Predicted Public Health and Economic Impact of Respiratory Syncytial Virus Vaccination with Variable Duration of Protection for Adults ≥60 Years in Belgium
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeling Approach
2.2. Assumptions Related to Vaccination
2.3. Model Input Data
2.3.1. Epidemiological Parameters
2.3.2. Cost Parameters
2.4. Public Health and Economic Outcomes
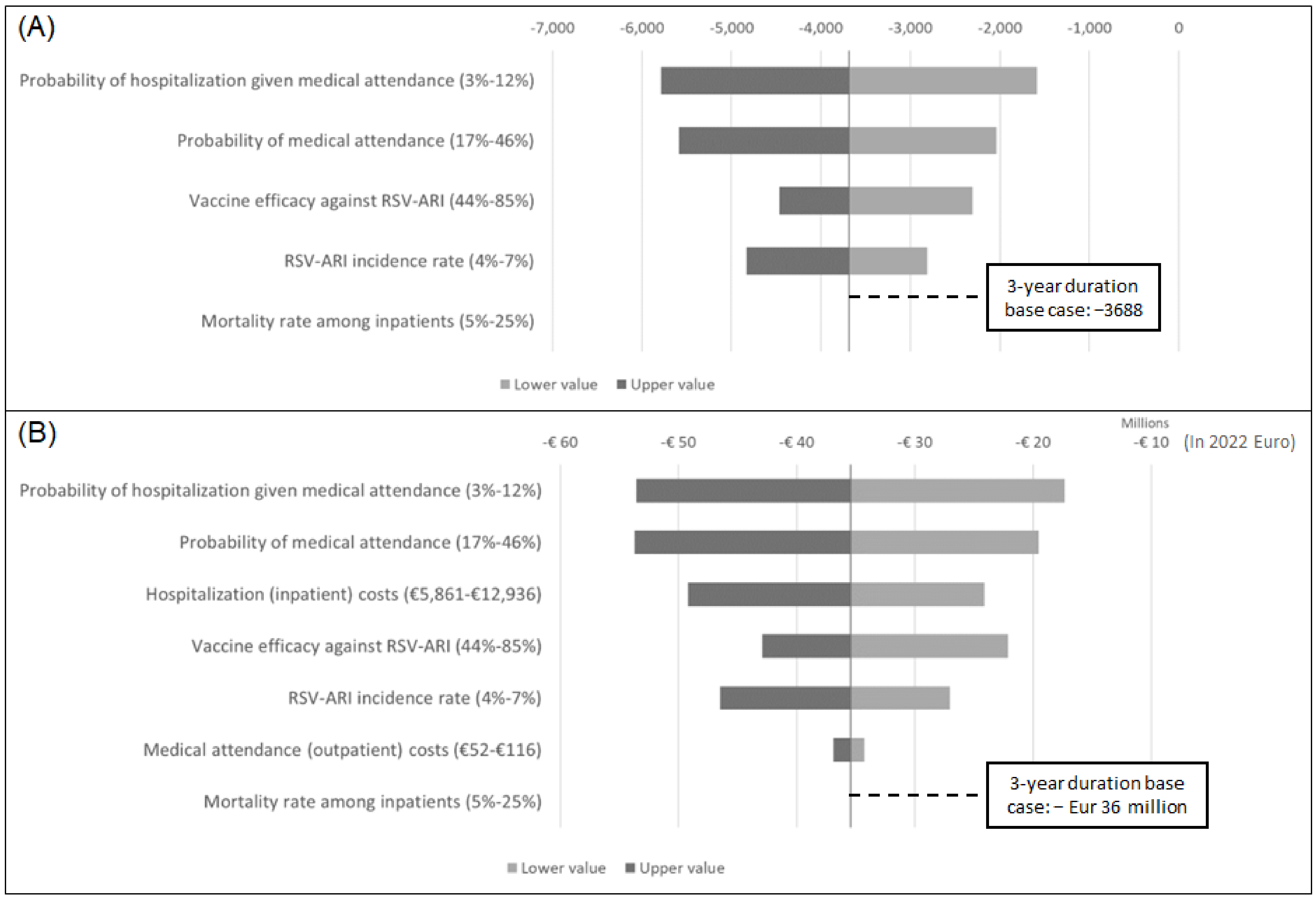
2.5. Sensitivity and Scenario Analyses
2.6. Model Verification and Validation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergeron, H.C.; Tripp, R.A. Immunopathology of RSV: An Updated Review. Viruses 2021, 13, 2478. [Google Scholar] [CrossRef]
- Belongia, E.A.; King, J.P.; Kieke, B.A.; Pluta, J.; Al-Hilli, A.; Meece, J.K.; Shinde, V. Clinical Features, Severity, and Incidence of RSV Illness During 12 Consecutive Seasons in a Community Cohort of Adults ≥60 Years Old. Open Forum Infect. Dis. 2018, 5, ofy316. [Google Scholar] [CrossRef]
- Prasad, N.; Walker, T.A.; Waite, B.; Wood, T.; Trenholme, A.A.; Baker, M.G.; McArthur, C.; Wong, C.A.; Grant, C.C.; Huang, Q.S.; et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Adults with Chronic Medical Conditions. Clin. Infect. Dis. 2021, 73, e158–e163. [Google Scholar] [CrossRef] [PubMed]
- Wyffels, V.; Kariburyo, F.; Gavart, S.; Fleischhackl, R.; Yuce, H. A Real-World Analysis of Patient Characteristics and Predictors of Hospitalization Among US Medicare Beneficiaries with Respiratory Syncytial Virus Infection. Adv. Ther. 2020, 37, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Widmer, K.; Griffin, M.R.; Zhu, Y.; Williams, J.V.; Talbot, H.K. Respiratory syncytial virus- and human metapneumovirus-associated emergency department and hospital burden in adults. Influenza Other Respir. Viruses 2014, 8, 347–352. [Google Scholar] [CrossRef]
- Ackerson, B.; An, J.; Sy, L.S.; Solano, Z.; Slezak, J.; Tseng, H.F. Cost of Hospitalization Associated With Respiratory Syncytial Virus Infection Versus Influenza Infection in Hospitalized Older Adults. J. Infect. Dis. 2020, 222, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Respiratory Syncytial Virus (RSV). Available online: https://epidemio.wiv-isp.be/ID/diseases/Pages/RSV.aspx (accessed on 10 May 2022).
- Korsten, K.; Adriaenssens, N.; Coenen, S.; Butler, C.; Ravanfar, B.; Rutter, H.; Allen, J.; Falsey, A.; Pircon, J.Y.; Gruselle, O.; et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): An international prospective cohort study. Eur. Respir. J. 2021, 57, 2002688. [Google Scholar] [CrossRef]
- Bruyndonckx, R.; Coenen, S.; Butler, C.; Verheij, T.; Little, P.; Hens, N.; Beutels, P.; Ieven, M.; Goossens, H.; the GRACE project Group. Respiratory syncytial virus and influenza virus infection in adult primary care patients: Association of age with prevalence, diagnostic features and illness course. Int. J. Infect. Dis. 2020, 95, 384–390. [Google Scholar] [CrossRef]
- DeMartino, J.; Mehta, N.; Foroughi, C.; Gaburo, K.; Radtke, T.; Kirson, N.; Krishnarajah, G. Annual economic burden of respiratory syncytial virus infections among the 60+ population in the United States. In Proceedings of the AMCP Nexus 2022, National Harbor, MD, USA, 11–14 November 2022; Academy of Managed Care Pharmacy: Alexandria, VI, USA, 2022; Volume 28, pp. S1–S137. [Google Scholar]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- A Study of an Adenovirus Serotype 26 Pre-Fusion Conformation-Stabilized F Protein (Ad26. RSV. preF) Based Respiratory Syncytial Virus (RSV) Vaccine in the Prevention of Lower Respiratory Tract Disease in Adults Aged 60 Years and Older (EVERGREEN). ClinicalTrial.gov ID: NCT04908683. Available online: https://clinicaltrials.gov/ct2/show/NCT04908683 (accessed on 16 August 2022).
- Efficacy Study of GSK’s Investigational Respiratory Syncytial Virus (RSV) Vaccine in Adults Aged 60 Years and above. Available online: https://clinicaltrials.gov/ct2/show/NCT04886596 (accessed on 16 August 2022).
- A Study to Evaluate the Safety and Immunogenicity of an Adjuvanted RSV Vaccine in Healthy Older Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT03572062 (accessed on 16 August 2022).
- A Study to Evaluate the Safety and Efficacy of mRNA-1345 Vaccine Targeting Respiratory Syncytial Virus (RSV) in Adults ≥60 Years of Age. Available online: https://clinicaltrials.gov/ct2/show/NCT05127434 (accessed on 16 August 2022).
- Herring, W.L.; Zhang, Y.; Shinde, V.; Stoddard, J.; Talbird, S.E.; Rosen, B. Clinical and economic outcomes associated with respiratory syncytial virus vaccination in older adults in the United States. Vaccine 2022, 40, 483–493. [Google Scholar] [CrossRef]
- Zeevat, F.; Luttjeboer, J.; Paulissen, J.H.J.; van der Schans, J.; Beutels, P.; Boersma, C.; Postma, M.J.; Investigators, R. Exploratory Analysis of the Economically Justifiable Price of a Hypothetical RSV Vaccine for Older Adults in the Netherlands and the United Kingdom. J. Infect. Dis. 2022, 226 (Suppl. 1), S102–S109. [Google Scholar] [CrossRef]
- Prem, K.; Zandvoort, K.V.; Klepac, P.; Eggo, R.M.; Davies, N.G.; Cook, A.R.; Jit, M.; Centre for the Mathematical Modelling of Infectious Diseases. Projecting contact matrices in 177 geographical regions: An update and comparison with empirical data for the COVID-19 era. PLoS Comput. Biol. 2021, 17, e1009098. [Google Scholar] [CrossRef]
- Drummond, M.F.S.M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Williams, K.; Gymnopoulou, E.; Bart, S.; Ervin, J.; Bastian, A.R.; Menten, J.; De Paepe, E.; Vandenberghe, S.; Chan, E.K.H.; et al. Efficacy and Safety of an Ad26.RSV.preF-RSV preF Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 609–620. [Google Scholar] [CrossRef]
- Safety and Efficacy of Bivalent RSV Prefusion F Vaccine in Adults ≥60 Years of Age. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/03-RSV-Adults-Gurtma-508.pdf (accessed on 20 March 2023).
- OECD. Influenza Vaccination Rates. 2018. Available online: https://www.oecd-ilibrary.org/content/data/e452582e-en (accessed on 16 August 2022).
- Cleemput, I.; Neyt, M.; Van De Sande, S.; Thiry, N. Belgian Guidelines for Economic Evaluations and Budget Impact Analyses. In KCE Reports, 2nd ed.; Belgian Health Care Knowledge Centre (KCE): Brussels, Belgium, 2012. [Google Scholar]
- Life Expectancy and Life Tables. Available online: https://statbel.fgov.be/en/themes/population/mortality-life-expectancy-and-causes-death/life-expectancy-and-life-tables#documents (accessed on 16 August 2022).
- Consumer Price Index and Health Index. Available online: https://statbel.fgov.be/en/open-data/consumer-price-index-and-health-index (accessed on 16 August 2022).
- Jansen, A.G.; Sanders, E.A.; Hoes, A.W.; van Loon, A.M.; Hak, E. Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur. Respir. J. 2007, 30, 1158–1166. [Google Scholar] [CrossRef]
- Subissi, L.; Bossuyt, N.; Reynders, M.; Gerard, M.; Dauby, N.; Bourgeois, M.; Delaere, B.; Quoilin, S.; Van Gucht, S.; Thomas, I.; et al. Capturing respiratory syncytial virus season in Belgium using the influenza severe acute respiratory infection surveillance network, season 2018/19. Euro Surveill. 2020, 25, 1900627. [Google Scholar] [CrossRef] [PubMed]
- TCT. Gegevens uit de Databank Medische Diagnose/Zorg & Kost, de dato 01 02 2022; Technische Cel Voor de Verwerking van de Gegevens met Betrekking tot Ziekenhuizen, RIZIV/INAMI, België. Available online: https://tct.fgov.be/webetct/etct-web/html/nl/index.jsp (accessed on 16 August 2022).
- Mao, Z.; Li, X.; Korsten, K.; Bont, L.; Butler, C.; Wildenbeest, J.; Coenen, S.; Hens, N.; Bilcke, J.; Beutels, P.; et al. Economic Burden and Health-Related Quality of Life of Respiratory Syncytial Virus and Influenza Infection in European Community-Dwelling Older Adults. J. Infect. Dis. 2022, 226 (Suppl. 1), S87–S94. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Ercius, A.K.; Smith, K.J. A predictive model of the economic effects of an influenza vaccine adjuvant for the older adult (age 65 and over) population. Vaccine 2009, 27, 2251–2257. [Google Scholar] [CrossRef]
- StatBel. Average Gross Monthly Wages. Available online: https://data.gov.be/fr/dataset/5632a04bda7d61aa3d52d666fce8d0a963f28130 (accessed on 16 August 2022).
- OECD. Labour Market Statistics: Labour Force Statistics by Sex and Age: Indicators, 2021st ed.; OECD: Paris, France, 2022. [Google Scholar]
- Bilcke, J.; Coenen, S.; Beutels, P. Influenza-like-illness and clinically diagnosed flu: Disease burden, costs and quality of life for patients seeking ambulatory care or no professional care at all. PLoS ONE 2014, 9, e102634. [Google Scholar] [CrossRef]
- Ieven, M.; Coenen, S.; Loens, K.; Lammens, C.; Coenjaerts, F.; Vanderstraeten, A.; Henriques-Normark, B.; Crook, D.; Huygen, K.; Butler, C.C.; et al. Aetiology of lower respiratory tract infection in adults in primary care: A prospective study in 11 European countries. Clin. Microbiol. Infect. 2018, 24, 1158–1163. [Google Scholar] [CrossRef]
- Tseng, H.F.; Sy, L.S.; Ackerson, B.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.A.; Shinde, V. Severe Morbidity and Short- and Mid- to Long-term Mortality in Older Adults Hospitalized with Respiratory Syncytial Virus Infection. J. Infect. Dis. 2020, 222, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Lui, G.C.; Wong, K.T.; Li, T.C.; Tse, E.C.; Chan, J.Y.; Yu, J.; Wong, S.S.; Choi, K.W.; Wong, R.Y.; et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin. Infect. Dis. 2013, 57, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Volling, C.; Hassan, K.; Mazzulli, T.; Green, K.; Al-Den, A.; Hunter, P.; Mangat, R.; Ng, J.; McGeer, A. Respiratory syncytial virus infection-associated hospitalization in adults: A retrospective cohort study. BMC Infect. Dis. 2014, 14, 665. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Stoecker, C.; Kim, L.; Gierke, R.; Pilishvili, T. Incremental Cost-Effectiveness of 13-valent Pneumococcal Conjugate Vaccine for Adults Age 50 Years and Older in the United States. J. Gen. Intern. Med. 2016, 31, 901–908. [Google Scholar] [CrossRef]
- De Burghgraeve, T.; Henrard, S.; Verboven, B.; Van Pottelbergh, G.; Vaes, B.; Mathei, C. The incidence of lower respiratory tract infections and pneumococcal vaccination status in adults in flemish primary care. Acta Clin. Belg. 2021, 76, 335–345. [Google Scholar] [CrossRef]
- Buyukkaramikli, N.C.; Rutten-van Molken, M.; Severens, J.L.; Al, M. TECH-VER: A Verification Checklist to Reduce Errors in Models and Improve Their Credibility. Pharmacoeconomics 2019, 37, 1391–1408. [Google Scholar] [CrossRef]
- Beck, E.; Biundo, E.; Devlin, N.; Doherty, T.M.; Garcia-Ruiz, A.J.; Postma, M.; Sheikh, S.; Smela, B.; Toumi, M.; Wasem, J.; et al. Capturing the value of vaccination within health technology assessment and health economics: Literature review and novel conceptual framework. Vaccine 2022, 40, 4008–4016. [Google Scholar] [CrossRef]
- Ackerson, B.; Tseng, H.F.; Sy, L.S.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.A.; Shinde, V. Severe Morbidity and Mortality Associated With Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin. Infect. Dis. 2019, 69, 197–203. [Google Scholar] [CrossRef]
- Care for the Elderly. Available online: https://www.healthybelgium.be/en/health-system-performance-assessment/specific-domains/care-for-the-elderly (accessed on 16 August 2022).
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir. Rev. 2021, 30, 200258. [Google Scholar] [CrossRef]
- Hodgson, D.; Pebody, R.; Panovska-Griffiths, J.; Baguelin, M.; Atkins, K.E. Evaluating the next generation of RSV intervention strategies: A mathematical modelling study and cost-effectiveness analysis. BMC Med. 2020, 18, 348. [Google Scholar] [CrossRef] [PubMed]
- Van Effelterre, T.; Hens, N.; White, L.J.; Gravenstein, S.; Bastian, A.R.; Buyukkaramikli, N.; Cheng, C.-Y.; Hartnett, J.; Krishnarajah, G.; Weber, K.; et al. Modeling Respiratory Syncytial Virus Adult Vaccination in the United States with a Dynamic Transmission Model. Clin. Infect. Dis. 2023, ciad161. [Google Scholar] [CrossRef] [PubMed]
- Devos, C.C.A.; Lefèvre, M.; Obyn, C.; Renard, F.; Bouckaert, N.; Gerkens, S.; de Noordhout, C.M.; Devleesschauwer, B.; Haelterman, M.; Léonard, C.; et al. Performance of the Belgian Health System—Report 2019; Health Services Research (HSR): Brussels, Belgium; Belgian Health Care Knowledge Centre (KCE): Brussels, Belgium, 2019. [Google Scholar]
- Structure of the Population. Available online: https://statbel.fgov.be/en/themes/population/structure-population (accessed on 16 August 2022).
- Bart, S.; Williams, K.; Gymnopoulou, E.; Falsey, A.R.; Ervin, J.; Bastian, A.R.; Menten, J.; De Paepe, E.; de Boer, H.; Vandenberghe, S.; et al. Safety and Tolerability of an Ad26.RSV.preF-based Vaccine in a Randomized, Double-blind, Placebo-controlled, Phase 2b Study in Adults Aged ≥65 Years. In Proceedings of the 8th ESWI Influenza Conference, Salzburg, Austria, 4–7 December 2021. [Google Scholar]
- Consumer Price Index for All Urban Consumers [CPI-U] Medical Care. Available online: https://www.bls.gov/cpi/data.htm (accessed on 16 August 2022).
- US Dollar (USD). Available online: https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/eurofxref-graph-usd.en.html (accessed on 24 May 2022).

| Vaccine Profile | Vaccine Efficacy against Any Symptomatic ARI (%) | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| One-year duration | 70 | - | - | - | - |
| Three-year duration | 70 | 57 | 57 | - | - |
| Five-year duration | 70 | 57 | 57 | 46 | 46 |
| Description | Value | 95% CI | Distribution | References |
|---|---|---|---|---|
| Symptomatic RSV-ARI incidence rate (≥60 years) | 5% | 4–7% | Beta | [8] |
| Probability of medical attendance among symptomatic RSV-ARI cases | 31% | 17–46% | Beta | [8] |
| Probability of hospitalization among medically attended cases | 8% | 3–12% | Beta | [8,27] |
| Inpatient mortality rate | 14% | 5–25% | Beta | [28] |
| Cost per hospitalization | EUR 9057 | EUR 5861–12,936 | Gamma | [29] |
| Cost per outpatient treatment | EUR 81 | EUR 52–116 | Gamma | [30] |
| Cost associated with non-medically attended symptomatic ARI episode (medication) | EUR 5 | EUR 3–7 | Gamma | [30] |
| Cost per vaccination-related local adverse events | EUR 0.02 | EUR 0.011–0.024 | Gamma | [31] |
| Cost per vaccination-related systemic adverse events | EUR 0.17 | EUR 0.11–0.24 | Gamma | [31] |
| Cost associated with productivity loss for a hospitalized patient | EUR 41 | EUR 27–59 | Gamma | [29,32,33] |
| Cost associated with productivity loss for a medically attended, non-hospitalized patient | EUR 21 | EUR 13–30 | Gamma | [32,33,34] |
| Caregiver productivity loss associated with a hospitalized patient | EUR 447 | EUR 289–693 | Gamma | [32,33,34] |
| Caregiver productivity loss for a medically attended, non-hospitalized patient | EUR 224 | EUR 145–319 | Gamma | [32,33,34] |
| One-Year b | Three-Year c | Five-Year d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Vaccination | Vaccination | Incremental | No Vaccination | Vaccination | Incremental | No Vaccination | Vaccination | Incremental | |
| Number vaccinated a | - | 1,729,516 | 1,729,516 | - | 1,729,516 | 1,729,516 | - | 1,729,516 | 1,729,516 |
| Symptomatic RSV-ARI cases | 147,232 | 86,322 | −60,910 | 425,786 | 271,058 | −154,728 | 682,947 | 458,345 | −224,601 |
| Medically attended RSV cases | 44,988 | 26,376 | −18,611 | 130,101 | 82,823 | −42,278 | 208,678 | 140,050 | −68,628 |
| RSV hospitalizations | 3509 | 2057 | −1452 | 10,149 | 6461 | −3688 | 16,278 | 10,925 | −5353 |
| RSV-attributable deaths | 477 | 280 | −197 | 1380 | 879 | −502 | 2214 | 1486 | −728 |
| RSV-related treatment costs | EUR 35,163,993 | EUR 20,616,649 | EUR −14,547,344 | EUR 98,809,396 | EUR 62,826,540 | EUR −35,982,857 | EUR 154,202,095 | EUR 103,168,482 | EUR −51,033,613 |
| Inpatient | EUR 31,315,416 | EUR 18,360,228 | EUR −12,995,188 | EUR 88,014,750 | EUR 55,962,919 | EUR −32,051,831 | EUR 137,355,954 | EUR 91,897,618 | EUR −45,458,336 |
| Outpatient | EUR 3,312,116 | EUR 1,941,893 | EUR −1,370,222 | EUR 9,308,994 | EUR 5,918,991 | EUR −3,390,004 | EUR 14,527,631 | EUR 9,719,671 | EUR −4,807,960 |
| Non-medically attended | EUR 536,461 | EUR 314,527 | EUR −221,934 | EUR 1,485,652 | EUR 944,631 | EUR −541,021 | EUR 2,318,511 | EUR 1,551,193 | EUR −767,318 |
| Vaccination-associated adverse event costs | EUR 0 | EUR 320,980 | EUR 320,980 | EUR 0 | EUR 320,980 | EUR 320,980 | EUR 0 | EUR 320,980 | EUR 320,980 |
| Number needed to vaccinate to prevent one | |||||||||
| Symptomatic RSV-ARI case | 28 | 11 | 8 | ||||||
| Medically attended RSV case | 93 | 37 | 25 | ||||||
| RSV hospitalization | 1191 | 469 | 323 | ||||||
| RSV-attributable death | 8760 | 3448 | 2376 | ||||||
| Results | ||||
|---|---|---|---|---|
| Mean | 25th Percentile a | 50th Percentile (Median) b | 75th Percentile c | |
| Symptomatic RSV-ARI averted | 153,704 | 138,871 | 153,221 | 167,639 |
| Medically attended RSV-ARI averted | 46,721 | 36,761 | 45,390 | 55,561 |
| RSV hospitalizations averted | 3609 | 2475 | 3409 | 4434 |
| RSV-attributable deaths averted | 495 | 290 | 446 | 634 |
| RSV-related treatment costs averted | EUR 35,091,821 | EUR 23,290,543 | EUR 32,369,949 | EUR 43,781,160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postma, M.J.; Cheng, C.-Y.; Buyukkaramikli, N.C.; Hernandez Pastor, L.; Vandersmissen, I.; Van Effelterre, T.; Openshaw, P.; Simoens, S. Predicted Public Health and Economic Impact of Respiratory Syncytial Virus Vaccination with Variable Duration of Protection for Adults ≥60 Years in Belgium. Vaccines 2023, 11, 990. https://doi.org/10.3390/vaccines11050990
Postma MJ, Cheng C-Y, Buyukkaramikli NC, Hernandez Pastor L, Vandersmissen I, Van Effelterre T, Openshaw P, Simoens S. Predicted Public Health and Economic Impact of Respiratory Syncytial Virus Vaccination with Variable Duration of Protection for Adults ≥60 Years in Belgium. Vaccines. 2023; 11(5):990. https://doi.org/10.3390/vaccines11050990
Chicago/Turabian StylePostma, Maarten J., Chih-Yuan Cheng, Nasuh C. Buyukkaramikli, Luis Hernandez Pastor, Ine Vandersmissen, Thierry Van Effelterre, Peter Openshaw, and Steven Simoens. 2023. "Predicted Public Health and Economic Impact of Respiratory Syncytial Virus Vaccination with Variable Duration of Protection for Adults ≥60 Years in Belgium" Vaccines 11, no. 5: 990. https://doi.org/10.3390/vaccines11050990
APA StylePostma, M. J., Cheng, C.-Y., Buyukkaramikli, N. C., Hernandez Pastor, L., Vandersmissen, I., Van Effelterre, T., Openshaw, P., & Simoens, S. (2023). Predicted Public Health and Economic Impact of Respiratory Syncytial Virus Vaccination with Variable Duration of Protection for Adults ≥60 Years in Belgium. Vaccines, 11(5), 990. https://doi.org/10.3390/vaccines11050990






