Efficacy of HPV Vaccination Regarding Vulvar and Vaginal Recurrences in Previously Treated Women: The Need for Further Evidence
Abstract
1. Introduction
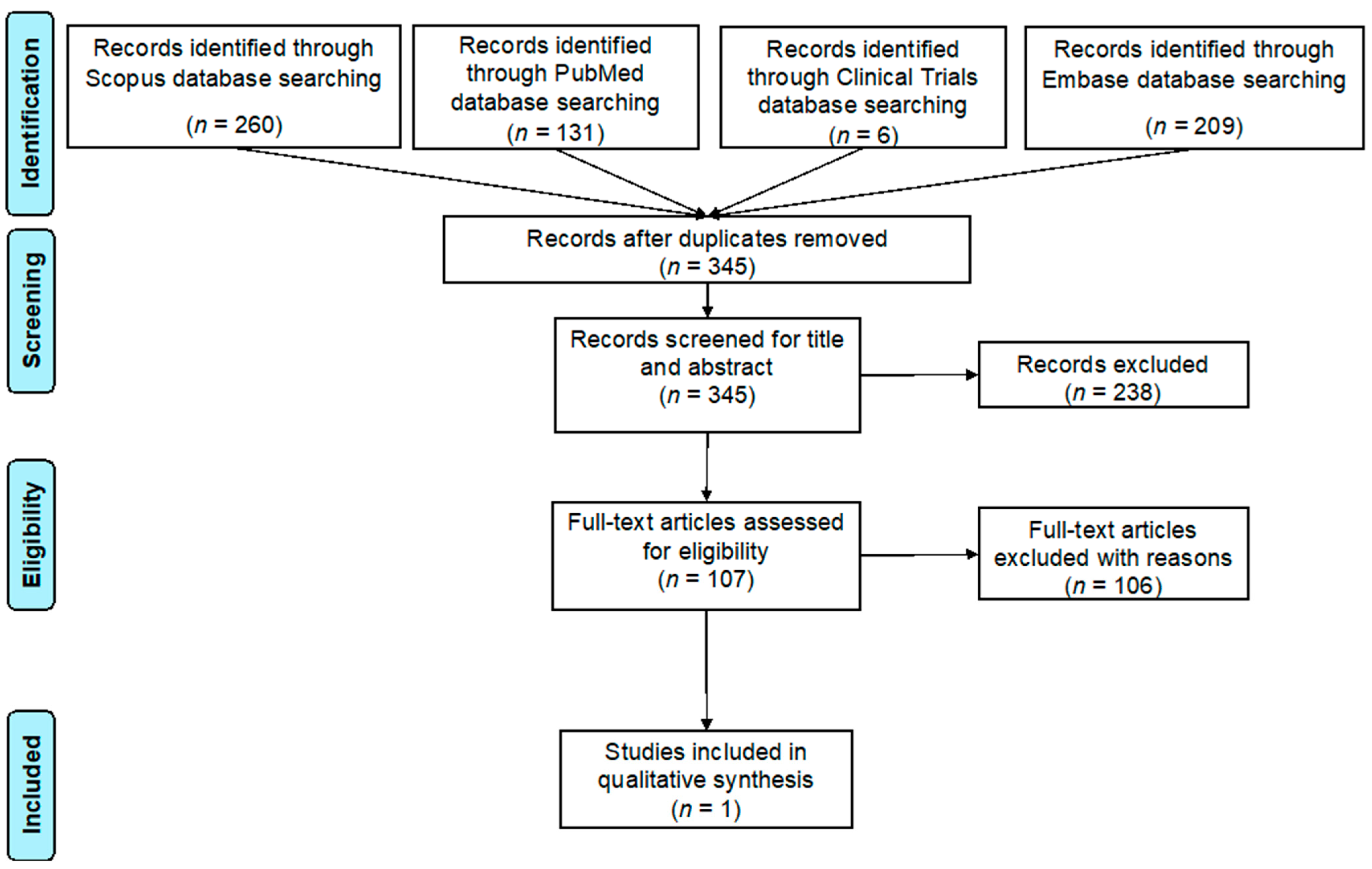
2. Materials and Methods
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ault, K.A. Epidemiology and Natural History of Human Papillomavirus Infections in the Female Genital Tract. Infect. Dis. Obstet. Gynecol. 2006, 2006, 40470. [Google Scholar] [CrossRef]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Boda, D.; Docea, A.O.; Calina, D.; Ilie, M.A.; Caruntu, C.; Zurac, S.; Neagu, M.; Constantin, C.; Branisteanu, D.E.; Voiculescu, V.; et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int. J. Oncol. 2018, 52, 637–655. [Google Scholar] [CrossRef]
- Lacey, C.J.; Lowndes, C.M.; Shah, K.V. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006, 24 (Suppl. S3), S35–S41. [Google Scholar] [CrossRef]
- Garbuglia, A.R.; Lapa, D.; Sias, C.; Capobianchi, M.R.; Del Porto, P. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front. Immunol. 2020, 11, 188. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gilks, C.B. Vulval squamous cell carcinoma and its precursors. Histopathology 2020, 76, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Wang, Z.; Zhang, Z. Prevalence of human papillomavirus DNA and p16INK4a positivity in vulvar cancer and vulvar intraepithelial neoplasia: A systematic review and meta-analysis. Lancet Oncol. 2023, 24, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Lösch, A.; Haider-Angeler, M.G.; Breitenecker, G.; Leodolter, S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J. Reprod. Med. 2000, 45, 613–615. [Google Scholar] [PubMed]
- European Medicines Agency. European Public Assessment Report for Cervarix. 2022. Available online: www.ema.europa.eu/en/medicines/human/EPAR/cervarix (accessed on 20 November 2022).
- European Medicines Agency. European Public Assessment Report for Gardasil. 2022. Available online: www.ema.europa.eu/en/medicines/human/EPAR/gardasil (accessed on 20 November 2022).
- European Medicines Agency. European Public Assessment Report for Gardasil 9. 2022. Available online: www.ema.europa.eu/en/medicines/human/EPAR/gardasil-9 (accessed on 20 November 2022).
- Karimi-Zarchi, M.; Allahqoli, L.; Nehmati, A.; Kashi, A.M.; Taghipour-Zahir, S.; Alkatout, I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef]
- Mlakar, J.; Oštrbenk Valenčak, A.; Kežar, J.; Beseničar-Pregelj, L.; Poljak, M. Assessment of Acceptability and Determinants of Uptake and Schedule Completion of Human Papillomavirus (HPV) Vaccine by 25 to 45 Years Old Women in Slovenia. Vaccines 2023, 11, 423. [Google Scholar] [CrossRef]
- Joura, E.A.; Leodolter, S.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Koutsky, L.A.; Garland, S.M.; Harper, D.M.; Tang, G.W.; Ferris, D.G.; et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: A combined analysis of three randomised clinical trials. Lancet 2007, 369, 1693–1702. [Google Scholar] [CrossRef]
- Dehlendorff, C.; Baandrup, L.; Kjaer, S.K. Real-World Effectiveness of Human Papillomavirus Vaccination against Vulvovaginal High-Grade Precancerous Lesions and Cancers. J. Natl. Cancer Inst. 2021, 113, 869–874. [Google Scholar] [CrossRef]
- De Vincenzo, R.; Caporale, N.; Bertoldo, V.; Ricci, C.; Evangelista, M.T.; Bizzarri, N.; Pedone Anchora, L.; Scambia, G.; Capelli, G. HPV and Cytology Testing in Women Undergoing 9-Valent HPV Opportunistic Vaccination: A Single-Cohort Follow Up Study. Vaccines 2021, 9, 643. [Google Scholar] [CrossRef]
- Pan, J.; Kavanagh, K.; Cuschieri, K.; Pollock, K.G.; Gilbert, D.C.; Millan, D.; Bell, S.; Graham, S.V.; Williams, A.R.W.; Cruickshank, M.E.; et al. Increased risk of HPV-associated genital cancers in men and women as a consequence of pre-invasive disease. Int. J. Cancer 2019, 145, 427–434. [Google Scholar] [CrossRef]
- Strander, B.; Hällgren, J.; Sparén, P. Effect of ageing on cervical or vaginal cancer in Swedish women previously treated for cervical intraepithelial neoplasia grade 3: Population based cohort study of long term incidence and mortality. BMJ 2014, 348, f7361. [Google Scholar] [CrossRef]
- Rositch, A.F.; Soeters, H.M.; Offutt-Powell, T.N.; Wheeler, B.S.; Taylor, S.M.; Smith, J.S. The incidence of human papillomavirus infection following treatment for cervical neoplasia: A systematic review. Gynecol Oncol. 2014, 132, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol. Oncol. 2018, 151, 229–234. [Google Scholar] [CrossRef]
- Ministry of Health in Spain. Government Recommendations’ for HPV. 2018. Available online: www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/vacunas/ciudadanos/vph.htm (accessed on 20 November 2022).
- Stephens, S.; Chatterjee, A.; Coles, V.; Crawford, R. The costs of treating vaginal and vulval cancer in England (2009–2015). BMC Public Health 2020, 20, 453. [Google Scholar] [CrossRef]
- Jones, R.W.; Rowan, D.M.; Stewart, A.W. Vulvar intraepithelial neoplasia: Aspects of the natural history and outcome in 405 women. Obstet. Gynecol. 2005, 106, 1319–1326. [Google Scholar] [CrossRef]
- Ghelardi, A.; Marrai, R.; Bogani, G.; Sopracordevole, F.; Bay, P.; Tonetti, A.; Lombardi, S.; Bertacca, G.; Joura, E.A. Surgical Treatment of Vulvar HSIL: Adjuvant HPV Vaccine Reduces Recurrent Disease. Vaccines 2021, 9, 83. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Benedetti Panici, P.; et al. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef]
- Pieralli, A.; Bianchi, C.; Auzzi, N.; Fallani, M.G.; Bussani, C.; Fambrini, M.; Cariti, G.; Scarselli, G.; Petraglia, F.; Ghelardi, A. Indication of prophylactic vaccines as a tool for secondary prevention in HPV-linked disease. Arch. Gynecol. Obstet. 2018, 298, 1205–1210. [Google Scholar] [CrossRef]
- Del Pino, M.; Martí, C.; Torras, I.; Henere, C.; Munmany, M.; Marimon, L.; Saco, A.; Torné, A.; Ordi, J. HPV Vaccination as Adjuvant to Conization in Women with Cervical Intraepithelial Neoplasia: A Study under Real-Life Conditions. Vaccines 2020, 8, 245. [Google Scholar] [CrossRef]
- Kang, W.D.; Choi, H.S.; Kim, S.M. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol. Oncol. 2013, 130, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.; Dessole, M.; Tinacci, E.; Saderi, L.; Muresu, N.; Capobianco, G.; Cossu, A.; Dessole, S.; Sotgiu, G.; Piana, A. Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience. Vaccines 2020, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Salom, E.M.; Penalver, M. Recurrent vulvar cancer. Curr. Treat. Options Oncol. 2002, 3, 143–153. [Google Scholar] [CrossRef]
- Nooij, L.S.; Brand, F.A.; Gaarenstroom, K.N.; Creutzberg, C.L.; de Hullu, J.A.; van Poelgeest, M.I. Risk factors and treatment for recurrent vulvar squamous cell carcinoma. Crit. Rev. Oncol. Hematol. 2016, 106, 1–13. [Google Scholar] [CrossRef]
- Joura, E.A.; Garland, S.M.; Paavonen, J.; Ferris, D.G.; Perez, G.; Ault, K.A.; Huh, W.K.; Sings, H.L.; James, M.K.; Haupt, R.M.; et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. bmj 2012, 344, e1401. [Google Scholar] [CrossRef]
- Garland, S.M.; Paavonen, J.; Jaisamrarn, U.; Naud, P.; Salmerón, J.; Chow, S.N.; Apter, D.; Castellsagué, X.; Teixeira, J.C.; Skinner, S.R.; et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial. Int. J. Cancer 2016, 139, 2812–2826. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz Karita, H.C.; Hauge, K.; Magaret, A.; Mao, C.; Schouten, J.; Grieco, V.; Xi, L.F.; Galloway, D.A.; Madeleine, M.M.; Wald, A. Effect of Human Papillomavirus Vaccine to Interrupt Recurrence of Vulvar and Anal Neoplasia (VIVA): A Trial Protocol. JAMA Netw. Open 2019, 2, e190819. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.R.; Graybill, W.S.; Pierce, J.Y. Morbidity and mortality of vulvar and vaginal cancers: Impact of 2-, 4-, and 9-valent HPV vaccines. Hum. Vaccin. Immunother. 2016, 12, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechini, A.; Moscadelli, A.; Velpini, B.; Bonito, B.; Orlando, P.; Putignano, P.; Posi, S.; Stacchini, L.; Bonanni, P.; Boccalini, S. Efficacy of HPV Vaccination Regarding Vulvar and Vaginal Recurrences in Previously Treated Women: The Need for Further Evidence. Vaccines 2023, 11, 1084. https://doi.org/10.3390/vaccines11061084
Bechini A, Moscadelli A, Velpini B, Bonito B, Orlando P, Putignano P, Posi S, Stacchini L, Bonanni P, Boccalini S. Efficacy of HPV Vaccination Regarding Vulvar and Vaginal Recurrences in Previously Treated Women: The Need for Further Evidence. Vaccines. 2023; 11(6):1084. https://doi.org/10.3390/vaccines11061084
Chicago/Turabian StyleBechini, Angela, Andrea Moscadelli, Beatrice Velpini, Benedetta Bonito, Paolo Orlando, Pasqua Putignano, Silvano Posi, Lorenzo Stacchini, Paolo Bonanni, and Sara Boccalini. 2023. "Efficacy of HPV Vaccination Regarding Vulvar and Vaginal Recurrences in Previously Treated Women: The Need for Further Evidence" Vaccines 11, no. 6: 1084. https://doi.org/10.3390/vaccines11061084
APA StyleBechini, A., Moscadelli, A., Velpini, B., Bonito, B., Orlando, P., Putignano, P., Posi, S., Stacchini, L., Bonanni, P., & Boccalini, S. (2023). Efficacy of HPV Vaccination Regarding Vulvar and Vaginal Recurrences in Previously Treated Women: The Need for Further Evidence. Vaccines, 11(6), 1084. https://doi.org/10.3390/vaccines11061084







