Cost-Effectiveness of the Use of Adjuvanted Quadrivalent Seasonal Influenza Vaccine in Older Adults in Ireland
Abstract
1. Introduction
2. Methods
2.1. Model Design and Parameters
2.2. Scenarios
2.3. Economic Evaluations
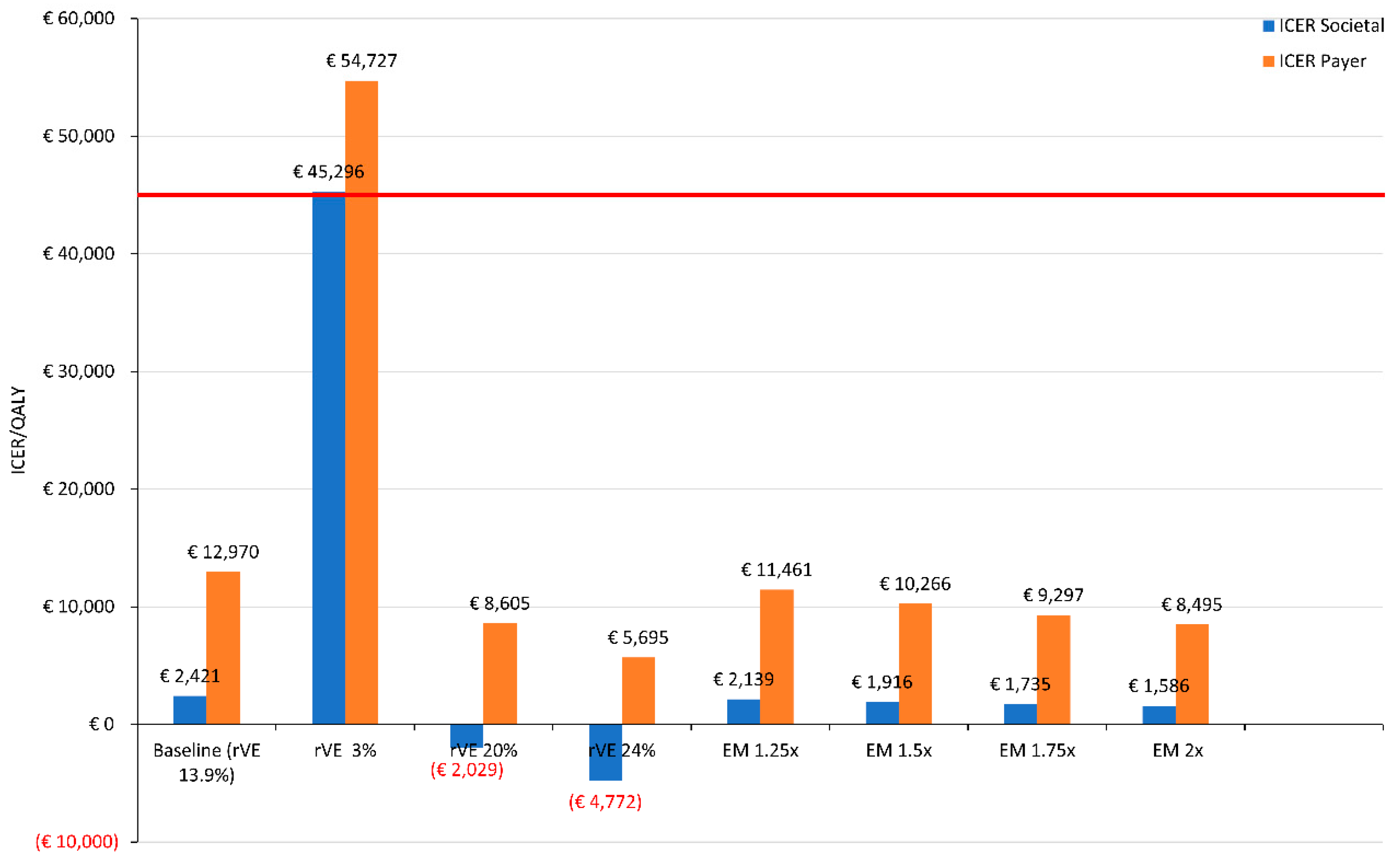
2.4. Sensitivity Analysis
2.5. Software
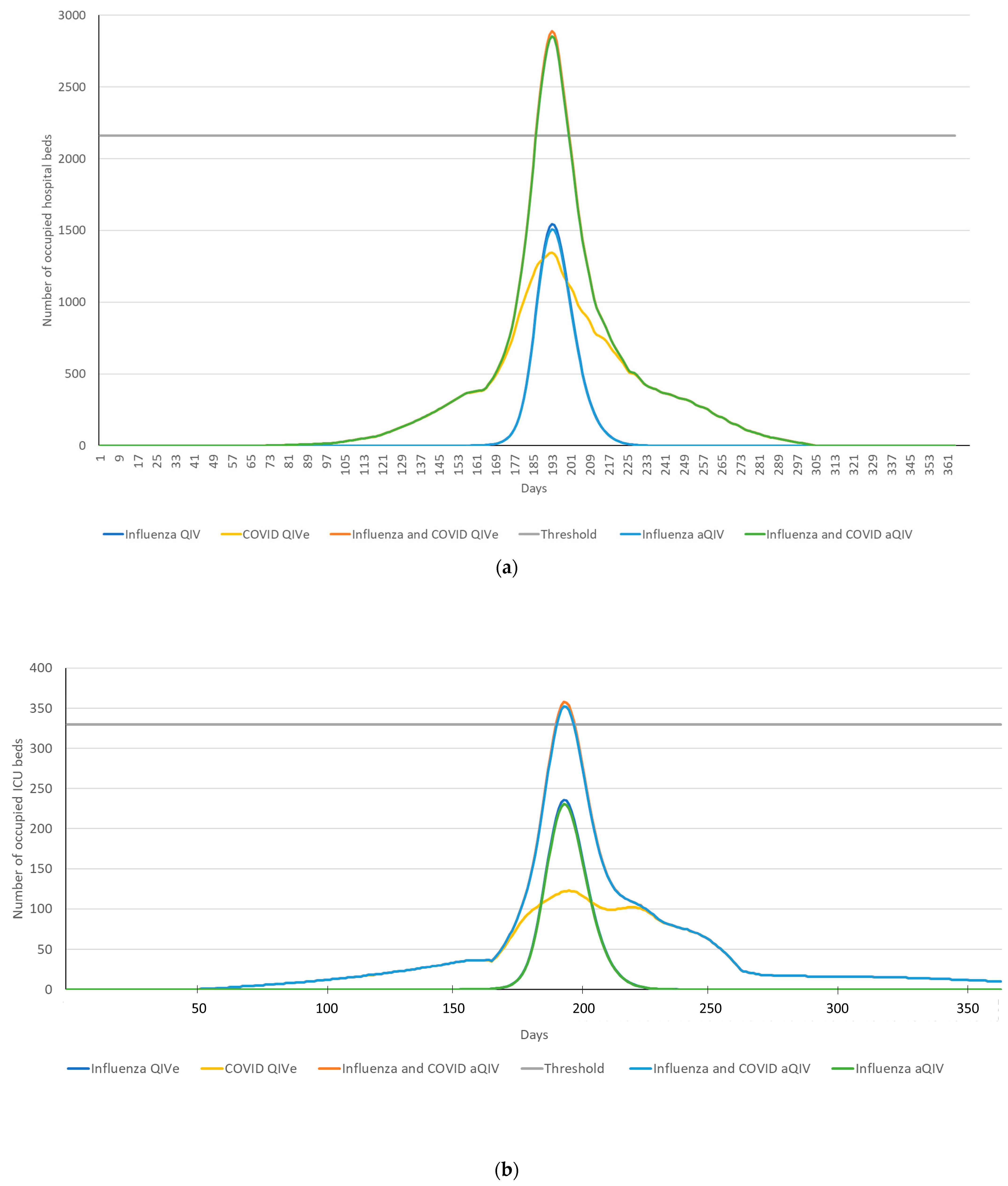
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HSE Immunisation Guidelines: Influenza. Available online: https://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/ (accessed on 15 December 2022).
- HSE Seasonal Influenza Vaccine Uptake in those Attending GP Clinics and Pharmacies for Vaccination, Ireland 1 September 2021 to 17 July 2022. Available online: https://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/surveillance/influenzaandadults65yearsandolder/Seasonal%20Flu%20Vacc%20Uptake_report_01%2009%202021%20-%20%2028%2007%202029_v1.0-%20final.pdf (accessed on 15 December 2022).
- HSE Seasonal Influenza Vaccine Uptake in Ireland in Persons Aged 65 Years and Older Attending GP Clinics and Pharmacies for Vaccination. Available online: https://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/surveillance/influenzaandadults65yearsandolder/Seasonal%20Flu%20Vaccination%20Uptake_65%20report_Sep18-Aug%2019.docx.pdf (accessed on 15 December 2022).
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Okoli, G.N.; Racovitan, F.; Abdulwahid, T.; Righolt, C.H.; Mahmud, S.M. Variable seasonal influenza vaccine effectiveness across geographical regions, age groups and levels of vaccine antigenic similarity with circulating virus strains: A systematic review and meta-analysis of the evidence from test-negative design studies after the 2009/10 influenza pandemic. Vaccine 2021, 39, 1225–1240. [Google Scholar] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun Ageing 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C.; Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef]
- Nichol, K.L. Influenza vaccination in the elderly: Impact on hospitalisation and mortality. Drugs Aging 2005, 22, 495–515. [Google Scholar] [CrossRef]
- Jester, B.J.; Uyeki, T.M.; Jernigan, D.B. Fifty Years of Influenza A(H3N2) Following the Pandemic of 1968. Am. J. Public Health 2020, 110, 669–676. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Wernecke, M.; MaCurdy, T.; et al. Relative Effectiveness of Influenza Vaccines Among the United States Elderly, 2018–2019. J. Infect Dis. 2020, 222, 278–287. [Google Scholar] [CrossRef]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of Adjuvanted Trivalent Inactivated Influenza Vaccine Versus Egg-derived Quadrivalent Inactivated Influenza Vaccines and High-dose Trivalent Influenza Vaccine in Preventing Influenza-related Medical Encounters in US Adults ≥ 65 Years During the 2017–2018 and 2018–2019 Influenza Seasons. Clin. Infect. Dis. 2021, 73, 816–823. [Google Scholar]
- Pelton, S.I.; Divino, V.; Shah, D.; Mould-Quevedo, J.; DeKoven, M.; Krishnarajah, G.; Postma, M.J. Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017-2018 Influenza Season. Vaccines 2020, 8, 446. [Google Scholar] [CrossRef]
- McConeghy, K.W.; Davidson, H.E.; Canaday, D.H.; Han, L.; Saade, E.; Mor, V.; Gravenstein, S. Cluster-randomized Trial of Adjuvanted Versus Nonadjuvanted Trivalent Influenza Vaccine in 823 US Nursing Homes. Clin. Infect. Dis. 2021, 73, e4237–e4243. [Google Scholar] [CrossRef]
- Cocchio, S.; Gallo, T.; Del Zotto, S.; Clagnan, E.; Iob, A.; Furlan, P.; Fonzo, M.; Bertoncello, C.; Baldo, V. Preventing the Risk of Hospitalization for Respiratory Complications of Influenza among the Elderly: Is There a Better Influenza Vaccination Strategy? A Retrospective Population Study. Vaccines 2020, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Lu, M.; Kelman, J.; Lu, Y.; Lindaas, A.; Loc, J.; Pratt, D.; Wei, Y.; Chillarige, Y.; Wernecke, M.; et al. Comparative Effectiveness of Influenza Vaccines Among US Medicare Beneficiaries Ages 65 Years and Older During the 2019–2020 Season. Clin. Infect. Dis. 2021, 73, e4251–e4259. [Google Scholar] [CrossRef]
- HSE Flu Vaccine—Overview. Available online: https://www2.hse.ie/conditions/flu/flu-vaccine/ (accessed on 15 December 2022).
- Fochesato, A.; Sottile, S.; Pugliese, A.; Márquez-Peláez, S.; Toro-Diaz, H.; Gani, R.; Alvarez, P.; Ruiz-Aragón, J. An Economic Evaluation of the Adjuvanted Quadrivalent Influenza Vaccine Compared with Standard-Dose Quadrivalent Influenza Vaccine in the Spanish Older Adult Population. Vaccines 2022, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aragón, J.; Márquez-Peláez, S.; Gani, R.; Alvarez, P.; Guerrero-Luduena, R. Cost-Effectiveness and Burden of Disease for Adjuvanted Quadrivalent Influenza Vaccines Compared to High-Dose Quadrivalent Influenza Vaccines in Elderly Patients in Spain. Vaccines 2022, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Ashraf, M.; Mould-Quevedo, J. Estimating the impact of influenza vaccination of low-risk 50–64-year-olds on acute and ICU hospital bed usage in an influenza season under endemic COVID-19 in the UK. Hum. Vaccin. Immunother. 2023, 19, 2187592. [Google Scholar] [CrossRef]
- Calabrò, G.E.; Boccalini, S.; Panatto, D.; Rizzo, C.; Di Pietro, M.L.; Abreha, F.M.; Ajelli, M.; Amicizia, D.; Bechini, A.; Giacchetta, I.; et al. The New Quadrivalent Adjuvanted Influenza Vaccine for the Italian Elderly: A Health Technology Assessment. Int. J. Environ. Res. Public Health 2022, 19, 4166. [Google Scholar] [CrossRef]
- Dhanasekaran, V.; Sullivan, S.; Edwards, K.M.; Xie, R.; Khvorov, A.; Valkenburg, S.A.; Cowling, B.J.; Barr, I.G. Human seasonal influenza under COVID-19 and the potential consequences of influenza lineage elimination. Nat. Commun. 2022, 13, 1721. [Google Scholar] [CrossRef]
- Baguelin, M.; Flasche, S.; Camacho, A.; Demiris, N.; Miller, E.; Edmunds, W.J. Assessing optimal target populations for influenza vaccination programmes: An evidence synthesis and modelling study. PLoS Med. 2013, 10, e1001527. [Google Scholar] [CrossRef]
- Baguelin, M.; Camacho, A.; Flasche, S.; Edmunds, W.J. Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: A cost-effectiveness study. BMC Med. 2015, 13, 236. [Google Scholar] [CrossRef]
- HSE Previous Influenza Seasons’ Surveillance Reports. Available online: https://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/surveillance/influenzasurveillancereports/previousinfluenzaseasonssurveillancereports/ (accessed on 16 January 2023).
- Central Statistics Office. Popuilation and Migration Estimates, April 2021. Available online: https://www.cso.ie/en/releasesandpublications/ep/p-pme/populationandmigrationestimatesapril2021/mainresults/ (accessed on 24 April 2023).
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J.; et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008, 5, e74. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.A.; Maschio, M.; Mould-Quevedo, J.F.; Drummond, M.; Weinstein, M.C. The cost-effectiveness of an adjuvanted quadrivalent influenza vaccine in the United Kingdom. Hum. Vaccin. Immunother. 2021, 17, 4603–4610. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Surveillance of Influenza and other Respiratory Viruses in the UK—Winter 2019 to 2020. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20220401215804mp_/https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/895233/Surveillance_Influenza_and_other_respiratory_viruses_in_the_UK_2019_to_2020_FINAL.pdf (accessed on 4 October 2022).
- Thorrington, D.; van Leeuwen, E.; Ramsay, M.; Pebody, R.; Baguelin, M. Assessing optimal use of the standard dose adjuvanted trivalent seasonal influenza vaccine in the elderly. Vaccine 2019, 37, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, F.G.; van Leeuwen, E.; Bernard-Stoecklin, S.; Casado, I.; Castilla, J.; Domegan, L.; Gherasim, A.; Hooiveld, M.; Kislaya, I.; Larrauri, A.; et al. Health and economic impact of seasonal influenza mass vaccination strategies in European settings: A mathematical modelling and cost-effectiveness analysis. Vaccine 2022, 40, 1306–1315. [Google Scholar] [CrossRef]
- Influenza Vaccines 2022/2023. Available online: https://www.kvsa.de/fileadmin/user_upload/Bilder/Content/Praxis/Verordnung/22_02_18_Preisinformation_Grippeimpfstoffe_2022-2023_Tabelle_Homepage.pdf (accessed on 17 March 2023). (In German).
- National Institute for Health and Care Excellence (NICE). Medicinal Forms—Influenza Vaccines. Available online: https://bnf.nice.org.uk/drugs/influenza-vaccine/medicinal-forms/ (accessed on 17 March 2023).
- Statista. Average Annual Wage in Ireland from 2000 to 2021. Available online: https://www.statista.com/statistics/416212/average-annual-wages-ireland-y-on-y-in-euros/ (accessed on 16 January 2023).
- Essink, B.; Fierro, C.; Rosen, J.; Figueroa, A.L.; Zhang, B.; Verhoeven, C.; Edelman, J.; Smolenov, I. Immunogenicity and safety of MF59-adjuvanted quadrivalent influenza vaccine versus standard and alternate B strain MF59-adjuvanted trivalent influenza vaccines in older adults. Vaccine 2020, 38, 242–250. [Google Scholar] [CrossRef]
- O’Mahony, J.F. Revision of Ireland’s Cost-Effectiveness Threshold: New State-Industry Drug Pricing Deal Should Adequately Reflect Opportunity Costs. PharmacoEconomics-Open 2021, 5, 339–348. [Google Scholar] [CrossRef]
- Government of Ireland. Spending Review 2021: Review of High-Tech Drug Expenditure. Available online: https://assets.gov.ie/193851/9490e808-1774-440d-843a-28c3a9dc195c.pdf (accessed on 15 December 2022).
- Statista. Number of Hospital Beds in Ireland from 2000 to 2020. Available online: https://www.statista.com/statistics/557287/hospital-beds-in-ireland/ (accessed on 16 January 2023).
- HSE National Adult Critical Care Capacity—Census Report 2020. Available online: https://www.hse.ie/eng/about/who/cspd/ncps/critical-care/national-adult-critical-care-capacity-census-2020.pdf (accessed on 16 January 2023).
- OECD Hospital Beds and Occupancy. Available online: https://www.oecd-ilibrary.org/sites/e5a80353-en/index.html?itemId=/content/component/e5a80353-en (accessed on 16 January 2023).
- Nguyen, V.H.; Mould-Quevedo, J.F. Estimating the Impact of Influenza Vaccination on Acute and ICU Hospital Bed Usage in an Influenza Season under Endemic COVID-19 in the US. Vaccines 2022, 10, 1908. [Google Scholar] [CrossRef]
- Kohli, M.A.; Maschio, M.; Cartier, S.; Mould-Quevedo, J.; Fricke, F.U. The Cost-Effectiveness of Vaccination of Older Adults with an MF59-Adjuvanted Quadrivalent Influenza Vaccine Compared to Other Available Quadrivalent Vaccines in Germany. Vaccines 2022, 10, 1386. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Preliminary Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States—2021–2022 Influenza Season. Available online: https://www.cdc.gov/flu/about/burden/2021-2022.htm (accessed on 16 January 2023).
- Mertz, D.; Fadel, S.A.; Lam, P.P.; Tran, D.; Srigley, J.A.; Asner, S.A. Herd effect from influenza vaccination in non-healthcare settings: A systematic review of randomised controlled trials and observational studies. Euro Surveill. 2016, 21, 30378. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Seasonal Influenza 2021−2022—Annual Epidemiological Report. Available online: https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-2021-2022-annual-epidemiological-report (accessed on 24 April 2023).
- Lee, S.S.; Viboud, C.; Petersen, E. Understanding the rebound of influenza in the post COVID-19 pandemic period holds important clues for epidemiology and control. Int. J. Infect. Dis. 2022, 122, 1002–1004. [Google Scholar] [CrossRef]
- Health Protection Surveillance Centre. Influenza Season 2022—2023. Available online: https://www.hpsc.ie/a-z/respiratory/influenza/seasonalinfluenza/surveillance/influenzasurveillancereports/20222023season/ (accessed on 24 April 2023).
| Current Vaccination Scenario | aQIV Scenario | |||
|---|---|---|---|---|
| Age Group | Low Risk | High Risk | Low Risk | High Risk |
| 6–23 months | 0 | 3.10% | 0 | 3.10% |
| 2–17 years | 27.60% | 48.6% | 27.60% | 48.6% |
| 18–49 years | - | 48.6% | - | 48.6% |
| 50–64 years | - | 48.6% | 40% | 48.6% |
| 65–74 years | 68% | 68% | 68% | 68% |
| ≥75 years | 80% | 80% | 80% | 80% |
| Age Group | QIV a | aQIV | QLAIV |
|---|---|---|---|
| 6–23 months | 62.5% | - | - |
| 2–17 years | 62.5% | - | 62.5% |
| 18–49 years | 54.0% | - | - |
| 50–64 years | 54.0% | - | - |
| 65–74 years | 47.8% | 55.0% | - |
| ≥75 years | 45.3% | 52.9% | - |
| Current Vaccination Scenario | aQIV Scenario | Difference: aQIV−Current Vaccination Scenario | |
|---|---|---|---|
| Symptomatic cases | 492,790 | 488,683 | −4107 |
| GP visits | 49,279 | 48,868 | −411 |
| Hospital visits | 6812 | 6656 | −156 |
| Deaths | 1183 | 1141 | −42 |
| Life years lost | 13,271 | 12,949 | −322 |
| QALYs lost from death | 10,096 | 9851 | −245 |
| QALYs lost from sickness | 3132 | 3102 | −30 |
| Total QALYs lost | 13,228 | 12,953 | −275 |
| GP costs (EUR) | 2,463,948 | 2,443,417 | −20,531 |
| Hospitalisation costs (EUR) | 34,061,699 | 33,278,426 | −783,273 |
| Loss of productivity costs (EUR) | 323,786,375 | 320,887,491 | −2,898,884 |
| Vaccine costs (EUR) | 12,859,341 | 17,227,268 | +4,367,927 |
| Vaccine administration costs (EUR) | 25,908,065 | 25,908,065 | 0 |
| Total costs (EUR) | 399,079,428 | 399,744,668 | +665,240 |
| Cost (EUR) | QALYs Gained (Discounted) | ICER | |
|---|---|---|---|
| Societal perspective a | 665,240 | 275 | 2420 |
| Payer perspective b | 3,564,123 | 275 | 12,970 |
| Scenario | Hospital Beds Used | ICU Beds Used | Excess Hospital Beds Used | Excess ICU Beds Used | Excess Hospital Beds Prevented | Excess ICU Beds Prevented |
|---|---|---|---|---|---|---|
| Baseline a | 2713 | 353 | 552 | 23 | Ref | Ref |
| aQIV scenario | 2680 | 348 | 518 | 18 | 34 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.H.; Ashraf, M.; Mould-Quevedo, J.F. Cost-Effectiveness of the Use of Adjuvanted Quadrivalent Seasonal Influenza Vaccine in Older Adults in Ireland. Vaccines 2023, 11, 933. https://doi.org/10.3390/vaccines11050933
Nguyen VH, Ashraf M, Mould-Quevedo JF. Cost-Effectiveness of the Use of Adjuvanted Quadrivalent Seasonal Influenza Vaccine in Older Adults in Ireland. Vaccines. 2023; 11(5):933. https://doi.org/10.3390/vaccines11050933
Chicago/Turabian StyleNguyen, Van Hung, Mansoor Ashraf, and Joaquin F. Mould-Quevedo. 2023. "Cost-Effectiveness of the Use of Adjuvanted Quadrivalent Seasonal Influenza Vaccine in Older Adults in Ireland" Vaccines 11, no. 5: 933. https://doi.org/10.3390/vaccines11050933
APA StyleNguyen, V. H., Ashraf, M., & Mould-Quevedo, J. F. (2023). Cost-Effectiveness of the Use of Adjuvanted Quadrivalent Seasonal Influenza Vaccine in Older Adults in Ireland. Vaccines, 11(5), 933. https://doi.org/10.3390/vaccines11050933






