SARS-CoV-2 Vaccine Breakthrough Infections of Omicron and Delta Variants in Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Recruitment
2.2. Data Collection
2.3. Laboratory Methods
2.3.1. SARS-CoV-2 RNA Detection
2.3.2. SARS-CoV-2 Lineage Determination
2.3.3. Anti-SARS-CoV-2 Antibody Detection
2.3.4. SARS-CoV-2 Neutralization Assay
2.4. Statistical Analysis
3. Results
3.1. Study Population
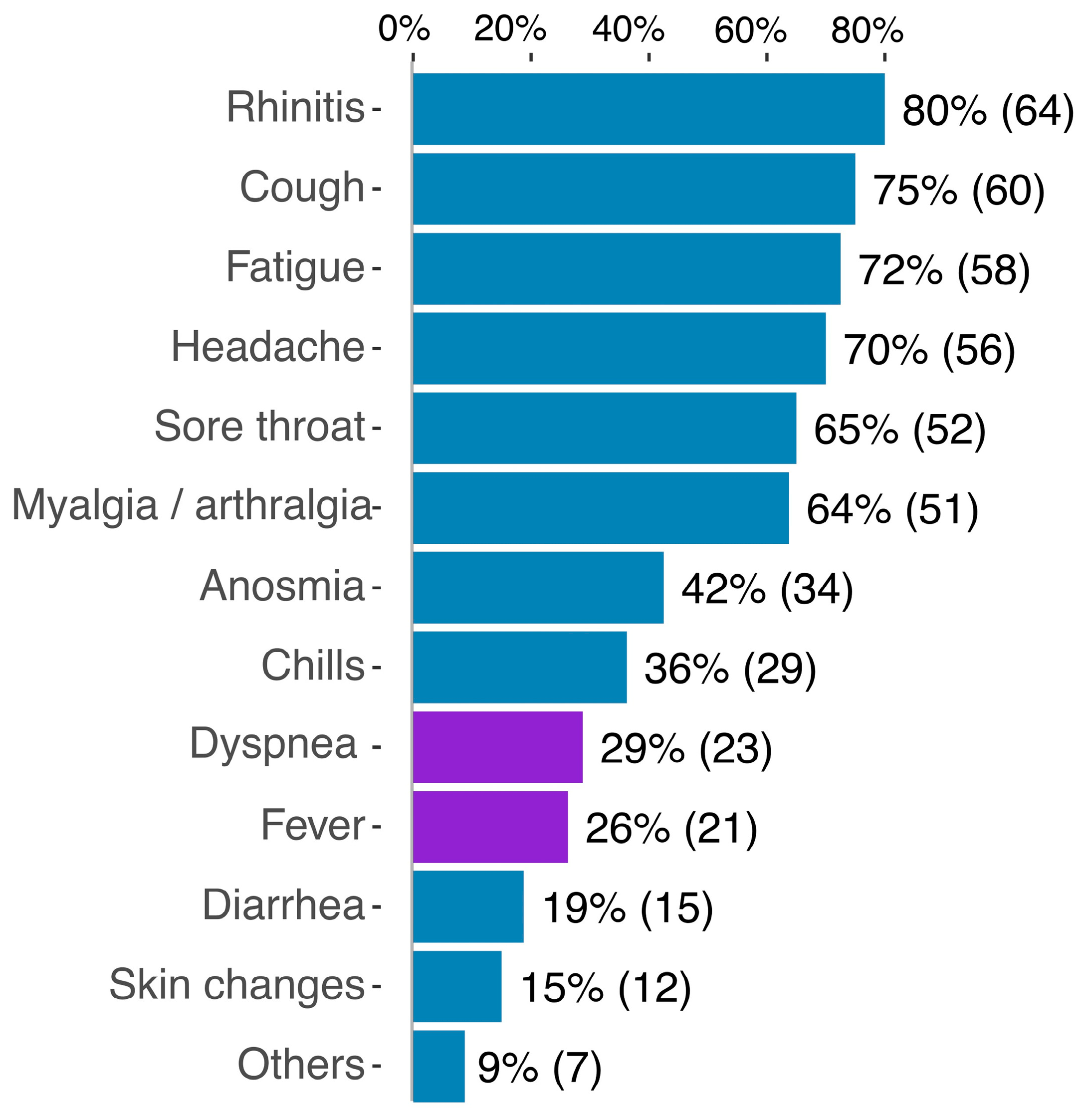
3.2. Clinical Results
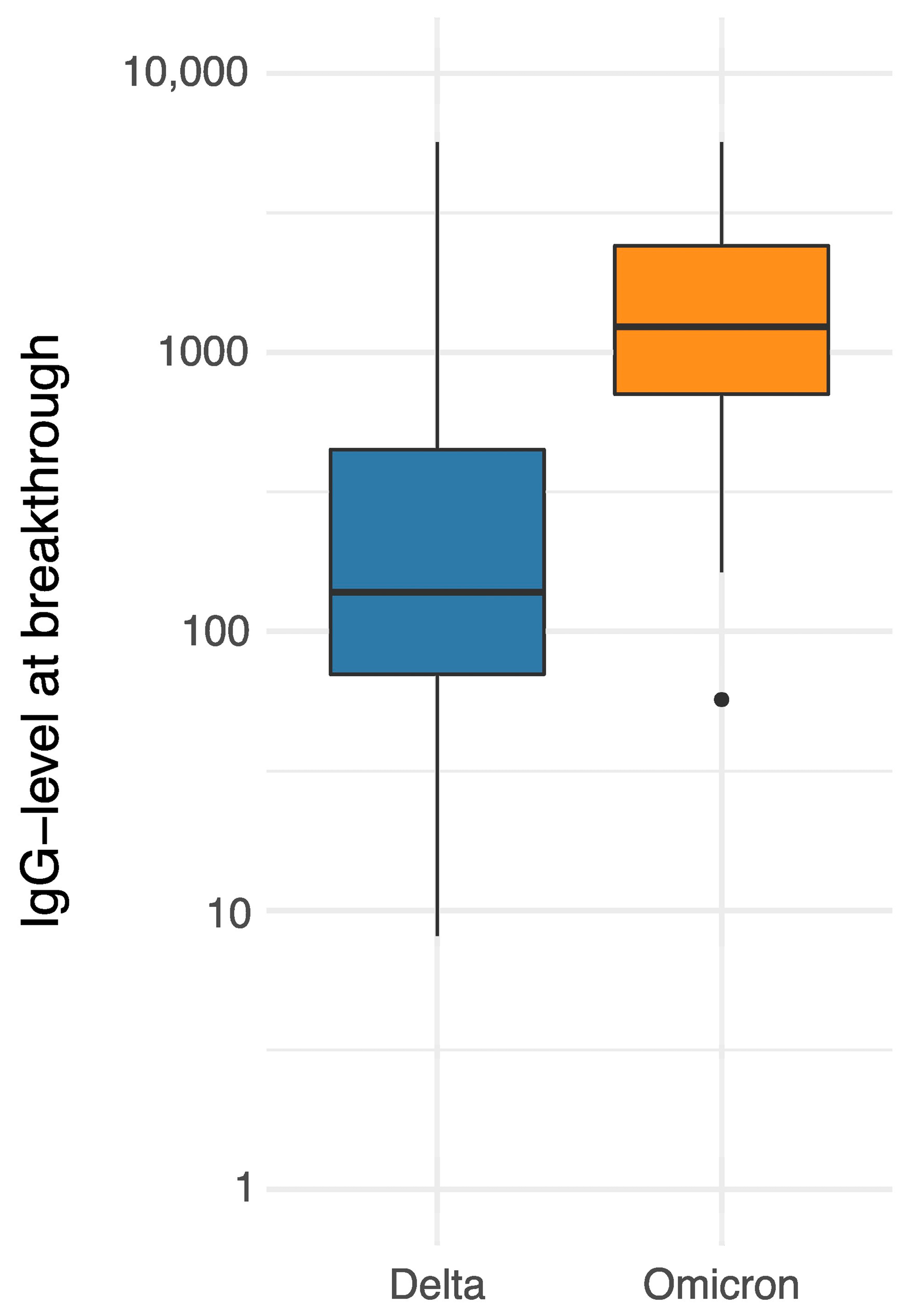
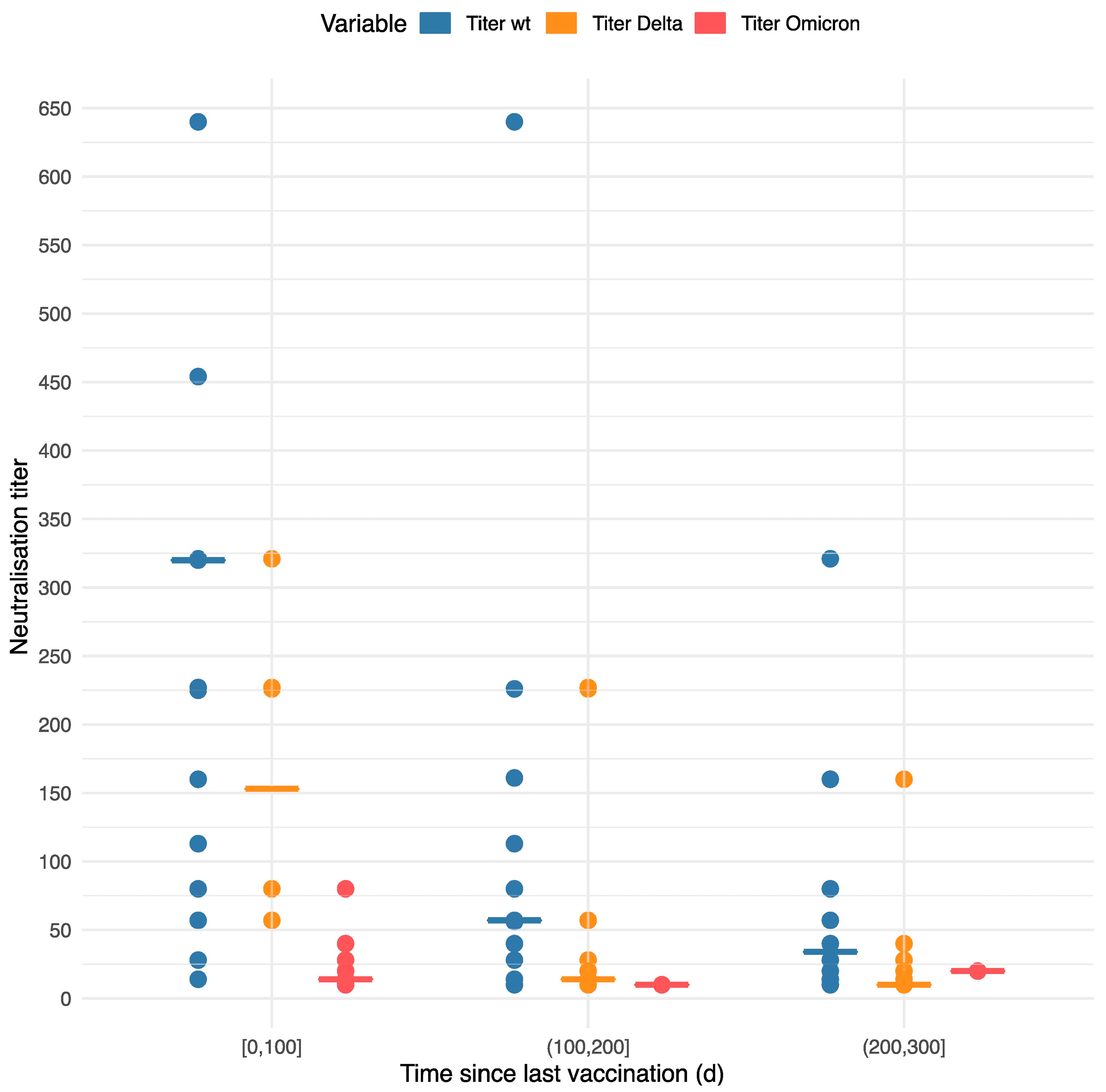
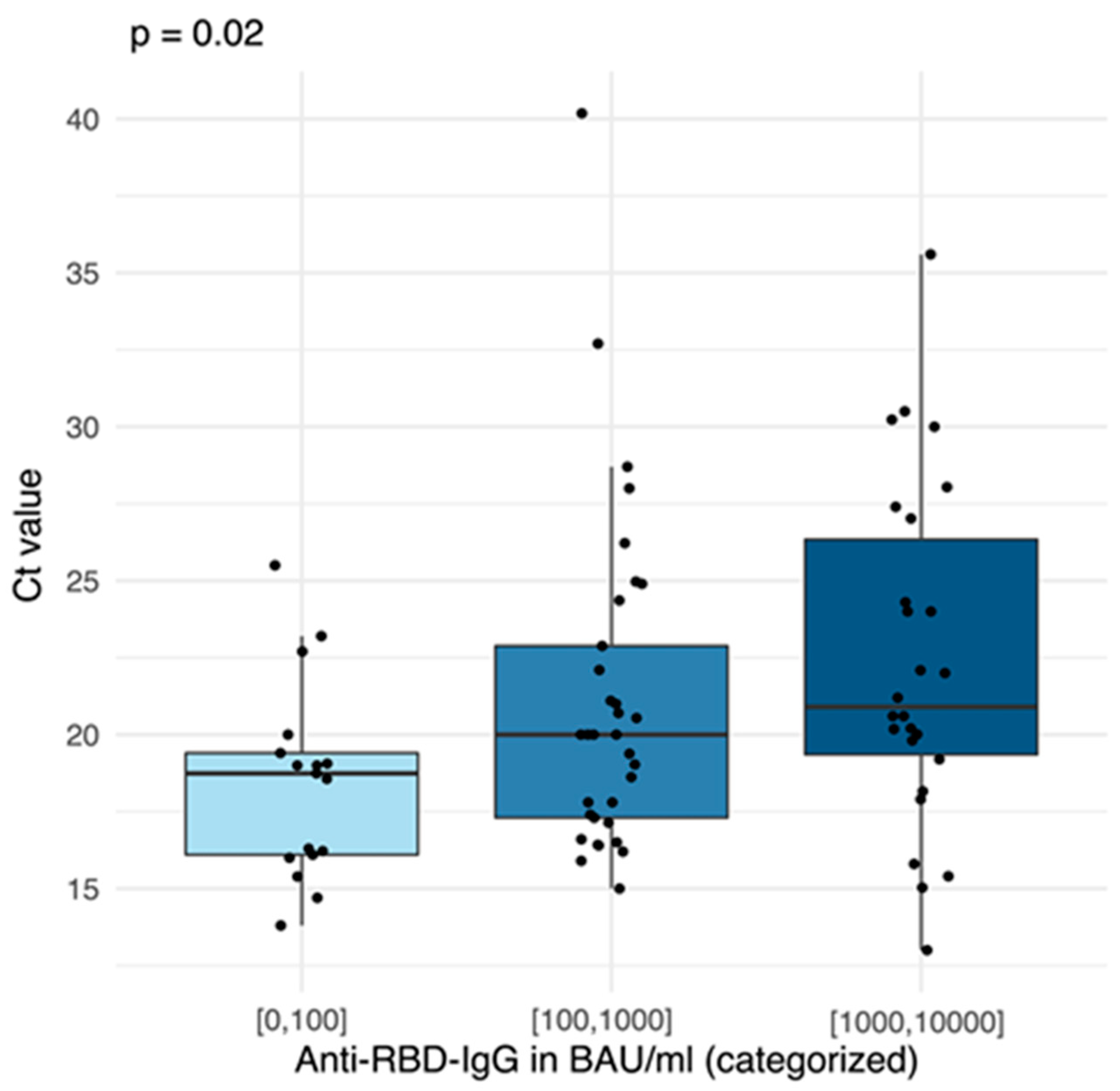
3.3. Laboratory Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 2 April 2022).
- Öffentlichkeitsarbeit, Staatsministerium des Innern-Referat Kommunikation und. Infektionsfälle in Sachsen—Coronavirus in Sachsen—Sachsen.de. Available online: https://www.coronavirus.sachsen.de/infektionsfaelle-in-sachsen-4151.html (accessed on 30 March 2022).
- RKI–Coronavirus SARS-CoV-2—7-Tage-Inzidenz der COVID-19-Fälle nach Kreisen sowie der Hospitalisierten COVID-19-Fälle nach Bundesländern. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Inzidenz-Tabellen.html (accessed on 2 April 2022).
- EMA. COVID-19 Vaccines: Authorised, 2021. European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised (accessed on 19 March 2022).
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Tracker European Centre for Disease Prevention and Control. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#summary-tab (accessed on 19 March 2022).
- RKI–Coronavirus SARS-CoV-2—Digitales Impfquotenmonitoring zur COVID-19-Impfung. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Impfquoten-Tab.html (accessed on 2 April 2022).
- Deutscher Bundestag—Impfpflicht für Gesund Heits- und Pflegepersonal ab 15. März Beschlossen. Available online: https://www.bundestag.de/dokumente/textarchiv/2021/kw49-de-infektionsschutzgesetz-impfpraevention-870424 (accessed on 2 April 2022).
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Ledda, C.; Costantino, C.; Motta, G.; Cunsolo, R.; Stracquadanio, P.; Liberti, G.; Maltezou, H.C.; Rapisarda, V. SARS-CoV-2 mRNA Vaccine Breakthrough Infections in Fully Vaccinated Healthcare Personnel: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R.; et al. Quantitative Detection and Viral Load Analysis of SARS-CoV-2 in Infected Patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Virologische Arbeitsmethoden Band II; VEB Gustav Fischer Verlag: Stuttgart, Germany, 1977.
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 11 October 2022).
- Ferastraoaru, D.; Hudes, G.; Jerschow, E.; Jariwala, S.; Karagic, M.; de Vos, G.; Rosenstreich, D.; Ramesh, M. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. J. Allergy Clin. Immunol. Pract. 2021, 9, 1152–1162.e3. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef] [PubMed]
- Möhlendick, B.; Čiučiulkaitė, I.; Elsner, C.; Anastasiou, O.E.; Trilling, M.; Wagner, B.; Zwanziger, D.; Jöckel, K.-H.; Dittmer, U.; Siffert, W. Individuals With Weaker Antibody Responses After Booster Immunization Are Prone to Omicron Breakthrough Infections. Front. Immunol. 2022, 13, 3165. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarini, N.; Sawyer, C.; Williams, C.; Sutton, D.; Roberts, C.; Simkin, F.; King, G.; McClure, V.; Cottrell, S.; Clayton, H.; et al. Epidemiological analysis of the first 1000 cases of SARS-CoV-2 lineage BA.1 (B.1.1.529, Omicron) compared with co-circulating Delta in Wales, UK. Influenza Other Respir. Viruses 2022, 16, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.S.; Peto, T.E.A. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N. Engl. J. Med. 2022, 8, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Avanzato, V.A.; Matson, M.J.; Seifert, S.N.; Pryce, R.; Williamson, B.N.; Anzick, S.L.; Barbian, K.; Judson, S.D.; Fischer, E.R.; Martens, C.; et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell 2020, 183, 1901–1912.e9. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, P.; Wang, N.; Wang, L.; Fan, K.; Zhu, Q.; Wang, K.; Chen, R.; Feng, R.; Jia, Z.; et al. Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron. Cell 2022, 185, 860–871.e13. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, M.; Verga, F.; Ghisetti, V.; Burdino, E.; Emanuele, T.; Bonora, S.; Di Perri, G.; Calcagno, A. Clinical Phenotype and Contagiousness of Early Breakthrough SARS-CoV-2 Infections after BNT162b2 COVID-19 mRNA Vaccine: A Parallel Cohort Study in Healthcare Workers. Vaccines 2021, 9, 1377. [Google Scholar] [CrossRef] [PubMed]
- Fröberg, J.; Gillard, J.; Philipsen, R.; Lanke, K.; Rust, J.; van Tuijl, D.; Teelen, K.; Bousema, T.; Simonetti, E.; van der Gaast-de Jongh, C.E.; et al. SARS-CoV-2 mucosal antibody development and persistence and their relation to viral load and COVID-19 symptoms. Nat. Commun. 2021, 12, 5621. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Klein, J.; Sundaram, M.E.; Liu, F.; Wong, P.; Silva, J.; Mao, T.; Oh, J.E.; Mohanty, S.; Huang, J.; et al. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat. Med. 2021, 27, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef] [PubMed]

| Demographics | n (%) |
|---|---|
| Sex female/male | 56 (69.1)/25 (30.9) |
| Mean age in years (min, max) 1 | 34.9 (18, 62) |
| Comorbidities | 47 (58.0) |
| Regular medication | 32 (39.5) |
| Occupation and workplace | |
| Doctor/nurse/other | 17 (21.0)/27 (33.3)/37 (45.7) |
| ICU/regular ward/other | 7 (8.6)/21 (25.9)/53 (65.4) |
| Patient-facing/non-patient-facing | 69 (85.2)/12 (14.8) |
| Chain of SARS-CoV-2 infection | |
| Known | 37 (45.7) |
| At workplace/outside of work | 5 (6.2)/32 (39.5) |
| Number and type of vaccinations | |
| Two/three vaccines | 81 (100.0)/33 (40.7) |
| First vaccine 1/2/3/4 2 | 62 (76.5)/17 (21.0)/1 (1.2)/1 (1.2) |
| Second vaccine 1/2/3/4 2 | 77 (95.1)/3 (3.7)/1 (1.2)/0 (0) |
| Third vaccine 1/2/3/4 2 | 32 (39.5)/0 (0)/1 (1.2)/0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regenhardt, E.; Kirsten, H.; Weiss, M.; Lübbert, C.; Stehr, S.N.; Remane, Y.; Pietsch, C.; Hönemann, M.; von Braun, A. SARS-CoV-2 Vaccine Breakthrough Infections of Omicron and Delta Variants in Healthcare Workers. Vaccines 2023, 11, 958. https://doi.org/10.3390/vaccines11050958
Regenhardt E, Kirsten H, Weiss M, Lübbert C, Stehr SN, Remane Y, Pietsch C, Hönemann M, von Braun A. SARS-CoV-2 Vaccine Breakthrough Infections of Omicron and Delta Variants in Healthcare Workers. Vaccines. 2023; 11(5):958. https://doi.org/10.3390/vaccines11050958
Chicago/Turabian StyleRegenhardt, Elisa, Holger Kirsten, Melanie Weiss, Christoph Lübbert, Sebastian N. Stehr, Yvonne Remane, Corinna Pietsch, Mario Hönemann, and Amrei von Braun. 2023. "SARS-CoV-2 Vaccine Breakthrough Infections of Omicron and Delta Variants in Healthcare Workers" Vaccines 11, no. 5: 958. https://doi.org/10.3390/vaccines11050958
APA StyleRegenhardt, E., Kirsten, H., Weiss, M., Lübbert, C., Stehr, S. N., Remane, Y., Pietsch, C., Hönemann, M., & von Braun, A. (2023). SARS-CoV-2 Vaccine Breakthrough Infections of Omicron and Delta Variants in Healthcare Workers. Vaccines, 11(5), 958. https://doi.org/10.3390/vaccines11050958






