COVID-19 Booster Dose Coverage and Hesitancy among Older Adults in an Urban Slum and Resettlement Colony in Delhi, India
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- India: WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data [Internet]. Available online: https://covid19.who.int/ (accessed on 12 February 2023).
- Paital, B.; Das, K.; Parida, S.K. Inter nation social lockdown versus medical care against COVID-19, a mild environmental insight with special reference to India. Sci. Total Environ. 2020, 728, 138914. [Google Scholar] [CrossRef]
- Paul, P.; El-Naas, A.; Hamad, O.; Salameh, M.A.; Mhaimeed, N.; Laswi, I.; Abdelati, A.A.; AlAnni, J.; Khanjar, B.; Al-Ali, D.; et al. Effectiveness of the pre-Omicron COVID-19 vaccines against Omicron in reducing infection, hospitalization, severity, and mortality compared to Delta and other variants: A systematic review. Hum. Vaccines Immunother. 2023, 19, 2167410. [Google Scholar] [CrossRef]
- Sharma, E.; Revinipati, S.; Bhandari, S.; Thakur, S.; Goyal, S.; Ghose, A.; Bajpai, S.; Muhammad, W.; Boussios, S. Efficacy and Safety of COVID-19 Vaccines—An Update. Diseases 2022, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, M.H.; Spinardi, J.; Zhang, L.; Oh, H.M.L.; Srivastava, A. Evidence synthesis and pooled analysis of vaccine effectiveness for COVID-19 mRNA vaccine BNT162b2 as a heterologous booster after inactivated SARS-CoV-2 virus vaccines. Hum. Vaccines Immunother. 2023, 19, 2165856. [Google Scholar] [CrossRef] [PubMed]
- Edara, V.V.; Manning, K.E.; Ellis, M.; Lai, L.; Moore, K.M.; Foster, S.L.; Floyd, K.; Davis-Gardner, M.E.; Mantus, G.; Nyhoff, L.E.; et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant. Cell Rep. Med. 2022, 3, 100529. [Google Scholar] [CrossRef]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef]
- Vasireddy, D.; Vanaparthy, R.; Mohan, G.; Malayala, S.V.; Atluri, P. Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know? J. Clin. Med. Res. 2021, 13, 317–325. [Google Scholar] [CrossRef]
- Deng, J.; Ma, Y.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Comparison of the Effectiveness and Safety of Heterologous Booster Doses with Homologous Booster Doses for SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 10752. [Google Scholar] [CrossRef]
- Singh, K.; Verma, A.; Lakshminarayan, M. India’s efforts to achieve 1.5 billion COVID-19 vaccinations: A narrative review. Osong Public Health Res. Perspect. 2022, 13, 316–327. [Google Scholar] [CrossRef]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Guidelines for COVID-19 Vaccination of Children between 15–18 Years and Precaution Dose to HCWs, FLWs & 60+ Population with Comorbidities. Ministry of Health and Family Welfare; [Internet]. Available online: https://www.mohfw.gov.in/pdf/GuidelinesforCOVID19VaccinationofChildrenbetween15to18yearsandPrecautionDosetoHCWsFLWs&60populationwithcomorbidities.pdf (accessed on 2 April 2023).
- Bhatnagar, T.; Chaudhuri, S.; Ponnaiah, M.; Yadav, P.D.; Sabarinathan, R.; Sahay, R.R.; Ahmed, F.; Aswathy, S.; Bhardwaj, P.; Bilimale, A.; et al. Effectiveness of BBV152/Covaxin and AZD1222/Covishield vaccines against severe COVID-19 and B.1.617.2/Delta variant in India, 2021: A multi-centric hospital-based case-control study. Int. J. Infect. Dis. 2022, 122, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Mani, K.; Lodha, R.; Bakhshi, S.; Mathur, V.P.; Gupta, P.; Kedia, S.; Sankar, J.; Kumar, P.; Kumar, A.; et al. SARS-CoV-2 Reinfection Rate and Estimated Effectiveness of the Inactivated Whole Virion Vaccine BBV152 against Reinfection Among Health Care Workers in New Delhi, India. JAMA Netw. Open 2022, 5, e2142210. [Google Scholar] [CrossRef]
- Parida, S.P.; Sahu, D.P.; Singh, A.K.; Alekhya, G.; Subba, S.H.; Mishra, A.; Padhy, B.M.; Patro, B.K. Adverse events following immunization of COVID-19 (Covaxin) vaccine at a tertiary care center of India. J. Med. Virol. 2022, 94, 2453–2459. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.; Raju, R.; Babji, S.; George, A.; Madhavan, R.; Xavier, J.V.L.; Chelladurai, J.S.D.; Nikitha, O.S.; Deborah, A.A.; Vijayakumar, S.; et al. Immunogenicity and safety of homologous and heterologous booster vaccination of ChAdOx1 nCoV-19 (COVISHIELD™) and BBV152 (COVAXIN®): A non-inferiority phase 4, participant and observer-blinded, randomised study. Lancet Reg. Health Southeast Asia 2023, 12, 100141. [Google Scholar] [CrossRef]
- Deshpande, G.R.; Yadav, P.D.; Abraham, P.; A Nyayanit, D.; Sapkal, G.N.; Shete, A.M.; Gupta, N.; Vadrevu, K.M.; Ella, R.; Panda, S.; et al. Booster dose of the inactivated COVID-19 vaccine BBV152 (Covaxin) enhances the neutralizing antibody response against Alpha, Beta, Delta and Omicron variants of concern. J. Travel Med. 2022, 29, taac039. [Google Scholar] [CrossRef]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Sharma, P.; Basu, S.; Mishra, S.; Mundeja, N.; Charan, B.S.; Singh, G.; Singh, M.M. COVID-19 Vaccine Acceptance and Its Determinants in the General Population of Delhi, India: A State Level Cross-Sectional Survey. Cureus 2022, 14, e26936. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Issa, J.; Hussain, S.; Tanasiewicz, M.; Wojtyczka, R.; Kubina, R.; Konwinska, M.D.; Riad, A. COVID-19 vaccine booster hesitancy (VBH) of healthcare professionals and students in Poland: Cross-sectional survey-based study. Front. Public Health 2022, 10, 938067. [Google Scholar] [CrossRef]
- Attia, S.; Mausbach, K.; Klugar, M.; Howaldt, H.-P.; Riad, A. Prevalence and Drivers of COVID-19 Vaccine Booster Hesitancy Among German University Students and Employees. Front. Public Health 2022, 10, 846861. [Google Scholar] [CrossRef]
- Klugar, M.; Riad, A.; Mohanan, L.; Pokorná, A. COVID-19 Vaccine Booster Hesitancy (VBH) of Healthcare Workers in Czechia: National Cross-Sectional Study. Vaccines 2021, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Omicron is supercharging the COVID vaccine booster debate. Nature 2021, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Sahay, R.R.; Yadav, P.D.; Nandapurkar, A.; Dhawde, R.; Suryawanshi, A.; Patil, D.Y.; Shete, A.M.; Sapkal, G.N.; Kulkarni, M.; Gurav, Y.K.; et al. Evaluation of immunogenicity post two doses of inactivated SARS-CoV-2 vaccine, Covaxin after six months. Hum. Vaccines Immunother. 2022, 18, 2156753. [Google Scholar] [CrossRef] [PubMed]
- Yadete, T.; Batra, K.; Netski, D.M.; Antonio, S.; Patros, M.J.; Bester, J.C. Assessing Acceptability of COVID-19 Vaccine Booster Dose among Adult Americans: A Cross-Sectional Study. Vaccines 2021, 9, 1424. [Google Scholar] [CrossRef]
- Qin, C.; Yan, W.; Tao, L.; Liu, M.; Liu, J. The Association between Risk Perception and Hesitancy toward the Booster Dose of COVID-19 Vaccine among People Aged 60 Years and Older in China. Vaccines 2022, 10, 1112. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Paul, E.; Fancourt, D. Predictors of uncertainty and unwillingness to receive the COVID-19 booster vaccine: An observational study of 22,139 fully vaccinated adults in the UK. Lancet Reg. Health Eur. 2022, 14, 100317. [Google Scholar] [CrossRef]
- Kumar, V.M.; Pandi-Perumal, S.R.; Trakht, I.; Thyagarajan, S.P. Strategy for COVID-19 vaccination in India: The country with the second highest population and number of cases. NPJ Vaccines 2021, 6, 60. [Google Scholar] [CrossRef]
- COVID VaccinationBooklet14SEP.pdf. [Internet]. Available online: https://www.mohfw.gov.in/pdf/COVIDVaccinationBooklet14SEP.pdf (accessed on 12 February 2023).
- Zhang, X.; Chen, L.-L.; Ip, J.D.; Chan, W.-M.; Hung, I.F.-N.; Yuen, K.-Y.; Li, X.; To, K.K.-W. Omicron sublineage recombinant XBB evades neutralising antibodies in recipients of BNT162b2 or CoronaVac vaccines. Lancet Microbe 2022, 4, e131. [Google Scholar] [CrossRef]
- Wong, M.K.; Brooks, D.J.; Ikejezie, J.; Gacic-Dobo, M.; Dumolard, L.; Nedelec, Y.; Steulet, C.; Kassamali, Z.; Acma, A.; Ajong, B.N.; et al. COVID-19 Mortality and Progress Toward Vaccinating Older Adults—World Health Organization, Worldwide, 2020–2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Miqueleiz, A.; Casado, I.; Navascués, A.; Burgui, C.; Ezpeleta, C.; Castilla, J.; Guevara, M.; The Working Group for the Study of COVID-19 in Navarra. Risk reduction of hospitalisation and severe disease in vaccinated COVID-19 cases during the SARS-CoV-2 variant Omicron BA.1-predominant period, Navarre, Spain, January to March 2022. Eurosurveillance 2023, 28, 2200337. [Google Scholar] [CrossRef]
- WHO. Urban Slum Population (%). [Internet]. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/2476 (accessed on 15 June 2023).
- World Bank. Population Living in Slums (% of Urban Population)—India. [Internet]. Available online: https://data.worldbank.org/indicator/EN.POP.SLUM.UR.ZS?locations=IN (accessed on 15 June 2023).
- Centre for Policy Research. Categorisation of Settlements in Delhi. [Internet]. Available online: https://cprindia.org/wp-content/uploads/2021/12/Categorisation-of-Settlement-in-Delhi.pdf (accessed on 15 June 2023).
- Tamysetty, S.; Babu, G.R.; Sahu, B.; Shapeti, S.; Ravi, D.; Lobo, E.; Varughese, C.S.; Bhide, A.; Madhale, A.; Manyal, M.; et al. Predictors of COVID-19 Vaccine Confidence: Findings from Slums of Four Major Metro Cities of India. Vaccines 2021, 10, 60. [Google Scholar] [CrossRef]
- Bhattacherjee, S.; Dasgupta, P.; Mukherjee, A.; Dasgupta, S. Vaccine hesitancy for childhood vaccinations in slum areas of Siliguri, India. Indian J. Public Health 2018, 62, 253–258. [Google Scholar] [CrossRef]
- Sharma, N.; Palo, S.K.; Bhimarasetty, D.M.; Kandipudi, K.L.P.; Purty, A.J.; Kumar, T.; Basu, S.; Alice, A.; Velavan, A.; Madhavan, S.; et al. Community Dynamics and Engagement Strategies in Establishing Demographic Development and Environmental Surveillance Systems: A Multi-Site Report from India. Healthcare 2023, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Nr, R.M.; Brahmajosyula, A.; Khamar, A.; Acharya, N.; Bilichod, L.P.; Kondath, D. Coverage of Coronavirus Disease-2019 (COVID-19) Booster Dose (Precautionary) in the Adult Population: An Online Survey. Cureus 2022, 14, e26912. [Google Scholar] [CrossRef]
- Achrekar, G.C.; Batra, K.; Urankar, Y.; Batra, R.; Iqbal, N.; Choudhury, S.A.; Hooda, D.; Khan, R.; Arora, S.; Singh, A.; et al. Assessing COVID-19 Booster Hesitancy and Its Correlates: An Early Evidence from India. Vaccines 2022, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.; Thakur, V.; Nath, N.; Malhotra, T.; Gupta, A.; Batlish, R. Adverse events following ChAdOx1 nCoV-19 Vaccine (COVISHIELD) amongst health care workers: A prospective observational study. Med. J. Armed Forces India 2021, 77 (Suppl. S2), S283–S288. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.; Johnson, A.; Mwanza, I.; Ngwira, H.; Schechter, J.; Odera, M.; Mbewe, D.N.; Moenga, R.; Muyingo, P.; Jalloh, R.; et al. Community Health Workers in Pandemics: Evidence and Investment Implications. Glob. Health Sci. Pract. 2022, 10, e2100648. [Google Scholar] [CrossRef]
- Stevenson, M.C.; Norrbom, C.; Savela, M.; Xiong, Y.L.; Lee, T.F.; Garcia, C.; Winstead, O.; Northrop, M.; Sandy, M. Community Health Workers in Time of Crisis: A COVID-19 Case Study. J. Humanist. Psychol. 2022, 1–30. [Google Scholar] [CrossRef]
- Accordino, S.; Canetta, C.; Blasi, F. Characteristics and outcomes of unvaccinated and vaccinated COVID-19 patients with acute respiratory failure treated with CPAP in a medical intermediate care unit. Eur. J. Intern. Med. 2023, 111, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Balian, S.; Bailey, B.; Abboud, S.; Kim, Y.; Humphries, D.; Kambali, S.; Kalangi, S.T.; Jarvis, J.; Dayal, L.; Beiz, H.; et al. Comparative admission rates and infection severity of COVID-19 among unvaccinated and vaccinated patients. J. Investig. Med. 2023, 71, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Economic Times. Jan 17 DGCI Approves COVID-19 Vaccine Covovax as Heterologous Booster Dose. [Internet]. Available online: https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/dgci-approves-covid-19-vaccine-covovax-as-heterologous-booster-dose/articleshow/97038353.cms (accessed on 2 April 2023).
- Harapan, H.; Wagner, A.L.; Yufika, A.; Winardi, W.; Anwar, S.; Gan, A.K.; Setiawan, A.M.; Rajamoorthy, Y.; Sofyan, H.; Vo, T.Q.; et al. Willingness-to-pay for a COVID-19 vaccine and its associated determinants in Indonesia. Hum. Vaccines Immunother. 2020, 16, 3074–3080. [Google Scholar] [CrossRef]
- Biasio, L.R. Vaccine hesitancy and health literacy. Hum. Vaccines Immunother. 2016, 13, 701–702. [Google Scholar] [CrossRef] [PubMed]
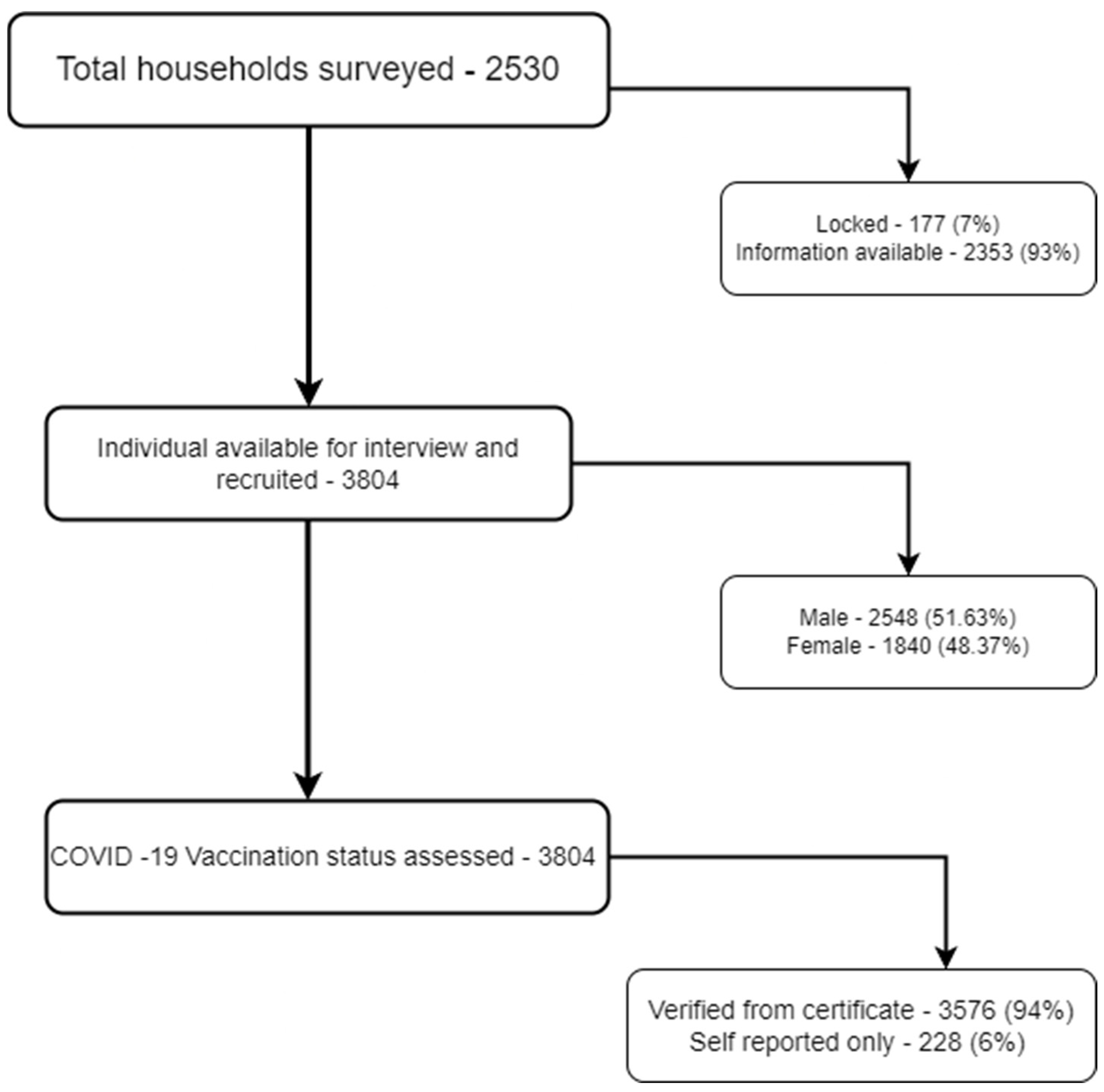
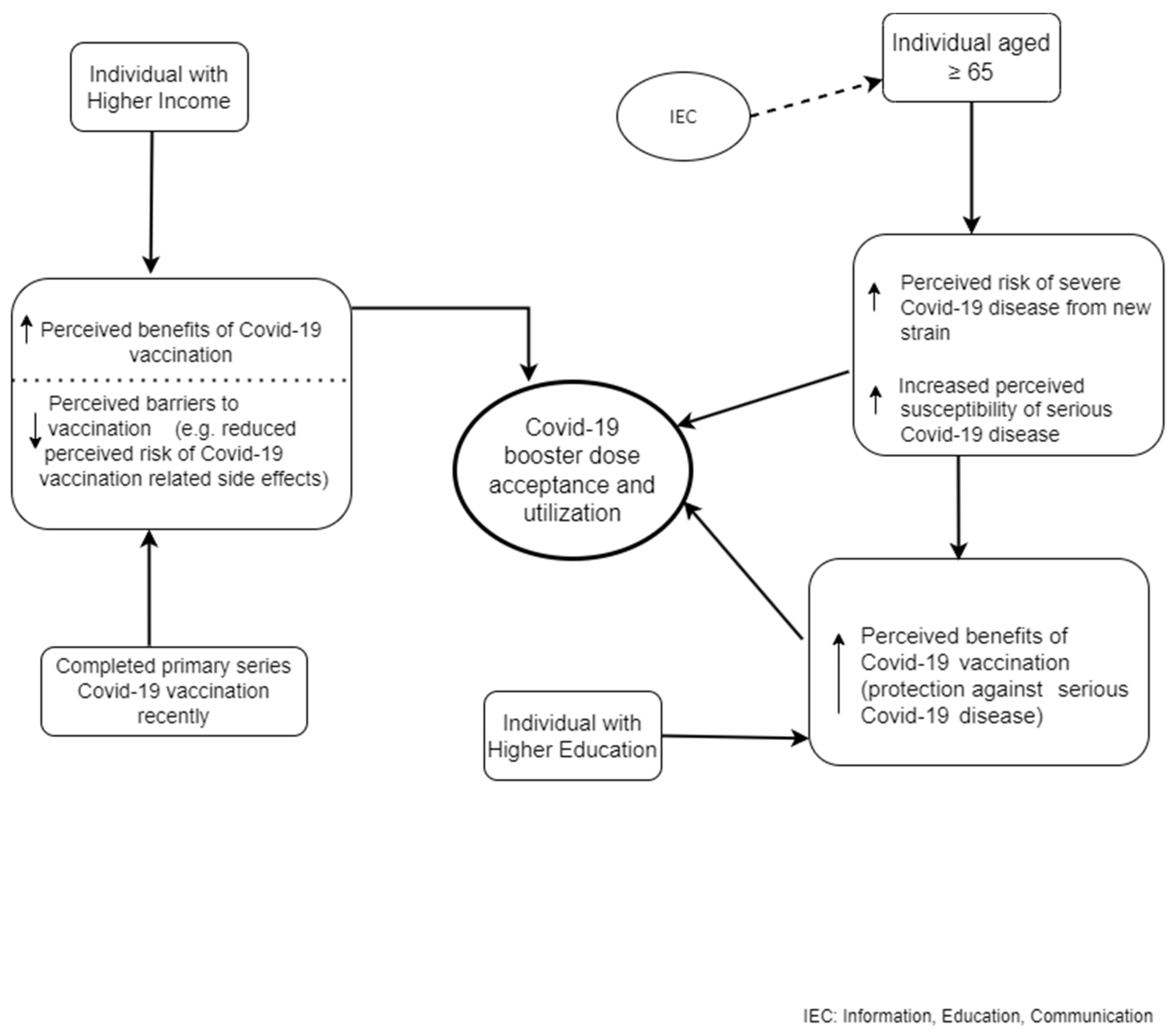
| Variables | Incomplete Dose c (n = 183) | Two Doses without Booster (n = 1404) | Booster Taken (n = 2217) | Total (n = 3804) |
|---|---|---|---|---|
| Age (in years) | ||||
| 50–65 | 79 (43.17) | 835 (59.47) | 1098 (49.53) | 2012 (52.89) |
| ≥66 | 104 (56.83) | 569 (40.53) | 1119 (50.47) | 1792 (47.11) |
| Sex | ||||
| Male | 92 (50.27) | 697 (49.64) | 1175 (53.00) | 1964 (51.63) |
| Female | 91 (49.73) | 707 (50.36) | 1042 (47.00) | 1840 (48.37) |
| Education | ||||
| Illiterate | 76 (41.53) | 485 (34.54) | 618 (27.88) | 1179 (30.99) |
| Primary | 39 (21.31) | 270 (19.23) | 374 (16.87) | 683 (17.95) |
| Secondary/High school | 36 (19.67) | 340 (24.22) | 546 (24.63) | 922 (24.24) |
| Inter certificate/Graduate and above | 32 (17.49) | 309 (22.01) | 679 (30.63) | 1020 (26.81) |
| PCI a (in Rupees) | ||||
| ≤4000 | 98 (53.55) | 693 (43.36) | 900 (40.60) | 1691 (44.45) |
| >4000 | 85 (46.45) | 711 (50.64) | 1317 (59.40) | 2113 (55.55) |
| Healthcare worker | ||||
| No | 182 (99.45) | 1386 (98.72) | 2178 (98.24) | 3746 (98.48) |
| Yes | 1 (0.55) | 18 (1.28) | 39 (1.76) | 58 (1.52) |
| Comorbidities | ||||
| None | 88 (48.09) | 957 (68.36) | 1372 (61.89) | 2417 (63.61) |
| ≥1 Disease present | 95 (51.91) | 443 (31.64) | 845 (38.11) | 1383 (36.39) |
| Type of COVID-19 vaccine | ||||
| Covaxin | 9 (14.52) | 193 (13.75) | 256 (11.55) | 458 (12.44) |
| Covishield | 51 (82.26) | 1199 (85.40) | 1958 (88.32) | 3208 (87.10) |
| Other | 2 (3.23) | 12 (0.85) | 3 (0.14) | 17 (0.46) |
| COVID-19 infection (confirmed) | ||||
| Never | 176 (96.17) | 1336 (95.16) | 2073 (93.50) | 3585 (94.24) |
| Only once | 7 (3.83) | 51 (3.63) | 113 (5.10) | 171 (4.50) |
| More than once | 0 (0.00) | 17 (1.21) | 31 (1.40) | 48 (1.26) |
| COVID-19-related oxygen/hospitalization requirement | ||||
| No | 182 (99.45) | 1379 (98.22) | 2176 (98.15) | 3737 (98.24) |
| Yes b | 1 (0.55) | 25 (1.78) | 41 (1.85) | 67 (1.76) |
| Year of last COVID-19 vaccine dose | ||||
| 2021 | 39 (62.90) | 559 (39.81) | 404 (18.22) | 1002 (27.21) |
| 2022 | 23 (37.10) | 845 (60.19) | 1813 (81.78) | 2681 (72.79) |
| Variables | Crude OR (95% CI) | p-Value | aOR (95% CI) | p-Value |
|---|---|---|---|---|
| Age (in years) | ||||
| 50–65 | Ref | Ref | ||
| >65 | 1.36 (1.19, 1.54) | <0.001 | 1.58 (1.37, 1.83) | <0.001 |
| Sex | ||||
| Female | Ref | Ref | ||
| Male | 1.12 (0.98, 1.28) | 0.07 | 0.92 (0.78, 1.07) | 0.28 |
| Education | ||||
| Illiterate | Ref | Ref | ||
| Primary | 1.07 (0.88, 1.29) | 1.07 (0.87, 1.32) | ||
| Secondary/High school | 1.27 (1.07, 1.51) | 1.33 (1.09, 1.64) | ||
| Inter certificate/Graduate and above | 1.76 (1.48, 2.09) | <0.001 | 1.77 (1.41, 2.22) | <0.001 |
| PCI a (in Rupees) | ||||
| ≤4000 | Ref | Ref | ||
| >4000 | 1.41 (1.23, 1.60) | <0.001 | 1.31 (1.12, 1.52) | <0.001 |
| Healthcare worker | ||||
| No | Ref | Ref | ||
| Yes | 1.36 (0.78, 2.35) | 0.26 | 0.94 (0.52, 1.68) | 0.84 |
| Comorbidities | ||||
| None | Ref | Ref | ||
| ≥1 disease | 1.20 (1.05, 1.37) | 0.008 | 1.25 (1.08, 1.45) | 0.003 |
| Type of COVID-19 vaccine (n = 3783) | ||||
| Covaxin | Ref | Ref | ||
| Covishield | 1.23 (1.01, 1.49) | 1.33 (1.08, 1.64) | ||
| Other | 0.17 (0.05, 0.59) | <0.001 | 0.16 (0.04, 0.58) | <0.001 |
| COVID-19 infection (confirmed) | ||||
| Never | Ref | Ref | ||
| Only once | 1.21 (0.67, 2.18) | 0.98 (0.52, 1.84) | ||
| More than once | 1.45 (1.05, 2.01) | 0.06 | 1.20 (0.83, 1.74) | 0.61 |
| COVID-19-related oxygen/hospitalization requirement | ||||
| No | Ref | Ref | ||
| Yes b | 1.20 (0.72, 1.98) | 0.46 | 0.91 (0.52, 1.60) | 0.75 |
| Year of last COVID-19 vaccine dose (n = 3683) | ||||
| 2021 | Ref | Ref | ||
| 2022 | 3.09 (2.65, 3.58) | <0.001 | 3.27 (2.80, 3.81) | <0.001 |
| Variables | N (col%) Willing to Pay (n = 321) | N (col%) Unwilling to Pay (n = 1114) | Crude OR (95% CI) | p-Value | aOR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Age (in years) | ||||||
| 50–65 | 177 (55.14) | 671 (60.23) | Ref | - | ||
| >65 | 144 (44.86) | 443 (39.77) | 0.81 (0.63, 1.04) | 0.10 | ||
| Sex | ||||||
| Female | 162 (50.47) | 542 (48.65) | Ref | - | ||
| Male | 159 (49.53) | 572 (51.35) | 1.07 (0.84, 1.37) | 0.56 | ||
| Education | ||||||
| Illiterate | 84 (26.17) | 408 (36.62) | Ref | Ref | ||
| Primary | 59 (18.38) | 221 (19.84) | 0.77 (0.53, 1.12) | 0.68 (0.46, 1.02) | ||
| Secondary/High school | 86 (26.79) | 263 (23.61) | 0.63 (0.45, 0.88) | 0.59 (0.41, 0.85) | ||
| Inter certificate/Graduate and above | 92 (28.66) | 222 (19.93) | 0.49 (0.35, 0.69) | 0.01 | 0.49 (0.33, 0.72) | 0.003 |
| PCI a (in Rupees) | ||||||
| ≤4000 | 121 (37.69) | 573 (51.44) | Ref | Ref | ||
| >4000 | 200 (62.31) | 541 (48.56) | 0.57 (0.44, 0.74) | 0.01 | 0.71 (0.53, 0.95) | 0.02 |
| Healthcare worker | ||||||
| No | 320 (99.69) | 1097 (98.47) | Ref | - | ||
| Yes | 1 (0.31) | 17 (1.53) | 4.96 (0.65, 37.40) | 0.12 | ||
| Comorbidities | ||||||
| None | 205 (64.06) | 804 (72.24) | Ref | Ref | ||
| ≥1 disease | 115 (35.94) | 309 (27.76) | 0.98 (0.45, 2.14) | 0.005 | 0.73 (0.55, 0.97) | 0.03 |
| Type of COVID-19 vaccine (n = 1392) | ||||||
| Covaxin | 27 (9.31) | 163 (14.79) | Ref | Ref | ||
| Covishield | 258 (88.97) | 931 (84.48) | 0.59 (0.39, 0.92) | 0.56 (0.36, 0.87) | ||
| Other | 5 (1.72) | 8 (0.73) | 0.26 (0.08, 0.87) | 0.02 | 0.35 (0.10, 1.18) | 0.024 |
| COVID-19 infection | ||||||
| Never | 307 (95.64) | 1063 (95.42) | Ref | - | ||
| Only once | 5 (1.56) | 13 (1.17) | 0.75 (0.26, 2.12) | |||
| More than once | 9 (2.80) | 38 (3.41) | 1.22 (0.58, 2.55) | 0.78 | ||
| COVID-19-related oxygen/hospitalization requirement | ||||||
| No | 315 (98.13) | 1097 (98.47) | Ref | - | ||
| Yes b | 6 (1.87) | 17 (1.53) | 0.81 (0.31, 2.08) | 0.67 | ||
| Year of last COVID-19 vaccine dose (n = 1392) | ||||||
| 2021 | 149 (51.38) | 422 (38.29) | Ref | Ref | ||
| 2022 | 141 (48.62) | 680 (61.71) | 1.70 (1.31, 2.21) | <0.001 | 1.52 (1.16, 1.98) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Basu, S.; Lalwani, H.; Rao, S.; Malik, M.; Garg, S.; Shrivastava, R.; Singh, M.M. COVID-19 Booster Dose Coverage and Hesitancy among Older Adults in an Urban Slum and Resettlement Colony in Delhi, India. Vaccines 2023, 11, 1177. https://doi.org/10.3390/vaccines11071177
Sharma N, Basu S, Lalwani H, Rao S, Malik M, Garg S, Shrivastava R, Singh MM. COVID-19 Booster Dose Coverage and Hesitancy among Older Adults in an Urban Slum and Resettlement Colony in Delhi, India. Vaccines. 2023; 11(7):1177. https://doi.org/10.3390/vaccines11071177
Chicago/Turabian StyleSharma, Nandini, Saurav Basu, Heena Lalwani, Shivani Rao, Mansi Malik, Sandeep Garg, Rahul Shrivastava, and Mongjam Meghachandra Singh. 2023. "COVID-19 Booster Dose Coverage and Hesitancy among Older Adults in an Urban Slum and Resettlement Colony in Delhi, India" Vaccines 11, no. 7: 1177. https://doi.org/10.3390/vaccines11071177
APA StyleSharma, N., Basu, S., Lalwani, H., Rao, S., Malik, M., Garg, S., Shrivastava, R., & Singh, M. M. (2023). COVID-19 Booster Dose Coverage and Hesitancy among Older Adults in an Urban Slum and Resettlement Colony in Delhi, India. Vaccines, 11(7), 1177. https://doi.org/10.3390/vaccines11071177






