Pulmonary Embolism after Vaccination with the COVID-19 Vaccine (Pfizer, BNT162b2): A Case Report
Abstract
:1. Introduction
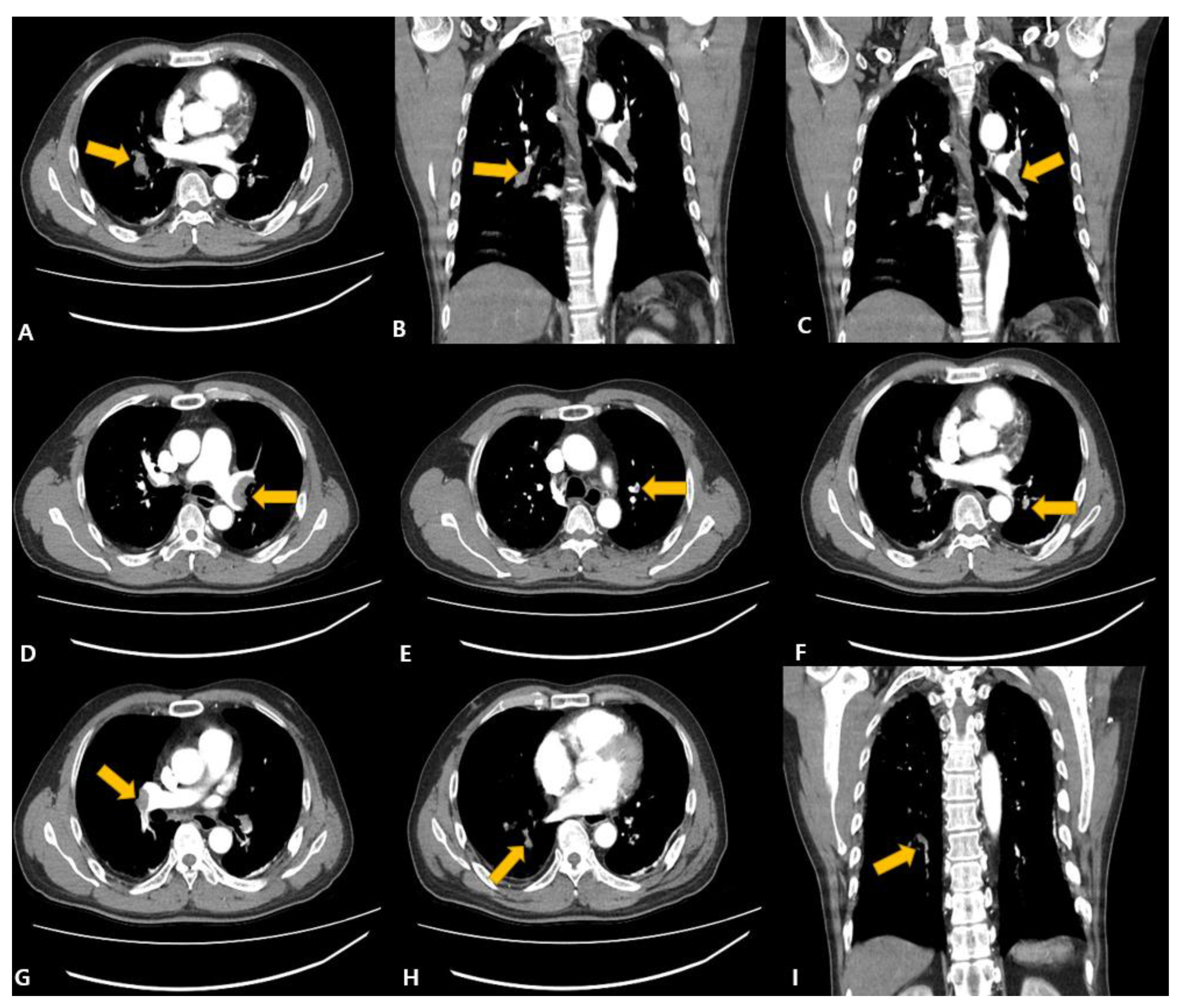
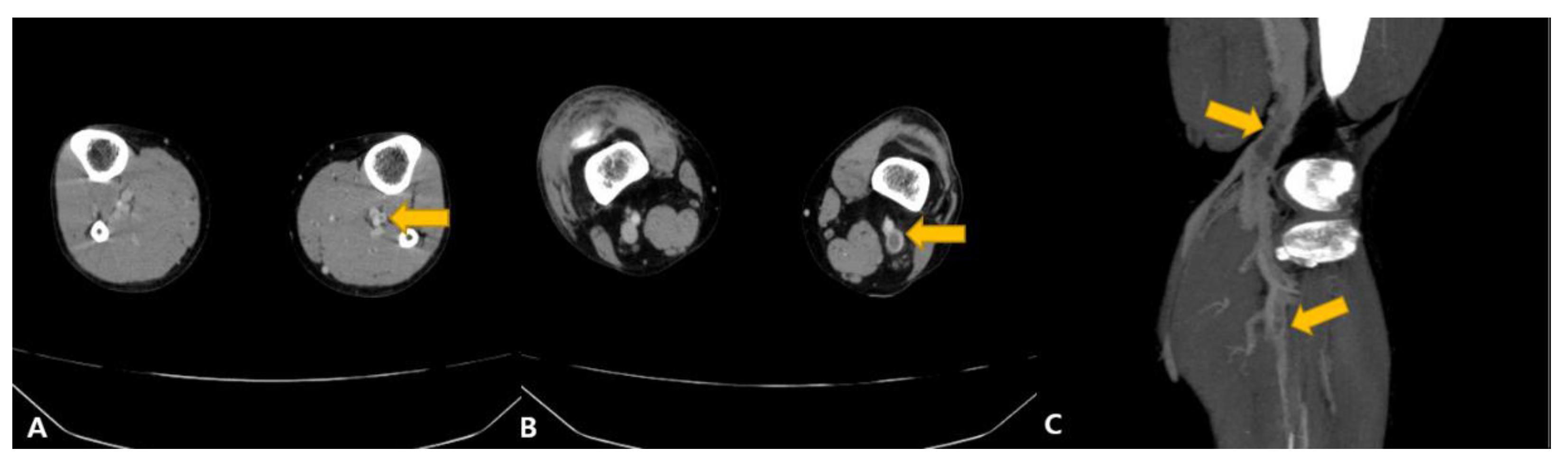
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, W.B.; Hwang, Y.H.; Cheong, H.J. COVID-19 Vaccination in Korea. Infect. Chemother. 2023, 55, 135–149. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-TTS-2021.1 (accessed on 15 March 2023).
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Sangli, M.S.; Virani, A.; Cheronis, N.; Vannatter, D.B.; Minich, D.C.; Noronha, M.S.; Bhagavatula, R.; Speredelozzi, D.; Sareen, M.; Kaplan, R.B. Thrombosis with Thrombocytopenia after the Messenger RNA–1273 Vaccine. Ann. Intern. Med. 2021, 174, 1480–1482. [Google Scholar] [CrossRef] [PubMed]
- Miri, C.; Bouchlarhem, A.; Boulouiz, S.; El Ouafi, N.; Bazid, Z. Pulmonary embolism with junctional tachycardia: A serious complication after COVID-19 vaccination. Ann. Med. Surg. 2022, 80, 103983. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqbali, J.S.; Al Rasbi, S.; Kashoub, M.S.; Al Hinaai, A.M.; Farhan, H.; Al Rawahi, B.; Al Alawi, A.M. A 59-year-old woman with extensive deep vein thrombosis and pulmonary thromboembolism 7 days following a first dose of the Pfiz-er-BioNTech BNT162b2 mRNA COVID-19 vaccine. Am. J. Case Rep. 2021, 22, e932946. [Google Scholar] [CrossRef] [PubMed]
- Tarbox, A.K.; Swaroop, M. Pulmonary embolism. Int. J. Crit Illn. Inj. Sci. 2013, 3, 69–72. [Google Scholar] [PubMed]
- National Academy of Medicine of Korea. 2022. Available online: https://www.namok.or.kr/bbs/index.php?code=notice&category=&gubun=&page=2&number=1182&mode=view&keyfield=&key= (accessed on 15 February 2023).
- Hwang, H.-G.; Lee, J.H.; Kim, S.-A.; Kim, Y.-K.; Yhim, H.-Y.; Hong, J.; Bang, S.-M. Incidence of Venous Thromboembolism: The 3rd Korean Nationwide Study. J. Korean Med. Sci. 2022, 37, e130. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2016. Available online: https://apps.who.int/iris/handle/10665/208262 (accessed on 15 March 2023).
- National Academy of Medicine of Korea. 2023. Available online: https://www.namok.or.kr/bbs/index.php?code=notice&category=&gubun=&page=1&number=1161&mode=view&keyfield=all&key= (accessed on 15 March 2023).
- Ishibashi, Y.; Takama, N.; Fujii, T.; Takizawa, D.; Amanai, S.; Kuno, T.; Aihara, K.; Koitabashi, N.; Ishii, H. Acute pulmonary thromboembolism after messenger RNA vaccination against coronavirus disease 2019: A case report. J. Cardiol. Cases 2023, 27, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.I.; Chen, C.F.; Chen, J.S.; Wu, Y.W.; Chiu, K.M.; Tu, C.M. Acute Pulmonary Embolism Following Moderna mRNA-1273 SARS-CoV-2 Vaccination—A Case Report and Literature Review. Acta Cardiol. Sin. 2022, 38, 539–541. [Google Scholar] [PubMed]
- Wiest, N.E.; Johns, G.S.; Edwards, E.A. Case of Acute Pulmonary Embolus after mRNA SARS-CoV-2 Immunization. Vaccines 2021, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Ogunkoya, J.O.; Ogunmola, M.I.; Ogunlade, A.F.; Ladele, A.E. COVID-19 Vaccination Associated Bilateral Pulmonary Em-bolism: Cause or Coincidence. Case Rep. Pulmonol. 2022, 2022, 9596285. [Google Scholar] [CrossRef] [PubMed]
- Su, P.-H.; Yu, Y.-C.; Chen, W.-H.; Lin, H.-C.; Chen, Y.-T.; Cheng, M.-H.; Huang, Y.-M. Case Report: Vaccine-Induced Immune Thrombotic Thrombocytopenia in a Pancreatic Cancer Patient after Vaccination with Messenger RNA−1273. Front. Med. 2021, 8, 772424. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Sohn, J.-H.; Lee, S.-H.; Lee, E.; Jeon, W.; Kim, Y. Cerebral Venous Thrombosis without Thrombocytopenia after COVID-19 Vaccination. J. Korean Neurol. Assoc. 2022, 40, 160–163. [Google Scholar] [CrossRef]
- Long, B.; Bridwell, R.; Gottlieb, M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am. J. Emerg. Med. 2021, 49, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Brazete, C.; Aguiar, A.; Furtado, I.; Duarte, R. Thrombotic events and COVID-19 vaccines. Int. J. Tuberc. Lung Dis. 2021, 25, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Berlit, P.; Diener, H.C.; Gerloff, C.; Greinacher, A.; Klein, C.; Petzold, G.C.; Piccininni, M.; Poli, S.; Röhrig, R.; et al. COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann. Neurol. 2021, 90, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, W.N.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Walker, V.; Denholm, R.; Akbari, A.; Omigie, E.; Hollings, S.; et al. CVD-COVID-UK consortium. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 2022, 19, e1003926. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency. 2022. Available online: https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019 (accessed on 15 March 2023).
| 1. National Academy of Medicine of Korea [8], Presentation Date: 11 August 2022 | |
|---|---|
| The Title of the Forum | COVID-19 Vaccine Safety Committee 4th Forum—3rd Study Results Presentation |
| Methods | • Design: Self-Controlled Case Series Minimize the involvement of confounding variables by comparing the incidence rate between the time when the effect of COVID-19 vaccination is estimated to occur (Risk interval) and the time when the effect is reduced (Post-vaccination Control interval). |
| Participants | 6.5 million (Pfizer vaccine) |
| Outcome | The Pfizer vaccine showed a significant increase in the incidence rate after one vaccination (IRR 1.29, 95% confidence interval 1.05–1.58, p-value = 0.017). |
| Conclusion | • As a result of the analysis of thrombosis-related diseases such as deep vein thrombosis, when comparing the risk period after vaccination and the control period after vaccination, an increase in the incidence rate of all vaccines was not confirmed in the composite outcome, which gathered all related diseases. • However, for the Pfizer vaccine, a slight increase in the consistent incidence of the total binding index and the post-vaccination risk period compared to the post-vaccination control period of individual diseases was detected. • A detailed analysis is required, but this does not match the results of overseas studies, and there is a possibility of overdiagnosis due to concerns about adverse reactions. Therefore, a close epidemiological evaluation of deep vein thrombosis and pulmonary embolism after Pfizer vaccination will be conducted in the future. |
| 2. National Academy of Medicine of Korea [12], Presentation Date: 28 February 2023 | |
| The Title of the Forum | COVID-19 Vaccine Safety Research Center 3rd Forum |
| Methods | Design: Self-Controlled Risk Interval Study and Target Trial Emulation Study |
| Participants | 2.4 million (Pfizer vaccine) |
| Outcome | There were no significant results. |
| Conclusion | • Previous analyses, such as deep vein thrombosis, did not confirm evidence to support causality. Therefore, in this reanalysis, the statistical association between domestic vaccination and deep vein thrombosis was evaluated using a strict case definition and two independent research methodologies. • In the self-controlled risk interval study and clinical trial simulation, no increase in the incidence of deep vein thrombosis was observed after vaccination. This is consistent with the results of some studies conducted abroad and a meta-analysis that synthesizes them. |
| Author/ Country | Age/ Gender | Vaccine | Underlying Disease (1) and Adverse Events (2) † | Blood (3) and Radiologic Observations (4) ǂ | Findings or Conclusions | ||
|---|---|---|---|---|---|---|---|
| Manufacturers | Dose | Time between Vaccination and Symptoms Onset | |||||
| 1. Sangli, S. et al. [4]/USA | 65/Male | Moderna (mRNA-1273) | 2 | 10 days | (1) Chronic hypertension, hyperlipidemia (2) Bilateral lower-extremity discomfort, intermittent headaches, and dyspnea | (3) Platelet: 14 × 109 cells/L (▼), D-dimer: 18.9 nmol/L (▲) (4) CT: large, bilateral, acute pulmonary emboli with right ventricular strain. Doppler: lower extremities revealed acute deep venous thromboses in both lower extremities. | This report presents the first report of VITT or TTS after a SARS-CoV-2 vaccine based on messenger RNA (mRNA) technology. Additionally, VITT or TTS, which was the cause of the adenovirus vector vaccine, may also occur in the mRNA vaccine. |
| 2. Miri, C. et al. [5]/Morocco | 49/Male | Pfizer (mRNA-BNT162b2) | 2 | 7 days | (1) No medical history (2) Dyspnea | (3) Platelet: Normal, Increased CRP (▲), Elevated D-dimer (▲); (4) CT: proximal right pulmonary embolism. | This patient had no risk factors predisposing them to the development of acute venous thrombosis, in particular pulmonary embolism, and he tested negative for COVID-19 infection; however, the development of this thrombosis due to the mRNA-1273 vaccine is the most reasonable explanation. |
| 3. Al-Maqbali, J.S. et al. [6]/Oman | 59/Female | Pfizer (mRNA-BNT162b2) | 1 | 7 days | (1) Type 2 diabetes mellitus, osteoarthritis (2) Chest pain, shortness of breath | (3) D-dimer: 24 mg/L (▲), Platelet: Normal; (4) Doppler: acute DVT involving the common femoral, superficial femoral, popliteal, posterior tibial, anterior tibial, and deep calf veins of the left lower limb; CT: saddle thrombus in the bifurcation of the pulmonary trunk and 40 extensive bilateral main pulmonary arteries emboli extending to the lobar segmental and subsegmental branches. | In the absence of an obvious explanation for the extensive DVT and bilateral PEs, and the proximity of COVID-19 vaccination, the authors believe that the patient’s presentation is probably related to a rare ADR of BNT162b2 mRNA COVID-19 (Pfizer-BioNTech). |
| 4. Ishibashi Y. et al. [13]/Japan | 74/Female | Moderna (mRNA-1273) | 3 | 1 month | (1) Hypertension, dyslipidemia, and hypothyroidism (2) Pale, cold sweating, and hypoxic | (3) D-dimer 9.0 μg/mL (▲); Platelet: Normal (348 K/UL); (4) CT: thrombi in both pulmonary arteries. | This report is of a case of bilateral pulmonary embolism in a patient with no known risk factors for thrombotic events or previous episodes of APE, after the booster dose of the Moderna mRNA COVID-19 vaccine. |
| 5. Cheong K.I. et al. [14]/Taiwan | 70/Male | Moderna (mRNA-1273) | 1 | 5 weeks | (1) Hypertension and an old cerebrovascular accident (2) Shortness of breath | (3) D-dimer: 4895 ng/mL (▲), Platelet: Normal; (4) Doppler: Left popliteal vein thrombosis. | COVID-19 vaccine may be the trigger for thrombosis in a patient with protein S deficiency. |
| 6. Wiest N.E. et al. [15]/USA | 66/Male | Moderna (mRNA-1273) | 2 | 9 days | (1) Hypertension, hyper-lipidemia, and renal cell carcinoma (2) Right flank pain and right pleuritic chest pain | (3) D-dimer 3840 ng/mL (▲), Platelet: Normal (176 × 109 cells/L); (4) CT: extensive multifocal pulmonary emboli involving both right and left lower lobe pulmonary arteries with evidence of right ventricular strain. | Thrombosis can occur after messenger RNA vaccination. Though commonly used for thrombosis, heparin may be ineffective. Non-heparin anticoagulants should be considered. |
| 7. Ogunkoya J.O. et al. [16]/Africa | 59/Male | Moderna (mRNA-1273) | 3 | 1 month | (1) No medical history (2) Dyspnea, cough | (3) Platelet: Normal (175 × 109 cells/L); (4) CT: pulmonary embolism of the right and left pulmonary arteries with features of possible early pulmonary hypertension. | This report describes the case of a 66-year-old male with no prior thromboembolic or hypercoagulable history who developed acute, bilateral pulmonary emboli promptly following his second Moderna SARS-CoV-2 immunization. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-J.; Yoo, S.-J. Pulmonary Embolism after Vaccination with the COVID-19 Vaccine (Pfizer, BNT162b2): A Case Report. Vaccines 2023, 11, 1075. https://doi.org/10.3390/vaccines11061075
Kim E-J, Yoo S-J. Pulmonary Embolism after Vaccination with the COVID-19 Vaccine (Pfizer, BNT162b2): A Case Report. Vaccines. 2023; 11(6):1075. https://doi.org/10.3390/vaccines11061075
Chicago/Turabian StyleKim, Eun-Ju, and Seok-Ju Yoo. 2023. "Pulmonary Embolism after Vaccination with the COVID-19 Vaccine (Pfizer, BNT162b2): A Case Report" Vaccines 11, no. 6: 1075. https://doi.org/10.3390/vaccines11061075
APA StyleKim, E.-J., & Yoo, S.-J. (2023). Pulmonary Embolism after Vaccination with the COVID-19 Vaccine (Pfizer, BNT162b2): A Case Report. Vaccines, 11(6), 1075. https://doi.org/10.3390/vaccines11061075





