Impact of Human Papillomavirus Vaccination on Male Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection Criteria
2.2. Selection of Studies and Data Extraction
2.3. Study Quality Assessment (Risk-of-Bias)
2.4. Glossary of Nomenclature
3. Results
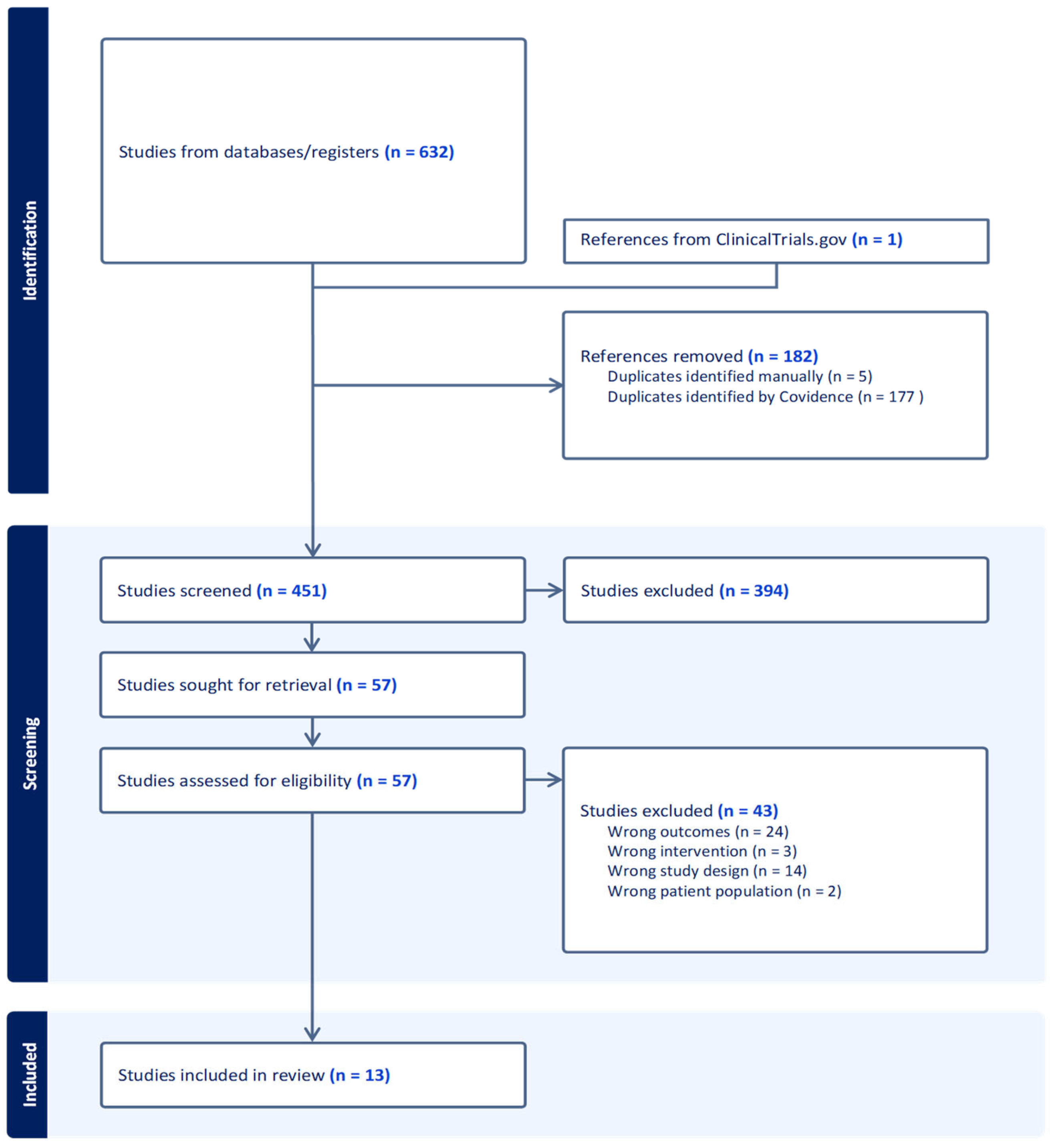
3.1. Number of Retrieved Papers
3.2. Studies’ General Characteristics (Table 1)
| Study | Study Design | Base Study | Study Site | Study Period | Intervention/Comparator | Number of Participants | Characteristics at Enrollment | Inclusion Criteria | Exclusion Criteria | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| [37] Giuliano, A. R., 2011 # | RCT NCT00090285 | - | Africa, Australia, Europe, and America (71 sites in 18 countries) | 2004 to 2008 | 4vHPV vaccine/placebo | 4065 participants (2032 vaccinated and 2033 placebo) | HIV negative | Heterosexual men aged 16 to 23 years old or MSM aged 16 to 26 years old, all with 1 to 5 sexual partners in their lifetime | Clinically detectable anogenital warts or genital lesions at screening or who had a history of such findings | 2.9 years |
| [31] Palefsky, J. M., 2011 ∆ | RCT | Giuliano A.R et al. [38] NCT00090285 | Australia, America, and Europe (7 countries) | 2004 to 2008 | 4vHPV vaccine/placebo | 598 participants (299 vaccinated and 299 placebo) | MSM+HIV negative; 16 to 26 years old | MSM aged 16 to 26 years old, with five or less sexual partners and anal intercourse/oral sex with other male in the last year | Medical history anogenital warts, genital lesions suggesting other STI, or intra-anal lesion related to AIN or condyloma. HIV-positive men at enrollment | 3 years |
| [17] Swedish, K. A., 2012 ∆ | Prospective Cohort | - | USA, New York City (1 site) | 2007 to 2010 | 4vHPV vaccination/unvaccination | 202 participants (88 vaccinated and 114 unvaccinated) | MSM+HIV negative; ≥18 years old | MSM aged 18 years old or older, HIV-negative, and with clinical history of biopsy-proven and treated HGAIN ** | Patients with HGAIN ** at the beginning and who had not received all 3 doses of 4vHPV vaccine | 3 years |
| [32] Goldstone, S. E., 2013 ∆ # | RCT | Giuliano A.R. et al. [38] NCT00090285 | Africa, Australia, Europe, America (71 sites in 18 countries) | 2004 to 2009 | 4vHPV vaccine/placebo | 4055 participants (2025 vaccinated and 2033 placebo) | Heterosexual males aged 16 to 24 years old MSM aged 16 to 27 years with a maximum of 6 lifetime sexual partners | For the HPV-naïve population, patients who were PCR-negative to all the 14 tested HPV types and seronegative to HPV-6/11/16/18. MSM with a normal anal cytology test. For ITT population, participants who received at least one dose of vaccine or placebo and returned for at least one follow-up visit | Immunodeficiency or HIV infection upon enrollment. Clinical history of genital lesions related to HPV infection or other sexually transmitted infection. Presence of lesions of unknown etiology | 3 years |
| [42] Swedish, K. A., 2014 ∆ | Prospective Cohort | Swedish K. A. et al. Clin Infect Dis 2012; 54:891–898. | USA, New York City (1 site) | 2007 to 2010 | 4vHPV vaccine/unvaccinated | 313 participants (116 vaccinated and 197 unvaccinated) | MSM+HIV negative; ≥26 years | MSM+HIV- aged up to 26 years old. No clinical history of anal condyloma, or recurrence free for at least a year | Patients who did not receive all 3 doses of vaccine and those vaccinated at other sites. Patients with anal or penile condyloma at the start of the study | 4 years |
| [40] Ferris, D., 2014 ∆ # | Prospective Cohort | Reisinger K. S. et al. Pediatr Infect Dis J 2007; 26(3): 201-209 NCT00092547 | Europe, America, Asia, Africa (72 sites in 17 countries) | 2005 to 2013 | 4vHPV vaccine | 775 participants (555 EVG and 220 CVG) | 9 to 15 years old | EVG: boys who received 4vHPV vaccine between the ages of 9 and 15 years old during the base study CVG: boys who received placebo in the base study and later received the 4vHPV vaccine. | Allergy to any vaccine component, thrombocytopenia, immunosuppression/previous immunosuppressive therapy, or previous receipt of an HPV vaccine | 8 years |
| [38] Coskuner, E. R., 2014 # | RCT | - | Turkey (1 site) | 2009 to 2013 | 4vHPV vaccine/placebo | 171 participants (91 vaccinated and 80 placebo) | Circumcised men with only female partners | Men with new onset of genital warts living in the same area for at least 1 year | Clinical history of treatment of pre-existing warts, chronic treatment for medical disorders or immunosuppression (including HIV) | 4 years |
| [33] Wilkin, T. J., 2018 ∆ | RCT NCT01461096 | - | USA and Brazil (24 sites in 2 countries) | 2012 to 2015 | 4vHPV vaccine/placebo | 577 participants (288 vaccinated and 287 unvaccinated) | HIV+; ≥27 years old | Men aged 27 years old or older, HIV+, who had had anal intercourse/oral sex with another male in the last year | History of HPV-related cancer, anal HSIL or condyloma treatment in the last 6 months. Prior HPV vaccination or allergy to vaccine components. Anticoagulant use, active drug, or alcohol use. Bleeding diatheses, systemic anti-neoplastic or immunomodulatory treatment | 3 to 4 years |
| [34] Mikamo, H., 2019 # | RCT NCT01862874 | - | Japan (24 sites) | 2013 to 2017 | 4vHPV vaccine/placebo | 1124 participants (562 vaccinated and 562 placebo) | Heterosexual or MSM aged 16 to 26 years old Participants refrained from sexual activity for 2 days before scheduled visits that included sample collection | Heterosexual men aged 16-26 years old, with 1 to 5 exclusively female partners; MSM aged 16-26 years who had had anal intercourse/oral sex with a male partner within the past year, and with 1 to 5 lifetime male/female partners. | History of genital warts or clinically present external genital warts at the beginning of the study | 3 years |
| [39] Olsson, S. E., 2020 ∆ # | Prospective cohort | P. Van Damme et al. Pediatrics 2015; 136: e28–e39 NCT00943722 | Europe, America, Asia, Africa (39 sites in 13 countries) | 2013 to 2018 | 9vHPV vaccine | 301 participants | 9 to 15 years old | Boys aged 9 to 15 years old who received all 3 vaccine doses during the base study | Clinical history of HPV infection. Allergy to any vaccine component, thrombocytopenia, immunosuppression/previous immunosuppressive therapy, or previous receipt of an HPV vaccine. Current enrollment in any other clinical study | 10 years |
| [35] Hidalgo-Tenorio, C., 2021 ∆ # | RCT | Hidalgo-Tenorio C. et al. AIDS Res Ther 2017; 14: 34 ISRCTN14732216 | Spain (1 site) | 2011 to 2017 | 4vHPV vaccine/placebo | 129 participants (66 4vHPV vaccinated and 63 placebo) | MSM+HIV+; >26 years old | MSM aged > 26 years old, HIV-positive, and not infected with all 4vHPV vaccine types at the same time, or not infected with genotypes 16 and 18 at the same time. Normal high resolution anoscopy, anal biopsy with condyloma alone, anal LSIL ** alone | MSM+HIV+ patients with simultaneous anal infection with the four genotypes addressed by the vaccine, and who at least had HPV genotypes 16 and 18. Active opportunist infection at enrollment. Patients who had HSIL **, or ASCC or had received treatment for these lesions. History of allergy to aluminum and/or yeast extract excipient | 4 years |
| [36] Gosens, K. C. M., 2021 ∆ | RCT NCT02087384 | - | Amsterdam, The Netherlands (3 sites) | 2014 to 2018 | 4vHPV vaccine/placebo | 126 participants (64 vaccinated and 62 placebo) | MSM+HIV+; ≥18 years old | HIV+MSM aged 18 years old or older who had a CD4+ cell count greater than 350 cells/µL, had biopsy-proven intra-anal HGAIN ** (successfully treated), and had lesions still in remission (maximum LGAIN **) at the time of enrollment | Previous HPV vaccination, allergy to any 4vHPV vaccine constituents, and other comorbidities. Medical history of anal carcinoma, current peri-anal HGAIN ** or peri-anal AIN2 or 3 at the time of enrollment. Immunosuppressive medication or immunodeficiency other than HIV, or a life expectancy inferior to a year | 1.5 years |
| [41] Goldstone, S. E., 2022 ∆ # | Prospective Cohort | Giuliano A.R. et al. [38] NCT00090285 | Australia, America, Europe, Asia, Africa (46 sites in 16 countries) | 2010 to 2017 | 4vHPV vaccine | 1803 participants (936 EVG and 867 CVG) | 16 to 26 years old | EVG: Heterosexual men aged 16 to 23 years old and homosexual men aged 16 to 26 years old who received 1 or 2 doses or more of the 4vHPV vaccine at the base study. CVG: Heterosexual men aged 16 to 23 years old and homosexual men aged 16 to 26 years old who received 1 or 2 doses or more of the 4vHPV vaccine, and were placebos in the base study | Enrollment in a study that involved/interfere with the collection of anogenital samples. History of anogenital warts or genital lesions | EVG: 9.5 years CVG: 4.7 years |
3.3. Outcomes Reported in the Included Studies of HPV-Related Anal Disease
| Study | Outcomes | Results | Limitations | Sponsorship/Conflicts of Interest | Risk-of-Bias |
|---|---|---|---|---|---|
| [31] Palefsky, J. M., 2011 | Efficacy of 4vHPV vaccine on the prevention of any grade of AIN or anal cancer related to infection with HPV-6/11/16/18 | The PP efficacy of the 4vHPV vaccine against AIN1 (including condyloma) was 91.1% and for AIN 2 or 3 of 91.7%. The ITT population efficacy was 50.3%, both for AIN1/2/3 | Narrow range of ages. Short follow-up time. Participants had limited sexual activity. Findings may not be generalizable to boys and men in the general population of similar ages | Sponsorship: Merck CI: Disclosed | Low |
| [17] Swedish, K. A., 2012 | Efficacy of 4vHPV vaccine in the prevention of recurrent HGAIN among MSM+HIV- | Hazard analysis revealed an increased risk of recurrent HGAIN **, but 4vHPV vaccination was associated with a decreased risk. The vaccinated patients have an incidence rate of 10.2 per 100 person years for recurrent HGAIN ** and unvaccinated patients have rates of 15.7 per 100 person-year. Comparing the risk of recurrent HGAIN ** on 4vHPV vaccinated individuals, the multivariable model for different years presents lower risk at 1 years (HR = 0.42) and similar risk at 2 and 3 years (HR = 0.50 and HR = 0.52, respectively). The Kaplan–Meier’s survival analysis indicates no recurrence of HGAIN ** in vaccinated individuals | The study depended on pre-existing medical records lacking race/ethnicity and oncogenic HPV infection status, and it was difficult to determine a “study entry” for unvaccinated participants. The sample comprises a mostly white population of non-smoking MSM with private insurance, so findings may not be generalizable to other populations | CI: Disclosed | Moderate |
| [32] Goldstone, S. E., 2013 | Efficacy of 4vHPV vaccine against AIN caused by HPV-6/11/16/18 and by 10 additional non-vaccine HPV types as well as efficacy regardless of whether HPV was detected in an AIN lesion. | A protective effect of vaccination on HPV 6/11/16/18-related AIN and anal cancer was identified. In the ITT, efficacy was 50.3% and, in the HPV,-naïve group, and was 89.6% regarding 6/11/16/18-related anal disease. Efficacy against AIN 1 in the naïve group was statistically significant (93.1%). Efficacy against anal disease endpoints caused by the ten non-vaccine types was not demonstrated | Young participants with limited number of sexual partners. Small sample of MSM; Short medium follow-up < 3 years. Low evidence due to low rate of events such as anal cancer | Sponsorship: Merck & Co, Inc. CI: Disclosed | Low |
| [40] Ferris, D., 2014 | Efficacy of 4vHPV vaccination against HPV-6/11/16/18-related disease | In both groups, there were no cases of HPV-6/11/16/18-related disease, including perianal cancer | Small sample size and observed attritions | Sponsorship: Merck Sharp & Dome CI: None | Low |
| [42] Swedish, K. A., 2014 | Efficacy of 4vHPV vaccine in the prevention of recurrent anal condyloma among MSM+HIV- | Anal condyloma cases appeared in 8.6% of 4vHPV vaccinated participants, and 18.8% unvaccinated participants. Vaccination was associated with decreased risk of anal condyloma (HR = 0.45) | Groups have considerable differences upon enrollment. Sample size of unvaccinated patients was larger, and they were followed over a longer period | CI: Disclosed | Moderate |
| [33] Wilkin, T. J., 2018 | Efficacy of 4vHPV vaccine on HSIL and anal cytological outcomes | Anal HSIL ** is not influenced by the 4vHPV vaccine (0% efficacy), and anal cytology was not statistically different between groups | Early suspension by DSMB due to protocol-defined futility rules. No access/approval for the nine-valent HPV vaccine at the beginning. No review of cytology or histology outcomes | CI: Disclosed | Moderate |
| [39] Olsson, S. E., 2020 | Incidence of the composite endpoint of HPV-6/11/16/18/31/33/45/52/58-related perianal cancer ≥ 6 months duration | According to the PP analysis, there were three HPV-related diseases, including perianal cancer | Control group from the base study | Sponsorship: Merck Sharp & Dome CI: Disclosed | Moderate |
| [35] Hidalgo-Tenorio, C., 2021 | Effectiveness of the 4vHPV vaccine to prevent anal ≥ HSILs by 4vHPV vaccine genotypes in MSM+HIV+ | Vaccinated and unvaccinated individuals have the same risk of development of ≥HSILs **; however, the 4vHPV vaccine showed a protective effect against HPV6 during the first year of follow-up. The factor associated with the risk of anal ≥ HSIL ** was receipt of the last dose of the vaccine less than 6 months earlier, in comparison to those vaccinated for a longer period | Strict eligibility criteria | CI: None | Low |
| [36] Gosens, K. C. M., 2021 | 1. Cumulative recurrence of intra/peri-anal HGAIN (biopsy-proven) at 12 months after last vaccination 2. Recurrence of intra/perianal HGAIN at time of last vaccination and at 6 months after last vaccination, cumulative occurrence of LGAIN, or anogenital condyloma, causative HPV genotype in recurrent HGAIN lesions, and safety of the 4vHPV vaccine | Cumulative HGAIN ** recurrence is similar between 4vHPV and placebo individuals. The recurrence of HGAIN ** has an incidence rate of 66.3 per 100 person years for 4vHPV vaccinated individuals and 56.5 for placebo. From the 78 recurrences of HGAIN **, only one was peri-anal, and no progression to anal cancer was identified during follow-up | Short follow-up time. Microscopical lesions were undetected/misdiagnosed (but equally distributed using randomization) | Sponsorship: Merck Sharp & Dome CI: Disclosed | Moderate |
| [41] Goldstone, S. E., 2022 | Incidence per 10,000 persons years of AIN or anal cancer related to HPV-6/11/16/18 in MSM | For the EVG, there were no new cases of HGAIN ** related to HPV-6, 11, 16, and 18 during the present study. For CVG, the incidence per 10,000 person years was lower during the long-term follow-up period than during the base study period for AIN and anal cancer related to HPV-6/11/16/18. In addition, the incidence of anal disease in both the EVG and CVG during the long-term follow-up period was significantly lower than in placebo recipients during the base study | High loss of participants | Sponsorship: Merck Sharp & Dome CI: Disclosed | Low |
3.4. Outcomes Reported in the Included Studies of HPV-Related Genital Disease
3.5. Study Quality Assessment (Risk-of-Bias)
| Study | Outcomes | Results | Limitations | Sponsorship/Conflicts of Interest | Risk-of-Bias |
|---|---|---|---|---|---|
| [37] Giuliano, A. R., 2011 | Reduction in the incidence (as compared with placebo) of EGL associated with HPV present in 4vHPV vaccine or to any HPV type. | The ITT efficacy to reduce recurrent EGL was 60.2% overall; in particular, 4vHPV vaccine has 67.2% of efficacy in preventing condyloma. The PP analysis showed an overall 83.8% efficacy and 89.4% 4vHPV vaccine efficacy for condyloma. Penile cancer was not evaluated due to lack of cases in the sample, and a low number of participants presented PIN | Short follow-up time and narrow age range of the subjects | Sponsorship: Merck & Co, Inc. CI: Disclosed | Moderate |
| [32] Goldstone, S. E., 2013 | Efficacy of 4vHPV vaccine against EGL caused by HPV-6/11/16/18 and by 10 additional non-vaccine HPV types. | 4vHPV vaccine had a protective effect against EGL. Although significant efficacy against non-vaccine HPV types was not seen. Efficacy was 9.3% lower at external genital sites when compared with efficacy against HPV 6/11/16/18 | Young participants with limited number of sexual partners. Small sample of MSM. Short medium follow-up < 3 years | Sponsorship: Merck & Co, Inc. CI: Disclosed | Low |
| [38] Coskuner, E. R., 2014 | Efficacy of 4vHPV vaccination on the recurrence of genital warts | There was no difference in recurrence between vaccinated and unvaccinated group, except for the marital status and number of lesions | Small sample size and only from a center. Absence of an initial assessment of vaccine-type antibodies or DNA. No data about previous HPV exposure | CI: None | Moderate |
| [40] Ferris, D., 2014 | Efficacy of 4vHPV vaccination against HPV-6/11/16/18-related disease | In both groups, there were no cases of HPV-6/11/16/18-related disease: genital condyloma, PIN, or penile cancer | Small sample size and observed attritions | Sponsorship: Merck Sharp & Dome CI: None | Low |
| [34] Mikamo, H., 2019 | Efficacy of 4vHPV vaccination on the reduction in the combined incidence of HPV-6/11/16/18-related condyloma acuminata, PIN, and penile, perianal, or perineal cancer. | Vaccination had 83.4% efficacy at interim analysis, and at the final analysis, 86.5% efficacy against combined incidence of HPV-6/11/16/18-related persistent infection and EGL. Only at the final analysis were cases of EGL detected in the placebo group (condyloma and PIN1) | High loss of participants and short follow-up time. This study does not have sufficient power to evaluate the disease endpoint (condyloma, PIN) | Sponsorship: Merck Sharp & Dome CI: Disclosed | Low |
| [39] Olsson, S. E., 2020 | Incidence of the composite endpoint of HPV-6/11/16/18/31/33/45/52/58-related PIN, genital warts and penile cancer of ≥6 months duration | According to the PP analysis, there were three HPV-related disease, including perianal cancer. | Control group from the base study | Sponsorship: Merck Sharp & Dome CI: Disclosed | Moderate |
| [35] Hidalgo-Tenorio, C., 2021 | Effectiveness of the 4vHPV vaccine to prevent anal ≥ HSILs, and EAGLs in MSM+HIV+ | Vaccinated and unvaccinated individuals have the same risk of development of ≥HSILs or EAGL; however, the 4vHPV vaccine showed a protective effect against HPV6 during the first year of follow-up | Strict eligibility criteria | CI: None | Low |
| [41] Goldstone, S. E., 2022 | Incidence per 10,000 persons years of external genital warts related to HPV-6/11 and external genital lesions related to HPV-6/11/16/18 in all participants | For the EVG, when compared to placebo group of the base study, results of incidence were null for external genital warts related to HPV-6/11 and external genital lesions related to HPV-6/11/16/18. For CVG, the incidence per 10,000 person years was lower during the long-term follow-up period than during the base study period for external genital warts related to HPV-6/11, and external genital lesions related to HPV-6/11/16/18. Comparing EVG and CVG in the present study, the incidence of the external genital warts related to HPV-6/11 and external genital lesions related to HPV-6/11/16/18 was similar to the incidence in the EVG | High loss of participants | Sponsorship: Merck Sharp & Dome CI: Disclosed | Low |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zizza, A.; Banchelli, F.; Guido, M.; Marotta, C.; Di Gennaro, F.; Mazzucco, W.; Pistotti, V.; D’Amico, R. Efficacy and safety of human papillomavirus vaccination in HIV-infected patients: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 4954. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Orsi, A.; Martini, M.; Icardi, G. HPV related diseases in males: A heavy vaccine-preventable burden. J. Prev. Med. Hyg. 2013, 54, 61–70. [Google Scholar] [PubMed]
- Passos, M.R.L. Infection with Human Papillomavirus (HPV). In Atlas of Sexually Transmitted Diseases: Clinical Aspects and Differential Diagnosis; Passos, M.R.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 239–319. [Google Scholar]
- Anic, G.M.; Giuliano, A.R. Genital HPV infection and related lesions in men. Prev. Med. 2011, 53 (Suppl. 1), S36–S41. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kothari, S.; Baay, M.; Garland, S.M.; Giuliano, A.R.; Nygård, M.; Velicer, C.; Tota, J.; Sinha, A.; Skufca, J.; et al. Real-world impact and effectiveness assessment of the quadrivalent HPV vaccine: A systematic review of study designs and data sources. Expert Rev. Vaccines 2022, 21, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.V.; Kothari, S.; Skufca, J.; Giuliano, A.R.; Sundström, K.; Nygård, M.; Koro, C.; Baay, M.; Verstraeten, T.; Luxembourg, A.; et al. Real-world impact and effectiveness of the quadrivalent HPV vaccine: An updated systematic literature review. Expert Rev. Vaccines 2022, 21, 1799–1817. [Google Scholar] [CrossRef]
- Fontes, A.; Andreoli, M.A.; Villa, L.L.; Assone, T.; Gaester, K.; Fonseca, L.A.M.; Duarte, A.J.; Casseb, J. High specific immune response to a bivalent anti-HPV vaccine in HIV-1-infected men in São Paulo, Brazil. Papillomavirus Res. 2016, 2, 17–20. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Wilkin, T.; Bautista, O.M.; Cheon, K.; Connor, L.; Dubey, S.; Luxembourg, A.; Rawat, S.; Shaw, A.; Velicer, C.; et al. Design of a phase III efficacy, immunogenicity, and safety study of 9-valent human papillomavirus vaccine in prevention of oral persistent infection in men. Contemp. Clin. Trials 2022, 115, 106592. [Google Scholar] [CrossRef]
- Van Damme, P.; Meijer, C.; Kieninger, D.; Schuyleman, A.; Thomas, S.; Luxembourg, A.; Baudin, M. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine 2016, 34, 4205–4212. [Google Scholar] [CrossRef]
- Patel, H.; Wagner, M.; Singhal, P.; Kothari, S. Systematic review of the incidence and prevalence of genital warts. BMC Infect. Dis. 2013, 13, 39. [Google Scholar] [CrossRef]
- Tejada, R.A.; Vargas, K.G.; Benites-Zapata, V.; Mezones-Holguín, E.; Bolaños-Díaz, R.; Hernández, A.V. Human papillomavirus vaccine efficacy in the prevention of anogenital warts: Systematic review and meta-analysis. Salud Publica Mex. 2017, 59, 84–94. [Google Scholar] [CrossRef]
- Cabo Beltran, O.R.; Rosales Ledezma, R. MVA E2 therapeutic vaccine for marked reduction in likelihood of recurrence of respiratory papillomatosis. Head Neck 2019, 41, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Fortes, H.R.; von Ranke, F.M.; Escuissato, D.L.; Araujo Neto, C.A.; Zanetti, G.; Hochhegger, B.; Souza, C.A.; Marchiori, E. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir. Med. 2017, 126, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef]
- Johnson, L.G.; Madeleine, M.M.; Newcomer, L.M.; Schwartz, S.M.; Daling, J.R. Anal cancer incidence and survival: The surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004, 101, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Palefsky, J.M.; Lensing, S.Y.; Belzer, M.; Lee, J.; Gaur, A.H.; Mayer, K.; Futterman, D.; Stier, E.A.; Paul, M.E.; Chiao, E.Y.; et al. High Prevalence of Anal High-Grade Squamous Intraepithelial Lesions, and Prevention Through Human Papillomavirus Vaccination, in Young Men Who Have Sex with Men Living with Human Immunodeficiency Virus. Clin. Infect. Dis. 2021, 73, 1388–1396. [Google Scholar] [CrossRef]
- Swedish, K.A.; Factor, S.H.; Goldstone, S.E. Prevention of Recurrent High-Grade Anal Neoplasia With Quadrivalent Human Papillomavirus Vaccination of Men Who Have Sex With Men: A Nonconcurrent Cohort Study. Clin. Infect. Dis. 2012, 54, 891–898. [Google Scholar] [CrossRef]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Alemany, L.; Cubilla, A.; Halec, G.; Kasamatsu, E.; Quirós, B.; Masferrer, E.; Tous, S.; Lloveras, B.; Hernández-Suarez, G.; Lonsdale, R.; et al. Role of Human Papillomavirus in Penile Carcinomas Worldwide. Eur. Urol. 2016, 69, 953–961. [Google Scholar] [CrossRef]
- Garland, S.M.; Kjaer, S.K.; Muñoz, N.; Block, S.L.; Brown, D.R.; DiNubile, M.J.; Lindsay, B.R.; Kuter, B.J.; Perez, G.; Dominiak-Felden, G.; et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin. Infect. Dis. 2016, 63, 519–527. [Google Scholar] [CrossRef]
- Petrosky, E.; Bocchini, J.A., Jr.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar] [PubMed]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Linertová, R.; Guirado-Fuentes, C.; Medina, J.M.; Imaz-Iglesia, I.; Rodríguez-Rodríguez, L.; Carmona-Rodríguez, M. Cost-effectiveness of extending the HPV vaccination to boys: A systematic review. J. Epidemiol. Community Health 2021, 75, 910–916. [Google Scholar] [CrossRef]
- Dibble, K.E.; Maksut, J.L.; Siembida, E.J.; Hutchison, M.; Bellizzi, K.M. A Systematic Literature Review of HPV Vaccination Barriers Among Adolescent and Young Adult Males. J. Adolesc. Young Adult Oncol. 2019, 8, 495–511. [Google Scholar] [CrossRef]
- Grandahl, M.; Nevéus, T. Barriers towards HPV Vaccinations for Boys and Young Men: A Narrative Review. Viruses 2021, 13, 1644. [Google Scholar] [CrossRef]
- Adeyanju, G.C.; Betsch, C.; Adamu, A.A.; Gumbi, K.S.; Head, M.G.; Aplogan, A.; Tall, H.; Essoh, T.A. Examining enablers of vaccine hesitancy toward routine childhood and adolescent vaccination in Malawi. Glob. Health Res. Policy 2022, 7, 28. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Darragh, T.M.; Colgan, T.J.; Thomas Cox, J.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int. J. Gynecol. Pathol. 2013, 32, 76–115. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Giuliano, A.R.; Goldstone, S.; Moreira, E.D.; Aranda, C.; Jessen, H.; Hillman, R.; Ferris, D.; Coutlee, F.; Stoler, M.H.; et al. HPV Vaccine against Anal HPV Infection and Anal Intraepithelial Neoplasia. N. Engl. J. Med. 2011, 365, 1576–1585. [Google Scholar] [CrossRef]
- Goldstone, S.E.; Jessen, H.; Palefsky, J.M.; Giuliano, A.R.; Moreira, E.D., Jr.; Vardas, E.; Aranda, C.; Hillman, R.J.; Ferris, D.G.; Coutlee, F.; et al. Quadrivalent HPV vaccine efficacy against disease related to vaccine and non-vaccine HPV types in males. Vaccine 2013, 31, 3849–3855. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, T.J.; Chen, H.C.; Cespedes, M.S.; Leon-Cruz, J.T.; Godfrey, C.; Chiao, E.Y.; Bastow, B.; Webster-Cyriaque, J.; Feng, Q.H.; Dragavon, J.; et al. A Randomized, Placebo-Controlled Trial of the Quadrivalent Human Papillomavirus Vaccine in Human Immunodeficiency Virus-Infected Adults Aged 27 Years or Older: AIDS Clinical Trials Group Protocol A5298. Clin. Infect. Dis. 2018, 67, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Mikamo, H.; Yamagishi, Y.; Murata, S.; Yokokawa, R.; Han, S.R.; Wakana, A.; Sawata, M.; Tanaka, Y. Efficacy, safety, and immunogenicity of a quadrivalent HPV vaccine in Japanese men: A randomized, Phase 3, placebo-controlled study. Vaccine 2019, 37, 1651–1658. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Pasquau, J.; Omar-Mohamed, M.; Sampedro, A.; López-Ruz, M.A.; López Hidalgo, J.; Ramírez-Taboada, J. Effectiveness of the Quadrivalent HPV Vaccine in Preventing Anal ≥ HSILs in a Spanish Population of HIV+ MSM Aged > 26 Years. Viruses 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Gosens, K.C.M.; van der Zee, R.P.; van Heukelom, M.L.S.; Jongen, V.W.; Cairo, I.; van Eeden, A.; van Noesel, C.J.M.; Quint, W.G.V.; Pasmans, H.; Dijkgraaf, M.G.W.; et al. HPV vaccination to prevent recurrence of anal intraepithelial neoplasia in HIV+ MSM. Aids 2021, 35, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Palefsky, J.M.; Goldstone, S.; Moreira, E.D., Jr.; Penny, M.E.; Aranda, C.; Vardas, E.; Moi, H.; Jessen, H.; Hillman, R.; et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011, 364, 401–411. [Google Scholar] [CrossRef]
- Coskuner, E.R.; Ozkan, T.A.; Karakose, A.; Dillioglugil, O.; Cevik, I. Impact of the quadrivalent HPV vaccine on disease recurrence in men exposed to HPV Infection: A randomized study. J. Sex. Med. 2014, 11, 2785–2791. [Google Scholar] [CrossRef]
- Olsson, S.E.; Restrepo, J.A.; Reina, J.C.; Pitisuttithum, P.; Ulied, A.; Varman, M.; Van Damme, P.; Moreira, E.D.; Ferris, D.; Block, S.; et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: Interim analysis after 8 years of follow-up. Papillomavirus Res. 2020, 10, 100203. [Google Scholar] [CrossRef]
- Ferris, D.; Samakoses, R.; Block, S.L.; Lazcano-Ponce, E.; Restrepo, J.A.; Reisinger, K.S.; Mehlsen, J.; Chatterjee, A.; Iversen, O.E.; Sings, H.L.; et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics 2014, 134, e657–e665. [Google Scholar] [CrossRef]
- Goldstone, S.E.; Giuliano, A.R.; Palefsky, J.M.; Lazcano-Ponce, E.; Penny, M.E.; Cabello, R.E.; Moreira, E.D., Jr.; Baraldi, E.; Jessen, H.; Ferenczy, A.; et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: Results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 2022, 22, 413–425. [Google Scholar] [CrossRef]
- Swedish, K.A.; Goldstone, S.E. Prevention of Anal Condyloma with Quadrivalent Human Papillomavirus Vaccination of Older Men Who Have Sex with Men. PLoS ONE 2014, 9, e93393. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Sternberg, M.; Dunne, E.F.; McQuillan, G.; Unger, E.R. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J. Infect. Dis. 2009, 200, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Viscidi, R.P.; Lee, J.-H.; Wu, Y.; Villa, L.L.; Lazcano-Ponce, E.; Carvalho da Silva, R.J.; Baggio, M.L.; Quiterio, M.; Salmerón, J.; et al. Human Papillomavirus (HPV) 6, 11, 16, and 18 Seroprevalence Is Associated with Sexual Practice and Age: Results from the Multinational HPV Infection in Men Study (HIM Study). Cancer Epidemiol. Biomark. Prev. 2011, 20, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-c.J.; Palefsky, J.M. HPV-Associated Anal Cancer in the HIV/AIDS Patient. Cancer Treat. Res. 2018, 177, 183–209. [Google Scholar] [PubMed]
- Markowitz, L.E.; Dunne, E.F.; Saraiya, M.; Chesson, H.W.; Curtis, C.R.; Gee, J.; Bocchini, J.A., Jr.; Unger, E.R. Human papillomavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 2014, 63, 1–30. [Google Scholar] [PubMed]
- Guidance on HPV Vaccination in EU Countries: Focus on Boys, People Living with HIV and 9-Valent HPV Vaccine Introduction. Available online: https://www.ecdc.europa.eu/en/publications-data/guidance-hpv-vaccination-eu-focus-boys-people-living-hiv-9vHPV-vaccine (accessed on 24 March 2023).
- Capra, G.; Nyitray, A.G.; Lu, B.; Perino, A.; Marci, R.; Schillaci, R.; Matranga, D.; Firenze, A.; Caleca, M.; Bellavia, C.; et al. Analysis of persistence of human papillomavirus infection in men evaluated by sampling multiple genital sites. Eur. Rev. Med. Pharm. Sci. 2015, 19, 4153–4163. [Google Scholar] [PubMed]
- Sauvageau, C.; Dufour-Turbis, C. HPV vaccination for MSM: Synthesis of the evidence and recommendations from the Québec Immunization Committee. Hum. Vaccines Immunother. 2016, 12, 1560–1565. [Google Scholar] [CrossRef]
- Rossi, C.; Vanhomwegen, C.; Laurent, F. HPV vaccination in boys and men: Update and recommendations. Rev. Med. Brux. 2018, 39, 352–358. [Google Scholar] [PubMed]
- Deshmukh, A.A.; Chiao, E.Y.; Cantor, S.B.; Stier, E.A.; Goldstone, S.E.; Nyitray, A.G.; Wilkin, T.; Wang, X.; Chhatwal, J. Management of precancerous anal intraepithelial lesions in human immunodeficiency virus-positive men who have sex with men: Clinical effectiveness and cost-effectiveness. Cancer 2017, 123, 4709–4719. [Google Scholar] [CrossRef]
- Grace, D.; Gaspar, M.; Paquette, R.; Rosenes, R.; Burchell, A.N.; Grennan, T.; Salit, I.E. HIV-positive gay men’s knowledge and perceptions of Human Papillomavirus (HPV) and HPV vaccination: A qualitative study. PLoS ONE 2018, 13, e0207953. [Google Scholar] [CrossRef]
- Ejaz, M.; Ekström, A.M.; Ahmed, A.; Haroon, A.; Ali, D.; Ali, T.S.; Salazar, M. Human Papillomavirus associated prevention: Knowledge, attitudes, and perceived risks among men who have sex with men and transgender women in Pakistan: A qualitative study. BMC Public Health 2022, 22, 378. [Google Scholar] [CrossRef]
- Amtmann, A.; Ahmed, I.; Zahner-Rimmel, P.; Mletzko, A.; Jordan, L.K.; Oberle, M.; Wedekind, H.; Christian, J.; Bergmann, S.M.; Becker, A.M. Virucidal effects of various agents-including protease-against koi herpesvirus and viral haemorrhagic septicaemia virus. J. Fish Dis. 2020, 43, 185–195. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, X.; Feng, Y.; Wu, S.; Jiang, Y. Safety and efficacy of human papillomavirus vaccination for people living with HIV: A systematic review and meta-analysis. Int. J. STD AIDS 2019, 30, 1105–1115. [Google Scholar] [CrossRef]
- Harder, T.; Wichmann, O.; Klug, S.J.; van der Sande, M.A.B.; Wiese-Posselt, M. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: A systematic review. BMC Med. 2018, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Colzani, E.; Johansen, K.; Johnson, H.; Pastore Celentano, L. Human papillomavirus vaccination in the European Union/European Economic Area and globally: A moral dilemma. Eurosurveillance 2021, 26, 2001659. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Minozzi, S.; Saulle, R.; Mitrova, Z.; Cinquini, M. Inter-rater agreement and time to complete the new Cochrane Risk-of-Bias tool (RoB 2.0). Abstracts of the Global Evidence Summit, Cape Town, South Africa. Cochrane Database Syst. Rev. 2017, 9 (Suppl. 1), 2111. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Rabbani, R.; Al-Yousif, N.; Robson, R.C.; Copstein, L.; Xia, J.; Pollock, M.; Mansour, S.; Ansari, M.T.; Tricco, A.C.; et al. Inter-rater reliability and concurrent validity of ROBINS-I: Protocol for a cross-sectional study. Syst. Rev. 2020, 9, 12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosado, C.; Fernandes, Â.R.; Rodrigues, A.G.; Lisboa, C. Impact of Human Papillomavirus Vaccination on Male Disease: A Systematic Review. Vaccines 2023, 11, 1083. https://doi.org/10.3390/vaccines11061083
Rosado C, Fernandes ÂR, Rodrigues AG, Lisboa C. Impact of Human Papillomavirus Vaccination on Male Disease: A Systematic Review. Vaccines. 2023; 11(6):1083. https://doi.org/10.3390/vaccines11061083
Chicago/Turabian StyleRosado, Catarina, Ângela Rita Fernandes, Acácio Gonçalves Rodrigues, and Carmen Lisboa. 2023. "Impact of Human Papillomavirus Vaccination on Male Disease: A Systematic Review" Vaccines 11, no. 6: 1083. https://doi.org/10.3390/vaccines11061083
APA StyleRosado, C., Fernandes, Â. R., Rodrigues, A. G., & Lisboa, C. (2023). Impact of Human Papillomavirus Vaccination on Male Disease: A Systematic Review. Vaccines, 11(6), 1083. https://doi.org/10.3390/vaccines11061083






