | p-Value | |
|---|---|---|---|---|
| Age (years), Median (IQR) | 46.00 (20.00) | 43.00 (19.00) | 56.00 (17.50) | <0.001 |
| Male, n (%) | 973 (65.3%) | 817 (65.9%) | 156 (61.9%) | 0.220 |
| Etiology of liver disease, n (%) | <0.001 | |||
| Hepatitis B/C | 1169 (78.4%) | 1006 (81.2%) | 163 (64.7%) | |
| Autoimmune liver diseases | 36 (2.4%) | 23 (1.9%) | 13 (5.2%) | |
| ALD | 18 (1.2%) | 14 (1.1%) | 4 (1.6%) | |
| NAFLD | 28 (1.9%) | 26 (2.1%) | 2 (0.8%) | |
| Hepatitis cirrhosis | 152 (10.2%) | 92 (7.4%) | 60 (23.8%) | |
| Autoimmune cirrhosis | 10 (0.7%) | 2 (0.2%) | 8 (3.2%) | |
| Alcoholic cirrhosis | 9 (0.6%) | 5 (0.4%) | 4 (1.6%) | |
| Fatty cirrhosis | 6 (0.4%) | 5 (0.4%) | 1 (0.4%) | |
| Other cirrhosis | 153 (10.3%) | 93 (7.5%) | 60 (23.8%) | |
| Other liver diseases | 33 (2.2%) | 20 (1.6%) | 13 (5.2%) | |
| Liver disease course (years), n (%) | <0.001 | |||
| <1 year | 230 (15.4%) | 214 (17.3%) | 16 (6.4%) | |
| 1–5 years | 376 (25.2%) | 297 (24.0%) | 79 (31.4%) | |
| 5–10 years | 293 (19.7%) | 236 (19.1%) | 57 (22.6%) | |
| >10 years | 592 (39.7%) | 492 (39.7%) | 100 (39.7%) | |
| Treatments, n (%) | 1235 (82.8%) | 996 (80.4%) | 239 (94.8%) | <0.001 |
| Antiviral drugs | 1010 (67.7%) | 833 (67.2%) | 177 (70.2%) | |
| Immunosuppressants | 14 (0.9%) | 7 (0.6%) | 7 (2.8%) | |
| Chinese patent medicines | 237 (15.9%) | 163 (13.2%) | 74 (29.4%) | |
| Other treatments | 81 (5.4%) | 52 (4.2%) | 29 (11.5%) | |
| Comorbidities, n (%) | 247 (16.6%) | 166 (13.4%) | 81 (32.1%) | 0.511 |
| Cardiovascular and cerebrovascular diseases | 128 (8.6%) | 89 (7.2%) | 39 (15.5%) | |
| Respiratory diseases | 15 (1.0%) | 9 (0.7%) | 6 (2.4%) | |
| Diabetes | 87 (5.8%) | 51 (4.1%) | 36 (14.3%) | |
| Chronic kidney disease | 19 (1.3%) | 12 (1.0%) | 7 (2.8%) | |
| Allergy history, n (%) | <0.001 | |||
| Yes | 174 (11.7%) | 127 (10.3%) | 47 (18.7%) | |
| No | 1317 (88.3%) | 1112 (89.8%) | 205 (81.4%) | |
| Living area, n (%) | 0.142 | |||
| Rural | 820 (55.0%) | 668 (53.9%) | 152 (60.3%) | |
| Township | 117 (7.9%) | 102 (8.2%) | 15 (86.0%) | |
| City | 554 (37.2%) | 469 (37.9%) | 85 (33.7%) | |
| Educational attainment, n (%) | <0.001 | |||
| No university degree | 1176 (78.9%) | 948 (76.5%) | 228 (90.5%) | |
| University degree | 315 (21.1%) | 291 (23.5%) | 24 (9.5%) | |
| Monthly household income (Chinese Yuan, CNY), n (%) | <0.001 | |||
| ≤2000 | 406 (27.2%) | 313 (25.3%) | 93 (36.9%) | |
| 2001–5000 | 672 (45.1%) | 571 (46.1%) | 101 (40.1%) | |
| 5001–10,000 | 258 (17.3%) | 235 (19.0%) | 23 (9.1%) | |
| >10,000 | 62 (4.2%) | 51 (4.1%) | 11 (4.4%) | |
| Marital status, n (%) | 0.106 | |||
| Unmarried | 67 (4.5%) | 62 (5.0%) | 5 (2.0%) | |
| Married (with spouse) | 1381 (92.6%) | 1141 (92.1%) | 240 (95.2%) | |
| Divorced or widowed | 43 (2.9%) | 36 (2.9%) | 7 (2.8%) | |
| Vaccine belief, n (%) | ||||
| Worry about infecting SARS-CoV-2 | 1092 (73.2%) | 901 (72.7%) | 191 (75.8%) | 0.315 |
| I think getting the COVID-19 vaccine is important for my health, n (%) | <0.001 | |||
| Agree | 1319 (88.5%) | 1115 (90.0%) | 204 (81.0%) | |
| Neutral | 148 (9.9%) | 104 (8.4%) | 44 (17.5%) | |
| Disagree | 24 (1.6%) | 20 (1.6%) | 4 (1.6%) | |
| I think the COVID-19 vaccine is effective, n (%) | 0.025 | |||
| Agree | 1342 (90.0%) | 1127 (91.0%) | 215 (85.3%) | |
| Neutral | 133 (8.9%) | 100 (8.1%) | 33 (13.1%) | |
| Disagree | 16 (1.1%) | 12 (1.0%) | 4 (1.6%) | |
| I think getting the COVID-19 vaccine is important for the health of those around me, n (%) | 0.004 | |||
| Agree | 1366 (91.6%) | 1146 (92.5%) | 220 (87.3%) | |
| Neutral | 111 (7.4%) | 80 (6.5%) | 31 (12.3%) | |
| Disagree | 14 (0.9%) | 13 (1.1%) | 1 (0.4%) | |
| I think the COVID-19 vaccine provided by the government is helpful in epidemic prevention, n (%) | 0.382 | |||
| Agree | 1390 (93.2%) | 1158 (93.5%) | 232 (92.1%) | |
| Neutral | 89 (6.0%) | 70 (5.7%) | 19 (7.5%) | |
| Disagree | 12 (0.8%) | 11 (0.9%) | 1 (0.4%) | |
| I think COVID-19 vaccination has adverse effects on CLD and treatment, n (%) | <0.001 | |||
| Agree | 548 (36.8%) | 446 (36.0%) | 102 (40.5%) | |
| Neutral | 397 (26.6%) | 297 (24.0%) | 100 (39.7%) | |
| Disagree | 546 (36.6%) | 496 (40.0%) | 50 (19.8%) | |
| I think the COVID-19 vaccine is safe, n (%) | 0.000 | |||
| Agree | 1283 (86.1%) | 1086 (87.7%) | 197 (78.2%) | |
| Neutral | 191 (12.8%) | 139 (11.2%) | 52 (20.6%) | |
| Disagree | 17 (1.1%) | 14 (1.1%) | 3 (1.2%) | |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Age ≥ 60 (vs. <60) | 3.597 (2.585–5.005) | <0.001 | 2.731 (1.811–4.12) | <0.001 |
| Types of CLD | ||||
| Hepatitis B/C (vs. not) | 0.424 (0.316–0.57) | <0.001 | 0.833 (0.537–1.292) | 0.415 |
| Cirrhosis (vs. not) | 5.977 (4.466–8.00) | <0.001 | 2.922 (1.972–4.331) | <0.001 |
| Autoimmune liver diseases (vs. not) | 2.876 (1.437–5.757) | 0.003 | 1.172 (0.453–3.034) | 0.744 |
| Liver disease course (vs. <1 year) | ||||
| 1–5 years | 3.558 (2.021–6.262) | <0.001 | 3.37 (1.782–6.374) | <0.001 |
| 5–10 years | 3.23 (1.8–5.796) | <0.001 | 3.236 (1.666–6.286) | 0.001 |
| >10 years | 2.718 (1.566–4.719) | <0.001 | 2.792 (1.495–5.216) | 0.001 |
| Treatments (vs. not) | 4.485 (2.523–7.975) | <0.001 | 2.471 (1.318–4.632) | 0.005 |
| Immunosuppressants | 5.029 (1.748–14.465) | <0.001 | 3.764 (1.083–13.08) | 0.037 |
| Chinese patent medicine | 2.744 (1.998–3.769) | <0.001 | 1.16 (0.777–1.733) | 0.467 |
| Comorbidities (vs. not) | 3.062 (2.244–4.178) | <0.001 | 2.004 (1.058–3.798) | 0.033 |
| Cardiovascular and cerebrovascular diseases | 2.366 (1.58–3.543) | <0.001 | 0.748 (0.379–1.475) | 0.401 |
| Respiratory diseases | 3.333 (1.176–9.45) | 0.024 | 0.491 (0.125–1.932) | 0.309 |
| Diabetes | 3.882 (2.474–6.092) | <0.001 | 1.101 (0.553–2.192) | 0.785 |
| Chronic kidney disease | 2.921 (1.139–7.495) | 0.026 | 0.963 (0.315–2.95) | 0.948 |
| Allergy history (vs. not) | 2.007 (1.392–2.896) | <0.001 | 1.732 (1.107–2.71) | 0.016 |
| No university degree (vs. have) | 2.916 (1.877–4.531) | <0.001 | 1.959 (1.187–3.231) | 0.008 |
| Not knowledgeable about COVID-19 vaccine considerations (vs. knowledgeable) | 3.158 (2.303–4.332) | <0.001 | 3.145 (2.253–4.391) | 0.001 |
| Thinking COVID-19 vaccination affects treatment (vs. not and neutral) | 3.54 (2.522–4.968) | <0.001 | 1.89 (1.284–2.783) | <0.001 |
| Not receiving doctors’ advice on vaccination (vs. receiving) | 4.508 (3.398–5.981) | <0.001 | 3.145 (2.253–4.391) | <0.001 |
| Vaccine Type | Number of Participants Who Completed the Third Dose of the Vaccine | Number of Participants Who Accepted the Vaccine | Number of Participants Who Refused the Vaccine | p-Value |
|---|---|---|---|---|
| Inactivated vaccines, n (%) | 668 (59.9%) | 529 (58.3%) | 139 (58.9%) | 0.409 |
| Recombinant protein vaccines, n (%) | 465 (39.2%) | 372 (41.0%) | 93 (39.4%) | |
| Mixed vaccines, n (%) | 11 (0.9%) | 7 (0.8%) | 4 (1.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yuan, W.; Zhan, H.; Kang, H.; Li, X.; Chen, Y.; Li, H.; Sun, X.; Cheng, L.; Zheng, H.; et al. SARS-CoV-2 Vaccine Uptake among Patients with Chronic Liver Disease: A Cross-Sectional Analysis in Hebei Province, China. Vaccines 2023, 11, 1293. https://doi.org/10.3390/vaccines11081293
Liu Y, Yuan W, Zhan H, Kang H, Li X, Chen Y, Li H, Sun X, Cheng L, Zheng H, et al. SARS-CoV-2 Vaccine Uptake among Patients with Chronic Liver Disease: A Cross-Sectional Analysis in Hebei Province, China. Vaccines. 2023; 11(8):1293. https://doi.org/10.3390/vaccines11081293
Chicago/Turabian StyleLiu, Yongmei, Wenfang Yuan, Haoting Zhan, Haiyan Kang, Xiaomeng Li, Yongliang Chen, Haolong Li, Xingli Sun, Linlin Cheng, Haojie Zheng, and et al. 2023. "SARS-CoV-2 Vaccine Uptake among Patients with Chronic Liver Disease: A Cross-Sectional Analysis in Hebei Province, China" Vaccines 11, no. 8: 1293. https://doi.org/10.3390/vaccines11081293
APA StyleLiu, Y., Yuan, W., Zhan, H., Kang, H., Li, X., Chen, Y., Li, H., Sun, X., Cheng, L., Zheng, H., Wang, W., Guo, X., Li, Y., & Dai, E. (2023). SARS-CoV-2 Vaccine Uptake among Patients with Chronic Liver Disease: A Cross-Sectional Analysis in Hebei Province, China. Vaccines, 11(8), 1293. https://doi.org/10.3390/vaccines11081293






