Human Papillomavirus Epidemiology and Prevention: Is There Still a Gender Gap?
Abstract
1. Introduction
2. Global Epidemiology of HPV and Its Related Diseases
3. Transmission
4. Clinical Manifestation
4.1. Benign
4.2. Malignant
5. Prevention
5.1. Primary Prevention
5.2. Secondary Prevention
5.3. Tertiary Prevention
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nøhr, B.; Kjaer, S.K.; Soylu, L.; Jensen, A. High-risk human papillomavirus infection in female and subsequent risk of infertility: A population-based cohort study. Fertil. Steril. 2019, 111, 1236–1242. [Google Scholar] [CrossRef]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef]
- Rosalik, K.; Tarney, C.; Han, J. Human Papilloma Virus Vaccination. Viruses 2021, 13, 1091. [Google Scholar] [CrossRef]
- Harden, M.E.; Munger, K. Human papillomavirus molecular biology. Mutat. Res. Rev. Mutat. Res. 2017, 772, 3–12. [Google Scholar] [CrossRef]
- Soheili, M.; Keyvani, H.; Soheili, M.; Nasseri, S. Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med. J. Islam. Repub. Iran. 2021, 35, 65. [Google Scholar] [CrossRef]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Goodman, A. HPV testing as a screen for cervical cancer. BMJ 2015, 350, h2372. [Google Scholar] [CrossRef]
- Roman, B.R.; Aragones, A. Epidemiology and incidence of HPV-related cancers of the head and neck. J. Surg. Oncol. 2021, 124, 920–922. [Google Scholar] [CrossRef]
- Ryndock, E.J.; Meyers, C. A risk for non-sexual transmission of human papillomavirus? Expert Rev. Anti Infect. Ther. 2014, 12, 1165–1170. [Google Scholar] [CrossRef]
- Iorga, L.; Dragos Marcu, R.; Cristina Diaconu, C.; Alexandra Stanescu, A.M.; Pantea Stoian, A.; Dorel Mischianu, D.L.; Surcel, M.; Bungau, S.; Constantin, T.; Boda, B.D.; et al. Penile carcinoma and HPV infection (Review). Exp. Ther. Med. 2020, 20, 91–96. [Google Scholar] [CrossRef]
- Manini, I.; Montomoli, E. Epidemiology and prevention of Human Papillomavirus. Ann Ig 2018, 30, 28–32. [Google Scholar] [PubMed]
- Sabeena, S.; Bhat, P.; Kamath, V.; Arunkumar, G. Possible non-sexual modes of transmission of human papilloma virus. J. Obs. Gynaecol. Res. 2017, 43, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Benedetti Panici, P.; et al. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef]
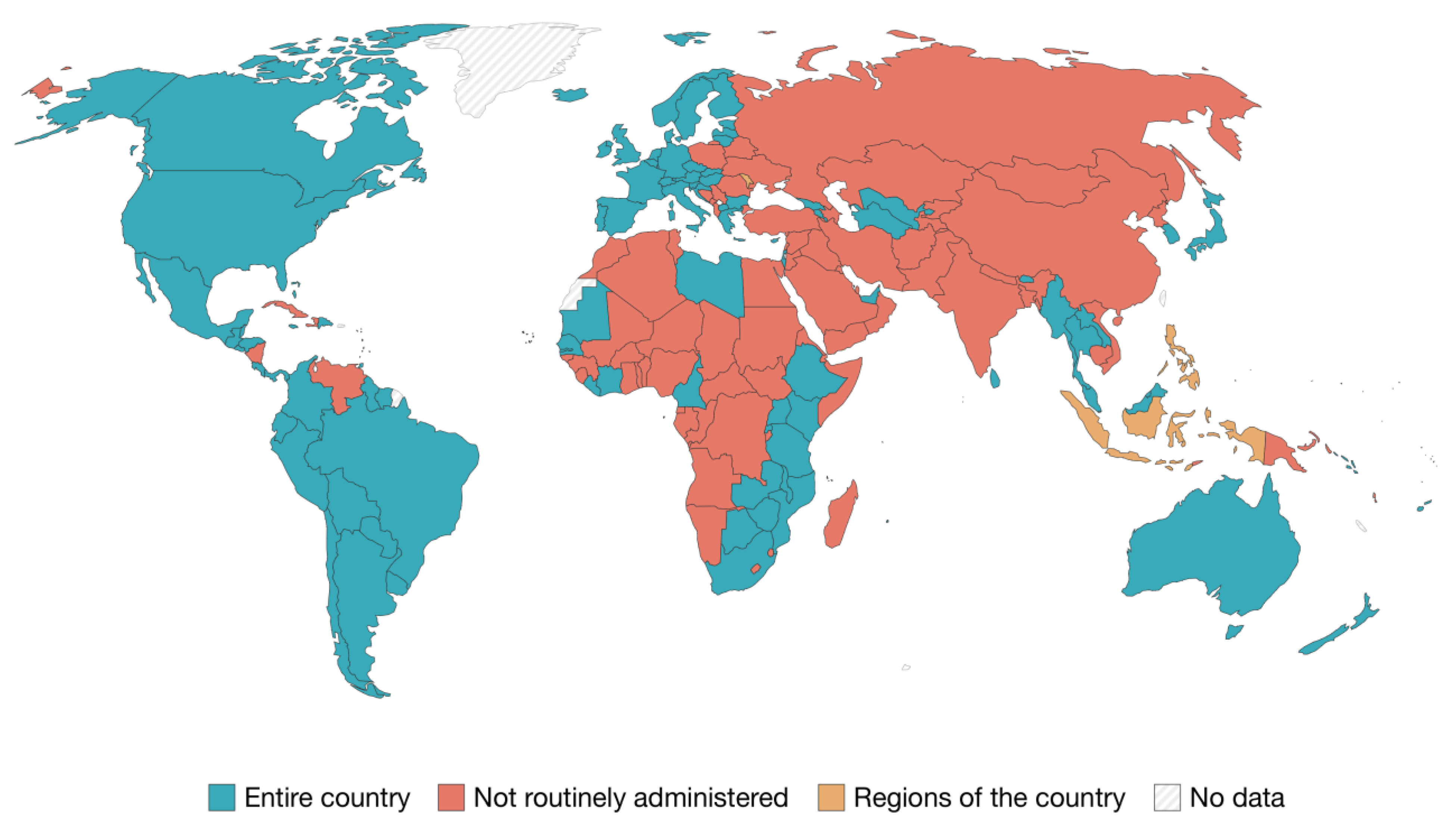
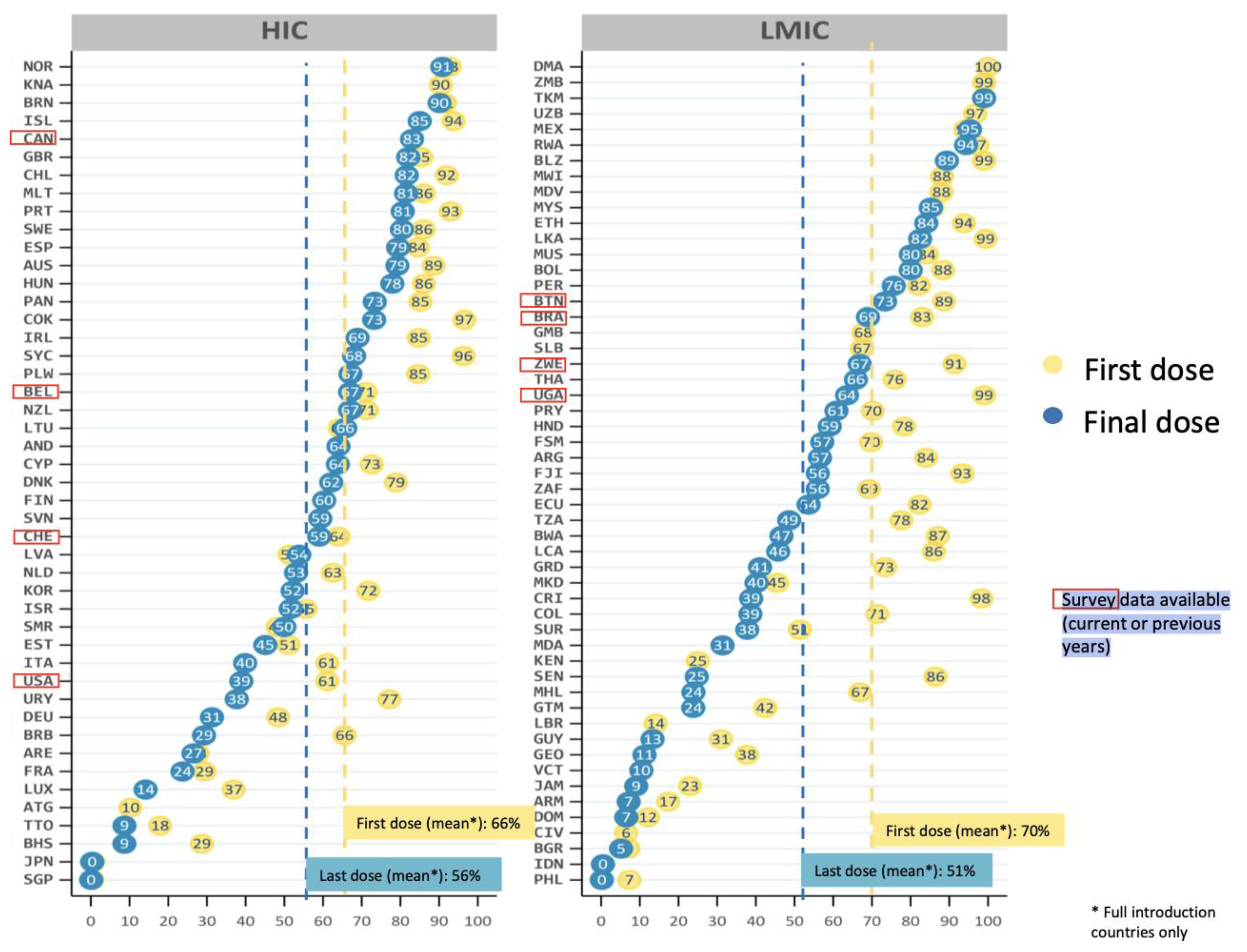
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar]
- Tao, Y.; Shao, H.; Zhang, T.; Pu, J.; Tang, C. Factors Influencing Men’s Attitudes toward HPV Vaccination in Males Included in the Chinese National Immunization Program. Vaccines 2022, 10, 1054. [Google Scholar] [CrossRef]
- Elst, L.; Albersen, M. HPV Vaccination: Does It Have a Role in Preventing Penile Cancer and Other Preneoplastic Lesions? Semin. Oncol. Nurs. 2022, 38, 151284. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2022, 12, 805695. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, H.; Liu, J.; Zhang, Z.; Fang, W.; Yang, Y.; Hong, S.; Xian, W.; Ma, Y.; Zhou, T.; et al. Incidence and risk factors of second primary cancer after the initial primary human papillomavirus related neoplasms. MedComm 2020, 1, 400–409. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 28–44. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer, World Health Organization. Incidence, Prevalence and Mortality Rates (World) in 2020. Available online: https://gco.iarc.fr/today/online-analysis-map?v=2020&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=23&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=0&include_nmsc_other=0&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&show_ranking=0&rotate=%255B10%252C0%255D (accessed on 11 February 2023).
- Santella, B.; Schettino, M.T.; Franci, G.; De Franciscis, P.; Colacurci, N.; Schiattarella, A.; Galdiero, M. Microbiota and HPV: The role of viral infection on vaginal microbiota. J. Med. Virol. 2022, 94, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, G.E.; Ricciardi, W. Verso un Mondo HPV Free: Strategie Internazionali, da Implementare a Livello Nazionale, per L’eliminazione del Cancro Cervicale: Il Valore Della Prevenzione e Della Vaccinazione Anti-HPV Negli Adolescenti Da: I Numeri del Cancro in Italia. AIOM-AIRTUM. 2022. Available online: https://www.aiom.it/wp-content/uploads/2022/12/2022_AIOM_NDC-web.pdf (accessed on 20 January 2023).
- Pimple, S.; Mishra, G. Cancer cervix: Epidemiology and disease burden. Cytojournal 2022, 19, 21. [Google Scholar] [CrossRef]
- Efua Sackey, M.; Markey, K.; Grealish, A. Healthcare professional’s promotional strategies in improving Human papillomavirus (HPV) vaccination uptake in adolescents: A systematic review. Vaccine 2022, 26, 2656–2666. [Google Scholar] [CrossRef] [PubMed]
- Sasidharanpillai, S.; Ravishankar, N.; Kamath, V.; Bhat, P.V.; Bhatt, P.; Arunkumar, G. Prevalence of Human Papillomavirus (HPV) DNA among Men with Oropharyngeal and Anogenital Cancers: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2021, 22, 1351–1364. [Google Scholar] [CrossRef]
- Lehtinen, M.; Gray, P.; Louvanto, K.; Vänskä, S. In 30 years, gender-neutral vaccination eradicates oncogenic human papillomavirus (HPV) types while screening eliminates HPV-associated cancers. Expert Rev. Vaccines 2022, 21, 735–738. [Google Scholar] [CrossRef]
- Carlander, A.F.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Lynggaard, C.D.; Jensen, J.S.; Buchwald, C.V.; Grønhøj, C. A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses 2021, 13, 1326. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Gabutti, G.; d’Anchera, E.; De Motoli, F.; Savio, M.; Stefanati, A. Human Papilloma Virus Vaccination: Focus on the Italian Situation. Vaccines 2021, 9, 1374. [Google Scholar] [CrossRef]
- Zamani, M.; Grønhøj, C.; Jensen, D.H.; Carlander, A.F.; Agander, T.; Kiss, K.; von Buchwald, C.; Friborg, J.; Andersen, E.; Nielsen, F.C.; et al. The current epidemic of HPV-associated oropharyngeal cancer: An 18-year Danish population-based study with 2,169 patients. Eur. J. Cancer 2020, 134, 52–59. [Google Scholar] [CrossRef]
- Del Mistro, A.; Frayle, H.; Menegaldo, A.; Favaretto, N.; Gori, S.; Nicolai, P.; Spinato, G.; Romeo, S.; Tirelli, G.; da Mosto, M.C.; et al. Age-independent increasing prevalence of human papillomavirus-driven oropharyngeal carcinomas in North-East Italy. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Seedat, R.Y. Juvenile-Onset Recurrent Respiratory Papillomatosis Diagnosis and Management—A Developing Country Review. Pediatric Health Med. Ther. 2020, 11, 39–46. [Google Scholar] [CrossRef]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef] [PubMed]
- Mastora, E.; Kitsou, C.; Evangelou, T.; Zikopoulos, A.; Zagorianakou, N.; Georgiou, I. Presence of HPV 16 and HPV 18 in Spermatozoa and Embryos of Mice. Vivo 2021, 35, 3203–3209. [Google Scholar] [CrossRef] [PubMed]
- Rombaldi, R.L.; Serafini, E.P.; Mandelli, J.; Zimmermann, E.; Losquiavo, K.P. Perinatal transmission of human papilomavirus DNA. Virol J. 2009, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Casalegno, J.S.; Le Bail Carval, K.; Eibach, D.; Valdeyron, M.L.; Lamblin, G.; Jacquemoud, H.; Mellier, G.; Lina, B.; Gaucherand, P.; Mathevet, P.; et al. High risk HPV contamination of endocavity vaginal ultrasound probes: An underestimated route of nosocomial infection? PLoS ONE 2012, 7, e48137. [Google Scholar] [CrossRef]
- Palma, S.; Gnambs, T.; Crevenna, R.; Jordakieva, G. Airborne human papillomavirus (HPV) transmission risk during ablation procedures: A systematic review and meta-analysis. Environ. Res. 2021, 192, 110437. [Google Scholar] [CrossRef]
- Sawchuk, W.S.; Weber, P.J.; Lowy, D.R.; Dzubow, L.M. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: Detection and protection. J. Am. Acad. Dermatol. 1989, 21, 41–49. [Google Scholar] [CrossRef]
- Garden, J.M.; O’Banion, M.K.; Bakus, A.D.; Olson, C. Viral disease transmitted by laser-generated plume (aerosol). Arch. Dermatol. 2002, 138, 1303–1307. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Chang, Y.C.; Liu, H.W.; Chu, T.Y. Long-term persistence of human papillomavirus in environments. Gynecol. Oncol. 2011, 121, 148–151. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G. Papillomavirus. In Global Water Pathogen Project, Part 3 Viruses; Rose, J.B., Jiménez-Cisneros, B., Meschke, J.S., Girones, R., Eds.; Michigan State University: Lansing, MI, USA; Unesco: Paris, France, 2016. [Google Scholar]
- Symonds, E.M. Viruses in raw sewage and their potential to indicate fecal pollution in coastal environments. Grad. Sch. Theses Diss. 2008. Available online: https://www.semanticscholar.org/paper/Viruses-in-raw-sewage-and-their-potential-to-fecal-Symonds/241a937ab52f8deb4da95ee3cf6b9bbccf65fede (accessed on 31 May 2023).
- Cantalupo, P.G.; Calgua, B.; Zhao, G.; Hundesa, A.; Wier, A.D.; Katz, J.P.; Grabe, M.; Hendrix, R.W.; Girones, R.; Wang, D.; et al. Raw sewage harbors diverse viral populations. mBio 2011, 2, e00180-11. [Google Scholar] [CrossRef] [PubMed]
- Bibby, K.; Peccia, J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Ment. Sci. Technol. 2013, 47, 1945–1951. [Google Scholar] [CrossRef]
- Fratini, M.; Di Bonito, P.; La Rosa, G. Oncogenic papillomavirus and polyomavirus in water environments: Is there a potential for waterborne transmission? Food Environ. Virol. 2014, 6, 1–12. [Google Scholar] [CrossRef]
- Di Bonito, P.; Della Libera, S.; Petricca, S.; Iaconelli, M.; Sanguinetti, M.; Graffeo, R.; Accardi, L.; La Rosa, G. A large spectrum of alpha and beta papillomaviruses are detected in human stool samples. J. Gen. Virol. 2015, 96, 607–613. [Google Scholar] [CrossRef]
- Dediol, I.; Buljan, M.; Vurnek-A Ivkoviä, M.; Bulat, V.; A Itum, M.; A Ubriloviä, A. Psychological burden of anogenital warts. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1035–1038. [Google Scholar] [CrossRef]
- Tyros, G.; Mastraftsi, S.; Gregoriou, S.; Nicolaidou, E. Incidence of anogenital warts: Epidemiological risk factors and real-life impact of human papillomavirus vaccination. Int. J. STD AIDS. 2021, 32, 4–13. [Google Scholar] [CrossRef]
- Fortes, H.R.; von Ranke, F.M.; Escuissato, D.L.; Araujo Neto, C.A.; Zanetti, G.; Hochhegger, B.; Souza, C.A.; Marchiori, E. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir. Med. 2017, 126, 116–121. [Google Scholar] [CrossRef]
- Seedat, R.Y.; Dikkers, F.G. Global epidemiology of HPV-associated recurrent respiratory papillomatosis and effect of vaccination. Future Virol. 2022, 17, 265–268. [Google Scholar]
- McLaughlin-Drubin, M.E.; Crum, C.P.; Münger, K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc. Natl. Acad. Sci. USA 2011, 108, 2130–2135. [Google Scholar] [CrossRef]
- Singh, R.K. Diffuse Non-Genital Cutaneous Warts. Am. J. Trop. Med. Hyg. 2021, 106, 378–379. [Google Scholar] [CrossRef] [PubMed]
- El Moussaoui, S.; Fernández-Campos, F.; Alonso, C.; Limón, D.; Halbaut, L.; Garduño-Ramirez, M.L.; Calpena, A.C.; Mallandrich, M. Topical Mucoadhesive Alginate-Based Hydrogel Loading Ketorolac for Pain Management after Pharmacotherapy, Ablation, or Surgical Removal in Condyloma Acuminata. Gels 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Pennycook, K.B.; McCready, T.A. Condyloma Acuminata. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Oyervides-Muñoz, M.A.; Pérez-Maya, A.A.; Rodríguez-Gutiérrez, H.F.; Gómez-Macias, G.S.; Fajardo-Ramírez, O.R.; Treviño, V.; Barrera-Saldaña, H.A.; Garza-Rodríguez, M.L. Understanding the HPV integration and its progression to cervical cancer. Infect. Genet. Evol. 2018, 61, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos-Villegas, E.G.; Pérez-yPérez, M.F.; Alvarez-Salas, L.M. Cervical Cancer, Papillomavirus, and miRNA Dysfunction. Front. Mol. Biosci. 2021, 8, 758337. [Google Scholar] [CrossRef]
- Merz, J.; Bossart, M.; Bamberg, F.; Eisenblaetter, M. Revised FIGO Staging for Cervical Cancer—A New Role for MRI. Rofo 2020, 192, 937–944. [Google Scholar] [CrossRef]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiology 2021, 88, 187–197. [Google Scholar] [CrossRef]
- Schlenker, B.; Schneede, P. The Role of Human Papilloma Virus in Penile Cancer Prevention and New Therapeutic Agents. Eur. Urol. Focus. 2019, 5, 42–45. [Google Scholar] [CrossRef]
- Kuasne, H.; Barros-Filho, M.C.; Busso-Lopes, A.; Marchi, F.A.; Pinheiro, M.; Muñoz, J.J.; Scapulatempo-Neto, C.; Faria, E.F.; Guimarães, G.C.; Lopes, A.; et al. Integrative miRNA and mRNA analysis in penile carcinomas reveals markers and pathways with potential clinical impact. Oncotarget 2017, 8, 15294–15306. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chien, C.Y.; Huang, T.L.; Chiu, T.J.; Wang, Y.M.; Fang, F.M.; Li, S.H. Low p16 Cytoplasmic Staining Predicts Poor Treatment Outcome in Patients with p16-Negative Locally Advanced Head and Neck Squamous Cell Carcinoma Receiving TPF Induction Chemotherapy. Biomedicines 2023, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Bird, S.W.; Bustamante, J.P.; Leon, L.E.; Nieto, P.A.; Addae, K.; Alegría-Mera, V.; Bravo, C.; Bravo, D.; Cardenas, J.P.; et al. A novel sequencing-based vaginal health assay combining self-sampling, HPV detection and genotyping, STI detection, and vaginal microbiome analysis. PLoS ONE 2019, 14, e0215945. [Google Scholar] [CrossRef]
- Eun, T.J.; Perkins, R.B. Screening for Cervical Cancer. Med. Clin. N. Am. 2020, 104, 1063–1078. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Schiller, J.T. Human Papillomavirus Vaccines. J. Infect. Dis. 2021, 224, S367–S378. [Google Scholar] [CrossRef]
- Gualano, M.R.; Bert, F.; Voglino, G.; Buttinelli, E.; D’Errico, M.M.; De Waure, C.; Di Giovanni, P.; Fantini, M.P.; Giuliani, A.R.; Marranzano, M.; et al. Collaborating Group. Attitudes towards compulsory vaccination in Italy: Results from the NAVIDAD multicentre study. Vaccine 2018, 36, 3368–3374. [Google Scholar] [CrossRef]
- Yazdani, Z.; Rafiei, A.; Valadan, R.; Ashrafi, H.; Pasandi, M.; Kardan, M. Designing a potent L1 protein-based HPV peptide vaccine: A bioinformatics approach. Comput. Biol. Chem. 2020, 85, 107209. [Google Scholar] [CrossRef] [PubMed]
- Soca Gallego, L.; Dominguez, A.; Parmar, M. Human Papilloma Virus Vaccine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Quang, C.; Chung, A.W.; Frazer, I.H.; Toh, Z.Q.; Licciardi, P.V. Single-dose HPV vaccine immunity: Is there a role for non-neutralizing antibodies? Trends Immunol. 2022, 43, 815–825. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Sampson, J.N.; Porras, C.; Schiller, J.T.; Kemp, T.; Herrero, R.; Wagner, S.; Boland, J.; Schussler, J.; Lowy, D.R.; et al. Costa Rica HPV Vaccine Trial (CVT) Group. Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial. J. Natl. Cancer Inst. 2020, 112, 1038–1046. [Google Scholar] [CrossRef]
- Panwar, K.; Godi, A.; Cocuzza, C.E.; Andrews, N.; Southern, J.; Turner, P.; Miller, E.; Beddows, S. Multiplex Human Papillomavirus L1L2 virus-like particle antibody binding assay. MethodsX 2022, 9, 101776. [Google Scholar] [CrossRef]
- Godi, A.; Panwar, K.; Haque, M.; Cocuzza, C.E.; Andrews, N.; Southern, J.; Turner, P.; Miller, E.; Beddows, S. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix® or Gardasil® vaccine. Vaccine 2019, 37, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Roser, M.; Ortiz-Ospina, E. Which Countries Include Human Papillomavirus (HPV) Vaccines in Their Vaccination Schedules? 2021. Available online: https://ourworldindata.org/grapher/human-papillomavirus-vaccine-immunization-schedule?country=BFA~ROU~ARM (accessed on 2 March 2023).
- Brotherton, J.M.; Malloy, M.; Budd, A.C.; Saville, M.; Drennan, K.T.; Gertig, D.M. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: Observational cohort of young women in Australia. Papillomavirus Res. 2015, 1, 59–72. [Google Scholar] [CrossRef]
- Wang, W.V.; Kothari, S.; Khoury, H.; Niccolai, L.; Garland, S.M.; Sundström, K.; de Pouvourville, G.; Bonanni, P.; Chen, Y.T.; Franco., E.L. A review of data systems for assessing the impact of HPV vaccination in selected high-income countries. Expert Rev. Vaccines 2023, 22, 161–179. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNICEF. Progress and Challenges with Achieving Universal Immunization Coverage 2020. 2020. Available online: https://cdn.who.int/media/docs/default-source/immunization/coverage/who-immuniz.pdf?sfvrsn=72fd7237_2&download=true (accessed on 18 May 2023).
- Akhatova, A.; Azizan, A.; Atageldiyeva, K.; Ashimkhanova, A.; Marat, A.; Iztleuov, Y.; Suleimenova, A.; Shamkeeva, S.; Aimagambetova, G. Prophylactic Human Papillomavirus Vaccination: From the Origin to the Current State. Vaccines 2022, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Dorji, T.; Tshomo, U.; Gyamtsho, S.; Tamang, S.T.; Wangmo, S.; Pongpirul, K. Gender-neutral HPV elimination, cervical cancer screening, and treatment: Experience from Bhutan. Int. J. Gynecol. Obstet. 2022, 156, 425–429. [Google Scholar] [CrossRef]
- Dykens, J.A.; Peterson, C.E.; Holt, H.K.; Harper, D.M. Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Front. Public Health 2023, 11, 1067299. [Google Scholar] [CrossRef]
- Amponsah-Dacosta, E.; Blose, N.; Nkwinika, V.V.; Chepkurui, V. Human Papillomavirus Vaccination in South Africa: Programmatic Challenges and Opportunities for Integration With Other Adolescent Health Services? Front. Public Health 2022, 10, 799984. [Google Scholar] [CrossRef]
- Chido-Amajuoyi, O.G.; Fokom Domgue, J.; Obi-Jeff, C.; Schmeler, K.; Shete, S. A call for the introduction of gender-neutral HPV vaccination to national immunisation programmes in Africa. Lancet Glob. Health 2019, 7, e20–e21. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Liu, F.; Lian, G.; Li, S.; He, Q.; Li, T. Human papillomavirus vaccination coverage and knowledge, perceptions and influencing factors among university students in Guangzhou, China. Hum. Vaccin. Immunother. 2021, 17, 3603–3612. [Google Scholar] [CrossRef]
- Simms, K.T.; Hanley, S.J.B.; Smith, M.A.; Keane, A.; Canfell, K. Impact of HPV vaccine hesitancy on cervical cancer in Japan: A modelling study. Lancet Public Health 2020, 5, e223–e234. [Google Scholar] [CrossRef]
- Nogueira-Rodrigues, A.; Flores, M.G.; Macedo Neto, A.O.; Braga, L.A.C.; Vieira, C.M.; de Sousa-Lima, R.M.; de Andrade, D.A.P.; Machado, K.K.; Guimarães, A.P.G. HPV vaccination in Latin America: Coverage status, implementation challenges and strategies to overcome it. Front. Oncol. 2022, 12, 984449. [Google Scholar] [CrossRef]
- Athanasiou, A.; Bowden, S.; Paraskevaidi, M.; Fotopoulou, C.; Martin-Hirsch, P.; Paraskevaidis, E.; Kyrgiou, M. HPV vaccination and cancer prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Biundo, E.; Courcier, S.; Damm, O.; Launay, O.; Maes, E.; Marcos, C.; Matthews, S.; Meijer, C.; Poscia, A.; et al. A report on the status of vaccination in Europe. Vaccine 2018, 36, 4979–4992. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Huu, N.H.; Thilly, N.; Derrough, T.; Sdona, E.; Claudot, F.; Pulcini, C.; Agrinier, N.; HPV Policy working group. Human papillomavirus vaccination coverage, policies, and practical implementation across Europe. Vaccine 2020, 38, 1315–1331. [Google Scholar] [CrossRef]
- Italian Communication Campaign on HPV Vaccination. Available online: https://www.salute.gov.it/portale/vaccinazioni/dettaglioCampagneVaccinazioni.jsp?lingua=italiano& (accessed on 28 March 2023).
- Giuliano, A.R.; Nyitray, A.G.; Albero, G. Male circumcision and HPV transmission to female partners. Lancet 2011, 377, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Geneva: World Health Organization. 2020. Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 29 March 2023).
- Sami, J.; Lemoupa Makajio, S.; Jeannot, E.; Kenfack, B.; Viñals, R.; Vassilakos, P.; Petignat, P. Smartphone-Based Visual Inspection with Acetic Acid: An Innovative Tool to Improve Cervical Cancer Screening in Low-Resource Setting. Healthcare 2022, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Khairkhah, N.; Bolhassani, A.; Najafipour, R. Current and future direction in treatment of HPV-related cervical disease. J. Mol. Med. 2022, 100, 829–845. [Google Scholar] [CrossRef]
- Cooper, D.B.; Dunton, C.J. Colposcopy, 16 July 2022. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bhatla, N.; Singhal, S. Primary HPV screening for cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 98–108. [Google Scholar] [CrossRef]
- Tota, J.E.; Bentley, J.; Blake, J.; Coutlée, F.; Duggan, M.A.; Ferenczy, A.; Franco, E.L.; Fung-Kee-Fung, M.; Gotlieb, W.; Mayrand, M.-H.; et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: Acting on evidence to change the current paradigm. Prev. Med. 2017, 98, 5–14. [Google Scholar] [CrossRef]
- Ebisch, R.M.F.; Rijstenberg, L.L.; Soltani, G.G.; van der Horst, J.; Vedder, J.E.M.; Hermsen, M.; Bosgraaf, R.P.; Massuger, L.F.A.G.; Meijer, C.J.L.M.; Heideman, D.A.M.; et al. Adjunctive use of p16 immunohistochemistry for optimizing management of CIN lesions in a high-risk human papillomavirus-positive population. Acta Obstet. Gynecol. Scand. 2022, 101, 1328–1336. [Google Scholar] [CrossRef]
- Stoler, M.H.; Wright, T.C., Jr.; Ferenczy, A.; Ranger-Moore, J.; Fang, Q.; Kapadia, M.; Ridder, R. Routine Use of Adjunctive p16 Immunohistochemistry Improves Diagnostic Agreement of Cervical Biopsy Interpretation: Results From the CERTAIN Study. Am. J. Surg. Pathol. 2018, 42, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; de Sanjosé, S.; Bruni, L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022, 154, 106900. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef] [PubMed]
- Profozić, Z.; Meštrović, T.; Savić, I.; Profozić, V. Prevalence of HPV Infection in Croatian Men during a 12-year Period: A Comparative Study of External Genital and Urethral Swabs. Cent. Eur. J. Public. Health 2016, 24, 321–325. [Google Scholar] [CrossRef]
- Pan, L.J.; Ma, J.H.; Zhang, F.L.; Pan, F.; Zhao, D.; Zhang, X.Y. HPV infection of the external genitalia in men whose female partners have cervical HPV infection. Zhonghua Nan Ke Xue 2018, 24, 516–519. [Google Scholar]
- Luttmer, R.; Dijkstra, M.G.; Snijders, P.J.F.; Jordanova, E.S.; King, A.J.; Pronk, D.T.; Meijer, C.J.; Heideman, D.A.M.; Doorbar, J.; Bleeker, M.C.G.; et al. Presence of human papillomavirus in semen of healthy men is firmly associated with HPV infections of the penile epithelium. Fertil. Steril. 2015, 104, 838–844.e8. [Google Scholar] [CrossRef]
- Tuan, L.A.; Prem, K.; Pham, Q.D.; Toh, Z.Q.; Tran, H.P.; Nguyen, P.D.; Mai, C.T.N.; Ly, L.T.K.; Cao, V.; Le-Ha, T.-D.; et al. Anal human papillomavirus prevalence and risk factors among men who have sex with men in Vietnam. Int. J. Infect. Dis. 2021, 112, 136–143. [Google Scholar] [CrossRef]
- Shapiro, G.K. HPV Vaccination: An Underused Strategy for the Prevention of Cancer. Curr. Oncol. 2022, 29, 3780–3792. [Google Scholar] [CrossRef]
- Kisling, L.A.; M Das, J. Prevention Strategies, 2022 May 8. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Swedish, K.A.; Factor, S.H.; Goldstone, S.E. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: A nonconcurrent cohort study. Clin. Infect. Dis. 2012, 54, 891–898. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Cantor, S.B.; Fenwick, E.; Chiao, E.Y.; Nyitray, A.G.; Stier, E.A.; Goldstone, S.E.; Wilkin, T.; Chhatwal, J. Adjuvant HPV vaccination for anal cancer prevention in HIV-positive men who have sex with men: The time is now. Vaccine 2017, 35, 5102–5109. [Google Scholar] [CrossRef]
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol. Oncol. 2018, 151, 229–234. [Google Scholar] [CrossRef]
- Michalczyk, K.; Misiek, M.; Chudecka-Głaz, A. Can Adjuvant HPV Vaccination Be Helpful in the Prevention of Persistent/Recurrent Cervical Dysplasia after Surgical Treatment?—A Literature Review. Cancers 2022, 14, 4352. [Google Scholar] [CrossRef]
- Di Donato, V.; Caruso, G.; Bogani, G.; Cavallari, E.N.; Palaia, G.; Perniola, G.; Ralli, M.; Sorrenti, S.; Romeo, U.; Pernazza, A.; et al. HPV Vaccination after Primary Treatment of HPV-Related Disease across Different Organ Sites: A Multidisciplinary Comprehensive Review and Meta-Analysis. Vaccines 2022, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Kin Cho Goon, P.; Scholtz, L.U.; Sudhoff, H. Recurrent respiratory papillomatosis (RRP)-time for a reckoning? Laryngoscope Investig. Otolaryngol. 2017, 2, 184–186. [Google Scholar] [CrossRef]
- Swedish, K.A.; Goldstone, S.E. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS ONE 2014, 9, e93393. [Google Scholar] [CrossRef] [PubMed]
- Goon, P.; Sauzet, O.; Schuermann, M.; Oppel, F.; Shao, S.; Scholtz, L.U.; Sudhoff, H.; Goerner, M. Recurrent Respiratory Papillomatosis (RRP)-Meta-analyses on the use of the HPV vaccine as adjuvant therapy. NPJ Vaccines 2023, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Husein-ElAhmed, H. Could the human papillomavirus vaccine prevent recurrence of ano-genital warts?: A systematic review and meta-analysis. Int. J. STD AIDS 2020, 31, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Jung, A.C.; Fakhry, C. Primary, secondary and tertiary prevention of human papillomavirus-driven head and neck cancers. Eur. J. Cancer 2017, 78, 105–115. [Google Scholar] [CrossRef]
- Rettig, E.M.; Wentz, A.; Posner, M.R.; Gross, N.D.; Haddad, R.I.; Gillison, M.L.; Fakhry, C.; Quon, H.; Sikora, A.G.; Stott, W.J.; et al. Prognostic implication of persistent human papillomavirus type 16 DNA detection in oral rinses for human papillomavirus-related oropharyngeal carcinoma. JAMA Oncol. 2015, 1, 907–915. [Google Scholar] [CrossRef]
- Fakhry, C.; Qualliotine, J.R.; Zhang, Z.; Agrawal, N.; Gaykalova, D.A.; Bishop, J.A.; Subramaniam, R.M.; Koch, W.M.; Chung, C.H.; Eisele, D.W.; et al. Serum antibodies to HPV16 early proteins warrant investigation as potential biomarkers for risk stratifica- tion and recurrence of HPV-associated oropharyngeal cancer. Cancer Prev. Res. 2016, 9, 135–141. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Li, G.; Hussey, C.S.; Vo, J.T.; Wei, Q.; Zhao, C.; Sturgis, E.M. Circulating human papillomavirus DNA as a marker for disease extent and recurrence among patients with oropharyngeal cancer. Cancer 2015, 121, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Wang, W.Y.; Chen, K.Y.; Wei, Y.H.; Liang, W.M.; Jan, J.S.; Jiang, R.-S. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N. Engl. J. Med. 2004, 350, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Twu, C.W.; Wang, W.Y.; Liang, W.M.; Jan, J.S.; Jiang, R.S.; Chao, J.; Jin, Y.T.; Lin, J.C. Comparison of the prognostic impact of serum anti-EBV anti- body and plasma EBV DNA assays in nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 130–137. [Google Scholar] [CrossRef]
- Chrysostomou, A.C.; Stylianou, D.C.; Constantinidou, A.; Kostrikis, L.G. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses 2018, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Bosco, R.; Messina, G.; Aiello, B.; Guarducci, G.; Nante, N. The Structures and Activities of Health Promotion in the Italian NHS. Healthcare 2023, 11, 148. [Google Scholar] [CrossRef]
- Palfrey, S. New initiatives to improve HPV vaccination rates. Hum. Vaccin. Immunother. 2016, 12, 1594–1598. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milano, G.; Guarducci, G.; Nante, N.; Montomoli, E.; Manini, I. Human Papillomavirus Epidemiology and Prevention: Is There Still a Gender Gap? Vaccines 2023, 11, 1060. https://doi.org/10.3390/vaccines11061060
Milano G, Guarducci G, Nante N, Montomoli E, Manini I. Human Papillomavirus Epidemiology and Prevention: Is There Still a Gender Gap? Vaccines. 2023; 11(6):1060. https://doi.org/10.3390/vaccines11061060
Chicago/Turabian StyleMilano, Giovanna, Giovanni Guarducci, Nicola Nante, Emanuele Montomoli, and Ilaria Manini. 2023. "Human Papillomavirus Epidemiology and Prevention: Is There Still a Gender Gap?" Vaccines 11, no. 6: 1060. https://doi.org/10.3390/vaccines11061060
APA StyleMilano, G., Guarducci, G., Nante, N., Montomoli, E., & Manini, I. (2023). Human Papillomavirus Epidemiology and Prevention: Is There Still a Gender Gap? Vaccines, 11(6), 1060. https://doi.org/10.3390/vaccines11061060







