Influenza Vaccination Implementation in Sri Lanka: A Cost-Effectiveness Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Setting
2.2. Model Design and Structure
2.3. Model Parameters
2.3.1. Influenza Incidence
2.3.2. Mortality
2.3.3. Vaccine Coverage and Effectiveness
| Parameter | Base Case | Range | Distribution | Source |
|---|---|---|---|---|
| Percentage of Population by Age Group | Beta | [13] | ||
| 0–4 | 8.59% | 6.87–10.31% | ||
| 5–64 | 83.58% | 80.29–86.86% | ||
| 65+ | 7.84% | 6.27–9.40% | ||
| Annual Influenza Incidence Rate per 100,000 | Beta | Calculation | ||
| 0–4 | 2419.1453 | |||
| 5–64 | 1389.2567 | |||
| 65+ | 1074.5235 | |||
| Annual Influenza Hospitalization Rate per 100,000 | Beta | Calculation | ||
| 0–4 | 81.1363 | |||
| 5–64 | 20.9187 | |||
| 65+ | 14.6561 | |||
| Annual Influenza Death Rate per 100,000 | Beta | Calculation | ||
| 0–4 | 2.7703 | |||
| 5–64 | 0.2802 | |||
| 65+ | 9.2901 | |||
| Annual Mortality Rate from Other Causes per 100,000 | Beta | Calculation | ||
| 0–4 | 155.9699 | |||
| 5–64 | 232.6198 | |||
| 65+ | 4923.9090 | |||
| Vaccine Coverage by Age Group | ||||
| 0–4 | 61.50% | 23.00–100.00% | Uniform | [28,29] |
| 5–64 | 14.90% | 0.80–45.00% | Beta | [30] |
| 65+ | 27.50% | 14.00–41.00% | Uniform | [28] |
| Vaccine Effectiveness Against Lab-Confirmed Influenza by Age Group | ||||
| 0–4 | 53.40% | 25.30–70.50% | PERT | [31] |
| 5–64 | 59.00% | 51.00–67.00% | PERT | [32] |
| 65+ | 49.00% | 33.00–62.00% | Triangular | [33] |
2.3.4. Cost Parameters
| Parameter | Base Case * | Range | Distribution | Source |
|---|---|---|---|---|
| Average Weight (kg) | ||||
| Newborn | 2.9 | [40] | ||
| 5 Year Old | 17 | [41] | ||
| Inpatient Direct Medical Costs | ||||
| Medical Visits per Day | 4159.80 (17.23) | 3543.17–4776.42 (14.68–19.79) | Uniform | [43] |
| Hospital Duration (days) | 3.7 | 2.96–4.44 | PERT | [44] |
| Oseltamivir 75 mg Capsule | 254.91 (1.06) | 203.93–305.89 (0.84–1.27) | Gamma | [45] |
| Transportation | 101.85 (0.42) | 52.38–174.61 (0.22–0.72) | Gamma | [42] |
| Outpatient Direct Medical Costs | ||||
| Medical Visit | 625.12 (2.59) | 506.99–743.25 (2.10–3.08) | Uniform | [43] |
| Amoxicillin 500 mg Capsule | 5.73 (0.02) | 5.45–5.91 (0.02–0.02) | Gamma | [45] |
| Paracetamol 500 mg Tablet | 1.22 (0.00) | 0.97–1.46 (0.00–0.01) | Gamma | [45] |
| Chlorpheniramine 500 mg Tablet | 0.16 (0.00) | 0.13–0.19 (0.00–0.00) | Gamma | [45] |
| Transportation | 101.85 (0.42) | 52.38–174.61 (0.22–0.72) | Gamma | [42] |
| Indirect Medical Costs | ||||
| Days Lost to Absenteeism and Presenteeism | 6.95 | 0–34.05 | Gamma | [50] |
| Daily per Capita Income | 541.82 (2.24) | 425.67–638.50 (1.76–2.65) | PERT | Calculation |
| Vaccine Costs | ||||
| Vaccine per Dose | [53] | |||
| 0–4 year age group | 248.38 (1.03) | 224.78–271.99 (0.93–1.13) | Uniform | |
| 5–64 year age group | 450.06 (1.86) | 224.78–675.35 (0.93–2.80) | Uniform | |
| 65+ year age group | 562.45 (2.33) | 449.55–675.35 (1.86–2.80) | Uniform | |
| Disposable 22 G Needle | 3.60 (0.01) | 2.88–4.71 (0.01–0.02) | Gamma | [52] |
| Disposable 2 m Syringe w/o needle | 5.32 (0.02) | 4.25–6.43 (0.02–0.03) | Gamma | [52] |
2.3.5. Disability-Adjusted life Years
2.4. Cost-Effectiveness Analysis and Sensitivity Analysis
2.5. Budget Impact Analysis
3. Results
3.1. Base-Case Analysis
3.2. Budget Impact Analysis
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, V.J.; Ho, Z.J.M.; Goh, E.H.; Campbell, H.; Cohen, C.; Cozza, V.; Fitzner, J.; Jara, J.; Krishnan, A.; Bresee, J.; et al. Advances in measuring influenza burden of disease. Influenza Other Respir. Viruses 2018, 12, 3–9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 7 February 2023).
- Newall, A.T.; Chaiyakunapruk, N.; Lambach, P.; Hutubessy, R.C.W. WHO guide on the economic evaluation of influenza vaccination. Influenza Other Respir. Viruses 2018, 12, 211–219. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.I. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef]
- Bresee, J.S.; Lafond, K.E.; McCarron, M.; Azziz-Baumgartner, E.; Chu, S.Y.; Ebama, M.; Hinman, A.R.; Xeuatvongsa, A.; Bino, S.; Richardson, D.; et al. The partnership for influenza vaccine introduction (PIVI): Supporting influenza vaccine program development in low and middle-income countries through public-private partnerships. Vaccine 2019, 37, 5089–5095. [Google Scholar] [CrossRef]
- Hirve, S.; Lambach, P.; Paget, J.; Vandemaele, K.; Fitzner, J.; Zhang, W. Seasonal influenza vaccine policy, use and effectiveness in the tropics and subtropics—A systematic literature review. Influenza Other Respir. Viruses 2016, 10, 254–267. [Google Scholar] [CrossRef]
- Peasah, S.K.; Azziz-Baumgartner, E.; Breese, J.; Meltzer, M.I.; Widdowson, M.A. Influenza cost and cost-effectiveness studies globally—A review. Vaccine 2013, 31, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Dickens, B.L.; Yoong, J.S.; I-Cheng Chen, M.; Teerawattananon, Y.; Cook, A.R. Cost-Effectiveness Analysis for Influenza Vaccination Coverage and Timing in Tropical and Subtropical Climate Settings: A Modeling Study. Value Health 2019, 22, 1345–1354. [Google Scholar] [CrossRef]
- Newman, L.P.; Bhat, N.; Fleming, J.A.; Neuzil, K.M. Global influenza seasonality to inform country-level vaccine programs: An analysis of WHO FluNet influenza surveillance data between 2011 and 2016. PLoS ONE 2018, 13, e0193263. [Google Scholar] [CrossRef]
- Rafeek, R.A.M.; Divarathna, M.V.M.; Noordeen, F. History and current trends in influenza virus infections with special reference to Sri Lanka. Virusdisease 2017, 28, 225–232. [Google Scholar] [CrossRef]
- Shapiro, D.; Bodinayake, C.K.; Nagahawatte, A.; Devasiri, V.; Kurukulasooriya, R.; Hsiang, J.; Nicholson, B.; De Silva, A.D.; Ostbye, T.; Reller, M.E.; et al. Burden and Seasonality of Viral Acute Respiratory Tract Infections among Outpatients in Southern Sri Lanka. Am. J. Trop. Med. Hyg. 2017, 97, 88–96. [Google Scholar] [CrossRef]
- Chintha Jayasinghe, S.G.; Jayamaha, J. Infleunza surveillance activities in Sri Lanka. In Proceedings of the 1st South East Asia Regional Group Meeting of the International Epidemiological Association, Colombo, Sri Lanka, 19–21 September 2019. [Google Scholar]
- Department of Census and Statistics, Sri Lanka. Estimates on Mid-year Population 2014–2022. Available online: http://www.statistics.gov.lk/Population/StaticalInformation/VitalStatistics/BySexandAgeGroups (accessed on 10 November 2022).
- Kumar, R. Public-private partnerships for universal health coverage? The future of "free health" in Sri Lanka. Global Health 2019, 15, 75. [Google Scholar] [CrossRef]
- Ministry of Health, Nutrition and Indigenous Medicine, Sri Lanka. Annual Health Bulletin 2014; Medical Statistics Unit Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2016; p. 221. Available online: http://www.health.gov.lk/moh_final/english/public/elfinder/files/publications/AHB/AHB2014.pdf (accessed on 15 November 2022).
- Ministry of Health, Nutrition and Indigenous Medicine, Sri Lanka. Annual Health Bulletin 2015; Medical Statistics Unit Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2017; p. 224. Available online: http://www.health.gov.lk/moh_final/english/public/elfinder/files/publications/AHB/2017/AHB%202015.pdf (accessed on 15 November 2022).
- Ministry of Health, Nutrition and Indigenous Medicine, Sri Lanka. Annual Health Bulletin of 2016; Medical Statistics Unit Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2016; p. 328. Available online: http://www.health.gov.lk/moh_final/english/public/elfinder/files/publications/AHB/2017/AHB_2016.pdf (accessed on 15 November 2022).
- Medical Statistics Unit, Ministry of Health and Indigenous Medicine, Sri Lanka. Annual Health Bulletin 2013; Ministry of Health and Indigenous Medicine: Colombo, Sri Lanka, 2013; p. 216. Available online: http://www.health.gov.lk/moh_final/english/public/elfinder/files/publications/AHB/AHB2013.pdf (accessed on 15 November 2022).
- Medical Statistics Unit, Ministry of Health and Indigenous Medicine, Sri Lanka. Annual Health Bulletin 2012; Ministry of Health and Indigenous Medicine: Colombo, Sri Lanka, 2012; p. 204. Available online: http://www.health.gov.lk/moh_final/english/public/elfinder/files/publications/AHB/Annual%20Health%20Bulletin%20-%202012.pdf (accessed on 15 November 2022).
- Epidemiology Unit, Ministry of Health, Sri Lanka. Epidemiological Component. 2013. Available online: https://www.epid.gov.lk/web/index.php?option=com_content&view=article&id=180&Itemid=494&lang=en (accessed on 10 December 2022).
- Ministry of Health, Nutrition and Indigenous Medicine, Sri Lanka. Annual Health Bulletin 2017; Medical Statistics Unit Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2019; p. 253. Available online: http://www.health.gov.lk/moh_final/english/public/elfinder/files/publications/AHB/2020/AHB_2017.pdf (accessed on 20 March 2023).
- Ministry of Health, Nutrition and Indigenous Medicine, Sri Lanka. Annual Health Bulletin 2019; Medical Statistics Unit Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2021; p. 355. Available online: http://www.health.gov.lk/moh_final/english/public/elfinder/files/publications/AHB/2020/AHB%202019.pdf (accessed on 20 March 2023).
- World Health Organization. A Manual for Estimating Disease Burden Associated with Seasonal Influenza; World Health Organization: Geneva, Switzerland, 2015; p. 124. [Google Scholar]
- Centers for Disease Control and Prevention. Preliminary Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2021–2022 Influenza Season. Available online: https://www.cdc.gov/flu/about/burden/2021-2022.htm (accessed on 7 February 2023).
- Ministry of Health, Sri Lanka. Indoor Morbidity and Mortality Report 2019. 2019. Available online: http://www.health.gov.lk/moh_final/english/others.php?pid=110 (accessed on 20 November 2022).
- Narayan, V.V.; Iuliano, A.D.; Roguski, K.; Bhardwaj, R.; Chadha, M.; Saha, S.; Haldar, P.; Kumar, R.; Sreenivas, V.; Kant, S.; et al. Burden of influenza-associated respiratory and circulatory mortality in India, 2010–2013. J. Glob. Health 2020, 10, 010402. [Google Scholar] [CrossRef]
- Ministry of Health, Nutrition and Indigenous Medicine, Sri Lanka. Deaths Registered by District, Age and Sex—2015. Available online: http://www.statistics.gov.lk/Population/VitalStatistics/Deaths/2015/table4.3 (accessed on 15 November 2022).
- Hirve, Siddhivinayak, Global Influenza Programme Department of Pandemic and Epidemic Diseases World Health Organization. Seasonal Influenza Vaccine Use in Low and Middle Income Countries in the Tropics and Subtropics: A Systematic Review; World Health Organization: Geneva, Switzerland, 2015; p. 97. [Google Scholar]
- United Nations Children’s Fund (UNICEF) Regional Office for South Asia. Sustaining Vaccination Coverage; United Nations Children’s Fund Regional Office for South Asia: Kathmandu, Nepal, 2019; Available online: https://www.unicef.org/rosa/reports/sustaining-vaccination-coverage (accessed on 7 February 2023).
- Sheldenkar, A.; Lim, F.; Yung, C.F.; Lwin, M.O. Acceptance and uptake of influenza vaccines in Asia: A systematic review. Vaccine 2019, 37, 4896–4905. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.J.; Choi, J.H.; Choi, Y.Y.; Choe, Y.J.; Kim, K.; Kim, Y.K.; Ahn, B.; Song, S.H.; Han, M.S.; Park, J.Y.; et al. Effectiveness of trivalent inactivated influenza vaccines in children during 2017–2018 season in Korea: Comparison of test-negative analysis by rapid and RT-PCR influenza tests. Int. J. Infect. Dis. 2020, 99, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.E.; McElhaney, J.; Smith, D.J.; Monto, A.S.; Nguyen-Van-Tam, J.S.; Osterhaus, A.D. Cochrane re-arranged: Support for policies to vaccinate elderly people against influenza. Vaccine 2013, 31, 6030–6033. [Google Scholar] [CrossRef]
- Jiang, M.; Li, P.; Wang, W.; Zhao, M.; Atif, N.; Zhu, S.; Fang, Y. Cost-effectiveness of quadrivalent versus trivalent influenza vaccine for elderly population in China. Vaccine 2020, 38, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- International Monetary Fund. World Economic Outlook. 2022. Available online: https://www.imf.org/en/Publications/WEO/weo-database/2022/October (accessed on 3 January 2023).
- Haacker, M.; Hallett, T.B.; Atun, R. On discount rates for economic evaluations in global health. Health Policy Plan. 2020, 35, 107–114. [Google Scholar] [CrossRef]
- Tillekeratne, L.G.; Bodinayake, C.K.; Nagahawatte, A.; Vidanagama, D.; Devasiri, V.; Arachchi, W.K.; Kurukulasooriya, R.; De Silva, A.D.; Ostbye, T.; Reller, M.E.; et al. Use of Rapid Influenza Testing to Reduce Antibiotic Prescriptions Among Outpatients with Influenza-Like Illness in Southern Sri Lanka. Am. J. Trop. Med. Hyg. 2015, 93, 1031–1037. [Google Scholar] [CrossRef]
- Tillekeratne, L.G.; Bodinayake, C.K.; Dabrera, T.; Nagahawatte, A.; Arachchi, W.K.; Sooriyaarachchi, A.; Stewart, K.; Watt, M.; Ostbye, T.; Woods, C.W. Antibiotic overuse for acute respiratory tract infections in Sri Lanka: A qualitative study of outpatients and their physicians. BMC Fam. Pract. 2017, 18, 37. [Google Scholar] [CrossRef]
- Tillekeratne, L.G.; Bodinayake, C.; Nagahawatte, A.; Kurukulasooriya, R.; Orlando, L.A.; Simmons, R.A.; Park, L.P.; Woods, C.W.; Reed, S.D. Use of clinical algorithms and rapid influenza testing to manage influenza-like illness: A cost-effectiveness analysis in Sri Lanka. BMJ Glob. Health 2019, 4, e001291. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.N.; Lanerolle, P.; Senarath, U.; Hills, A.P.; Wickramasinghe, V.P. Birth anthropometry from a tertiary care hospital in Sri Lanka: Differs from the WHO growth standards. Asia Pac. J. Clin. Nutr. 2020, 29, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, V.; Yadav, S.; Agrawal, K.K.; Tamboli, S.; Banerjee, M.; Cherian, A.; Goyal, J.P.; Khadilkar, A.; Kumaravel, V.; Mohan, V.; et al. Revised IAP growth charts for height, weight and body mass index for 5- to 18-year-old Indian children. Indian Pediatr. 2015, 52, 47–55. [Google Scholar] [CrossRef]
- Tillekeratne, L.G.; Bodinayake, C.K.; Nagahawatte, A.; Vidanagama, D.; Devasiri, V.; Arachchi, W.K.; Kurukulasooriya, R.; De Silva, A.D.; Ostybe, T.; Reller, M.E.; et al. An under-recognized influenza epidemic identified by rapid influenza testing, southern Sri Lanka, 2013. Am. J. Trop. Med. Hyg. 2015, 92, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO-CHOICE Estimates of Cost for Inpatient and Outpatient Health Service Delivery. 2021. Available online: https://www.who.int/publications/m/item/who-choice-estimates-of-cost-for-inpatient-and-outpatient-health-service-delivery (accessed on 7 January 2023).
- Perera, C.; Rannan-Eliya, R.P.; Senanayake, S.; Dalpatadu, S.; de Silva, H.; Wijesinghe, R. Public Hospital Inpatient Discharge Survey 2005; Institute for Health Policy: Colombo, Sri Lanka, 2009; p. 20. Available online: https://www.ihp.lk/publications/docs/HSR0901.pdf (accessed on 12 November 2022).
- State Pharmaceuticals Corporation of Sri Lanka. Products. Available online: https://www.spc.lk/products.php (accessed on 27 January 2022).
- Centers for Disease Control and Prevention. Antiviral Dosage. Available online: https://www.cdc.gov/flu/professionals/antivirals/antiviral-dosage.htm (accessed on 1 February 2023).
- France, Nicole. Paracetamol. Available online: https://www.drugs.com/paracetamol.html#dosage (accessed on 1 February 2023).
- Cunha, J.P. Chlorpheniramine. Available online: https://www.rxlist.com/consumer_chlorpheniramine_chlortrimeton/drugs-condition.htm (accessed on 1 February 2023).
- Mayo Clinic. Amoxicillin (Oral Route). Available online: https://www.mayoclinic.org/drugs-supplements/amoxicillin-oral-route/proper-use/drg-20075356 (accessed on 1 February 2023).
- Fernando, M.; Caputi, P.; Ashbury, F. Impact on Employee Productivity From Presenteeism and Absenteeism: Evidence From a Multinational Firm in Sri Lanka. J. Occup. Environ. Med. 2017, 59, 691–696. [Google Scholar] [CrossRef]
- Department of Census and Statistics, Ministry of Economic Policies and Plan Implementation, Sri Lanka. Household Income and Expenditure Survey—2019; Medical Statistics Unit Ministry of Health, Nutrition and Indigenous Medicine: Colombo, Sri Lanka; p. 8. Available online: http://www.statistics.gov.lk/IncomeAndExpenditure/StaticalInformation/HouseholdIncomeandExpenditureSurvey2019FinalResults (accessed on 7 February 2023).
- Gunawardane, D.A.; Karnon, J.; Rowel, D.D.S.; Lucas, N.; Gamaethige, N.; Mathota, C.; Ratnasiri UD, P.; Hemachandra, N.; Jayakody, H. Cost Analysis of Sri Lanka Every Newborn Action Plan to End Preventable Morbidity and Mortality; Family Health Bureau, Ministry of Health: Colombo, Sri Lanka, 2018; p. 71. Available online: https://www.researchgate.net/publication/324415360_Cost_Analysis_of_Sri_Lanka_Every_Newborn_Action_Plan_to_End_Preventable_Morbidity_and_Mortality (accessed on 10 January 2023).
- Pan American Health Organization. Expanded Program of Immunization Vaccine Prices for Year 2019; Pan American Health Organization: Washington, DC, USA, 2019; p. 1. Available online: https://www.paho.org/en/file/51813/download?token=j1jy-Y8Q (accessed on 15 January 2023).
- World Health Organization. Life Tables by Country Sri Lanka. 2020. Available online: https://apps.who.int/gho/data/view.main.LT62220?lang=en (accessed on 20 November 2022).
- United Nations Statistics Division. Population by Age, Sex and Urban/Rural Residence. 2022. Available online: http://data.un.org/Data.aspx?d=POP&f=tableCode%3a22%3bcountryCode%3a144&c=2,3,6,8,10,12,14,15,16&s=_countryEnglishNameOrderBy:asc,refYear:desc,areaCode:asc&v=1 (accessed on 3 January 2023).
- Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease Study 2016 (GBD 2016) Disability Weights. 2016. Available online: https://ghdx.healthdata.org/record/ihme-data/gbd-2016-disability-weights (accessed on 7 February 2023).
- World Health Organization. The World Health Report: 2002: Reducing Risks, Promoting Healthy Life: Overview; World Health Organization: Geneva, Switzerland, 2002; Available online: https://apps.who.int/iris/handle/10665/67454 (accessed on 7 February 2023).
- United Nations Children’s Fund (UNICEF) Sri lanka. Budget Brief: Health Sector Sri Lanka 2021; State Ministry of Primary Health Care: Colombo, Sri Lanka, 2022; p. 30. Available online: https://www.unicef.org/srilanka/reports/budget-brief-health-sector (accessed on 28 March 2023).
- Ott, J.J.; Klein Breteler, J.; Tam, J.S.; Hutubessy, R.C.; Jit, M.; de Boer, M.R. Influenza vaccines in low and middle income countries: A systematic review of economic evaluations. Hum. Vaccin. Immunother. 2013, 9, 1500–1511. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Liao, Q.; Guo, V.Y.W.; Xin, Y.; Lam, C.L.K. Cost-effectiveness analysis of vaccinations and decision makings on vaccination programmes in Hong Kong: A systematic review. Vaccine 2017, 35, 3153–3161. [Google Scholar] [CrossRef]
- Shields, G.E.; Elvidge, J.; Davies, L.M. A systematic review of economic evaluations of seasonal influenza vaccination for the elderly population in the European Union. BMJ Open 2017, 7, e014847. [Google Scholar] [CrossRef]
- Ting, E.E.K.; Sander, B.; Ungar, W.J. Systematic review of the cost-effectiveness of influenza immunization programs. Vaccine 2017, 35, 1828–1843. [Google Scholar] [CrossRef]
- Leidner, A.J.; Murthy, N.; Chesson, H.W.; Biggerstaff, M.; Stoecker, C.; Harris, A.M.; Acosta, A.; Dooling, K.; Bridges, C.B. Cost-effectiveness of adult vaccinations: A systematic review. Vaccine 2019, 37, 226–234. [Google Scholar] [CrossRef]
- D’Angiolella, L.S.; Lafranconi, A.; Cortesi, P.A.; Rota, S.; Cesana, G.; Mantovani, L.G. Costs and effectiveness of influenza vaccination: A systematic review. Ann. Ist. Super Sanita 2018, 54, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Dilokthornsakul, P.; Lan, L.M.; Thakkinstian, A.; Hutubessy, R.; Lambach, P.; Chaiyakunapruk, N. Economic evaluation of seasonal influenza vaccination in elderly and health workers: A systematic review and meta-analysis. EClinicalMedicine 2022, 47, 101410. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.W.; Simasathien, S.; Watanaveeradej, V.; Bhoomiboonchoo, P.; Fernandez, S.; Jarman, R.G.; Klungthong, C.; Gibbons, R.V.; Kerdpanich, P.; Piboonbanakit, D.; et al. Influenza vaccine effectiveness in the tropics: Moderate protection in a case test-negative analysis of a hospital-based surveillance population in Bangkok between August 2009 and January 2013. PLoS ONE 2015, 10, e0134318. [Google Scholar] [CrossRef] [PubMed]
- Rafeek, R.A.M.; Divarathna, M.V.M.; Morel, A.J.; Noordeen, F. Clinical and epidemiological characteristics of influenza virus infection in hospitalized children with acute respiratory infections in Sri Lanka. PLoS ONE 2022, 17, e0272415. [Google Scholar] [CrossRef] [PubMed]

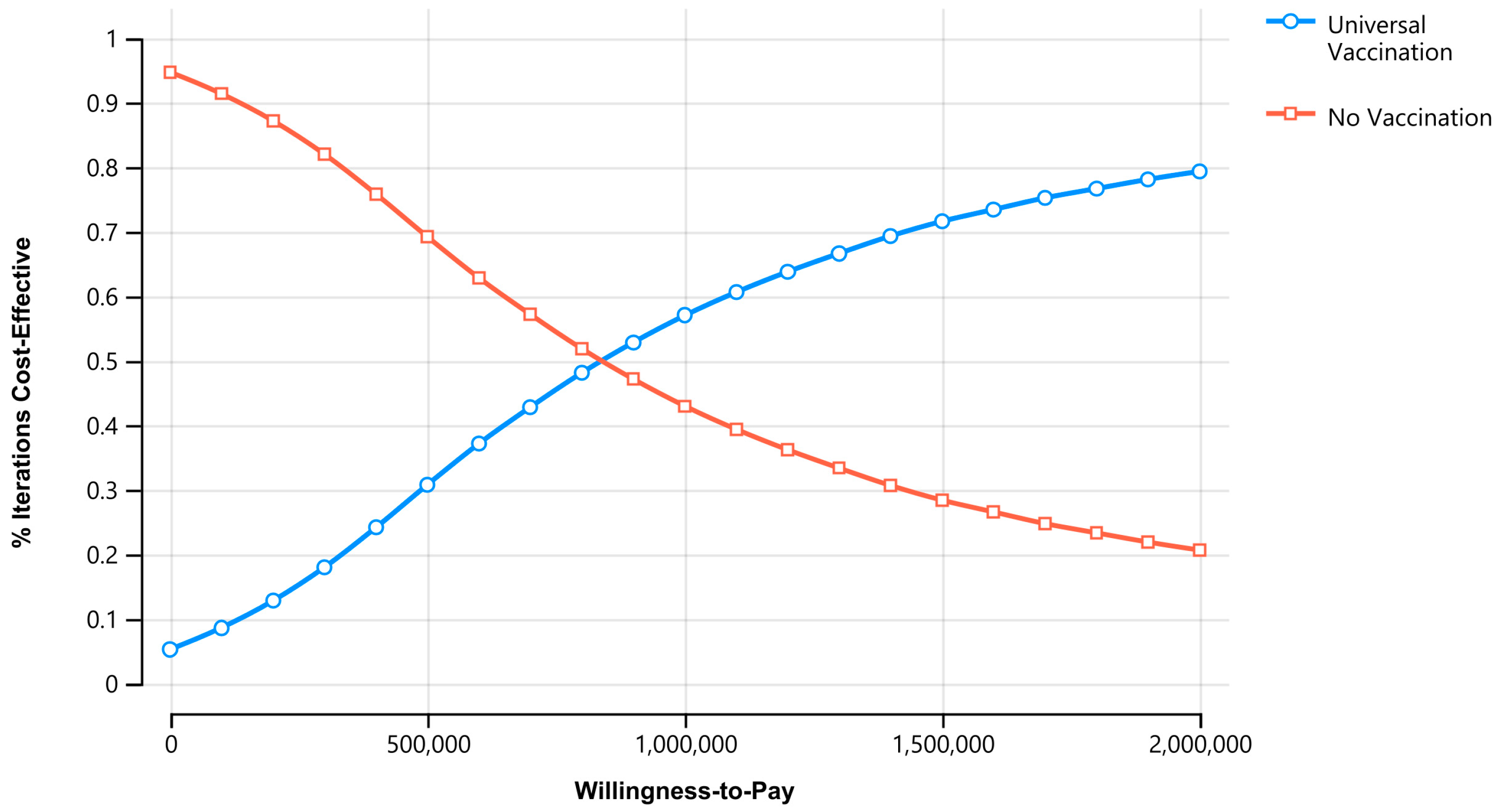
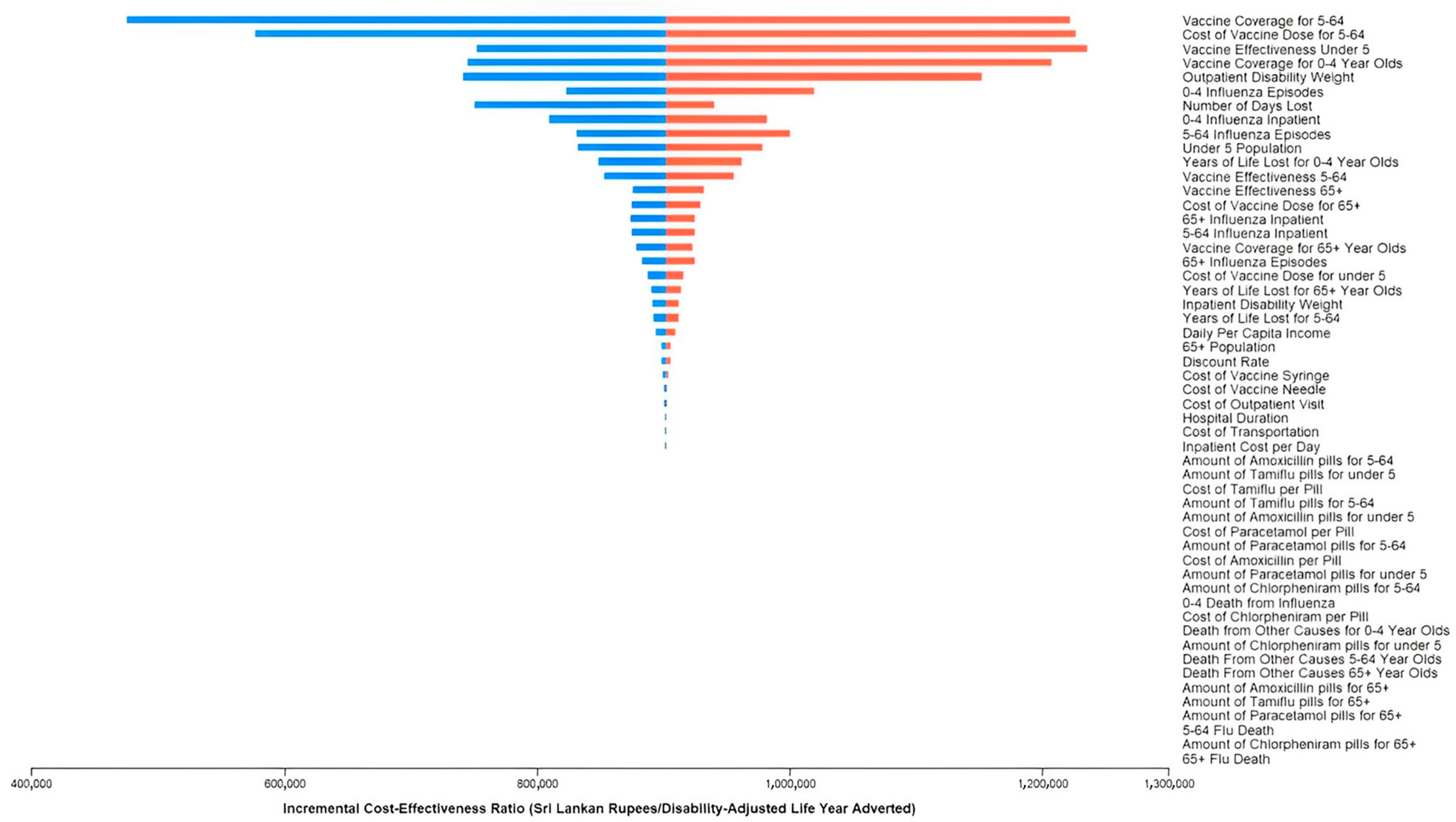
| Parameter | Base Case | Range | Distribution | Source |
|---|---|---|---|---|
| Disability Weight per Influenza Episode | Normal | [56] | ||
| Nonhospitalized | 0.051 | 0.032–0.074 | ||
| Hospitalized | 0.133 | 0.088–0.190 | ||
| Years of Life Lost by Age Group | PERT | Calculation | ||
| 0–4 year age group | 76.44 | 61.15–91.72 | ||
| 5–64 year age group | 48.67 | 38.93–58.40 | ||
| 65+ year age group | 12.53 | 10.02–15.03 | ||
| Parameters | No Vaccination | Universal Vaccination | Universal Vaccination vs. No Vaccination |
|---|---|---|---|
| Health Outcomes | |||
| Influenza Cases | 167,847 | 147,137 | −20,710 |
| 0–4 year olds | 23,002 | 15,696 | −7306 |
| 5–64 year olds | 135,245 | 123,103 | −12,142 |
| 65+ year olds | 9600 | 8338 | −1262 |
| Hospitalizations | 2867 | 2429 | −438 |
| 0–4 year olds | 774 | 529 | −245 |
| 5–64 year olds | 1967 | 1790 | −177 |
| 65+ year olds | 126 | 110 | −16 |
| Outpatients | 164,980 | 144,708 | −20,272 |
| 0–4 year olds | 22,228 | 15,167 | −7061 |
| 5–64 year olds | 133,278 | 121,313 | −11,965 |
| 65+ year olds | 9474 | 8228 | −1246 |
| Deaths from Influenza | 130 | 110 | −20 |
| 0–4 year olds | 26 | 18 | −8 |
| 5–64 year olds | 25 | 23 | −2 |
| 65+ year olds | 79 | 69 | −10 |
| DALYs | 13,072.89 | 11,105.06 | −2013.63 |
| Total Cost + | 793,871,356.06 (3289,158.75) | 2,555,577,215.23 (10,588,238.38) | 1,761,705,859.18 (7,299,079.63) |
| Incremental Cost per DALY adverted + | 874,890.55 (3624.84) | ||
| 0–4 Year Olds | 5–64 Year Olds | 65+ Year Olds | 0–4 Year Olds and 65+ Year Olds | Total | |
|---|---|---|---|---|---|
| No Vaccination | |||||
| Population Size | 1,905,000 | 18,538,000 | 1,738,000 | 3,643,000 | 22,181,000 |
| Number of Influenza Cases | 22,997 | 135,246 | 9603 | 32,600 | 167,846 |
| Indirect Medical Costs | 86,597,692.12 (358,790.57) | 509,287,654.66 (2,110,074.80) | 36,160,134.37 (149,818.26) | 36,160,134.37 (508,608.83) | 36,160,134.37 (2,618,683.63) |
| Number of Hospitalizations | 774 | 1966 | 126 | 900 | 2866 |
| Hospitalization Costs | 12,706,505.31 (52,645.45) | 34,477,676.51 (142,847.52) | 2,282,455.85 (9456.65) | 14,988,961.16 (62,102.09) | 49,466,637.67 (204,949.61) |
| Number of Outpatient Visits | 22,223 | 133,280 | 9477 | 31,700 | 164,980 |
| Outpatient Costs | 16,584,539.29 (68,712.87) | 103,477,649.37 (428,727.42) | 7,522,776.11 (31,168.28) | 24,107,315.40 (99,881.15) | 127,584,964.77 (528,608.57) |
| Number of Deaths | 26 | 25 | 79 | 105 | 130 |
| Total Costs | 115,888,736.73 (480,148.89) | 647,242,980.53 (2,681,649.74) | 45,965,366.33 (190,443.18) | 161,854,103.06 (670,592.07) | 809,097,083.59 (3,352,241.81) |
| Universal Vaccination | |||||
| Number of Influenza Cases | 15,710 | 123,154 | 8339 | 24,049 | 147,203 |
| Indirect Medical Costs | 59,159,420.15 (245,108.64) | 463,756,368.98 (1,921,430.10) | 31,400,951.48 (130,100.06) | 90,560,371.63 (375,208.70) | 554,316,740.61 (2,296,638.80) |
| Number of Hospitalizations | 529 | 1791 | 110 | 639 | 2430 |
| Hospitalization Costs | 8,683,201.42 (35,976.14) | 31,412,256.45 (130,146.90) | 1,982,493.31 (8213.84) | 10,665,694.73 (44,189.98) | 42,077,951.18 (174,336.89) |
| Number of Outpatient Visits | 15,181 | 121,363 | 8229 | 23,410 | 144,773 |
| Outpatient Costs | 11,329,643.81 (46,940.85) | 94,225,800.08 (390,395.26) | 6,532,653.44 (27,066.02) | 17,862,297.26 (74,006.87) | 112,088,097.34 (464,402.13) |
| Number of Vaccines Given | 1,171,575 | 2,762,162 | 477,950 | 1,649,525 | 4,411,687 |
| Vaccination Costs | 301,447,885.94 (1,248,955.44) | 1,267,787,378.03 (5,252,682.21) | 273,087,042.74 (1,131,451.12) | 574,534,928.68 (2,380,406.57) | 1,842,322,306.71 (7,633,088.77) |
| Number of Deaths | 18 | 23 | 69 | 87 | 110 |
| Total Costs | 380,620,151.33 (1,576,981.07) | 1,857,181,803.53 (7,694,654.47) | 313,003,140.98 (1,296,831.04) | 693,623,292.30 (2,875,549.76) | 2,550,805,095.84 (10,568,466.59) |
| No Vaccination v. Universal Vaccination | |||||
| Number of Influenza Cases | 7287 | 12,092 | 1264 | 8551 | 20,643 |
| Indirect Costs | 27,438,271.97 (113,681.94) | 45,531,285.68 (188,644.70) | 4,759,182.89 (19,718.19) | 32,197,454.86 (133,400.13) | 77,728,740.54 (322,044.83) |
| Number of Hospitalizations | 245 | 175 | 16 | 261 | 436 |
| Hospitalization Costs | 4,023,303.89 (16,669.31) | 3,065,420.06 (12,700.61) | 299,962.54 (1,242.80) | 4,323,266.43 (17,912.11) | 7,388,686.49 (30,612.72) |
| Number of Outpatient Visits | 7042 | 11,917 | 1248 | 8290 | 20,207 |
| Outpatient Costs | 5,254,895.48 (21,772.02) | 9,251,849.29 (38,332.16) | 990,122.66 (4,102.26) | 6,245,018.14 (25,874.29) | 15,496,867.43 (64,206.44) |
| Vaccination Costs | −301,447,885.94 (−1,248,955.44) | −1,267,787,378.03 (−5,252,682.21) | −273,087,042.74 (−1,131,451.12) | −574,534,928.68 (−2,380,406.57) | −1,842,322,306.71 (−7,633,088.77) |
| Number of Deaths | 8 | 2 | 10 | 18 | 20 |
| Total Costs | −264,731,414.60 (−1,096,832.18) | −1,209,938,823.00 (−5,013,004.74) | −267,037,774.65 (−1,106,387.86) | −531,769,189.25 (−2,204,957.69) | −1,741,708,012.25 (−7,216,224.78) |
| Total Costs as a Percentage of Health Spending | 0.04% | 0.16% | 0.04% | 0.07% | 0.23% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neighbors, C.E.; Myers, E.R.; Weerasinghe, N.P.; Wijayaratne, G.B.; Bodinayake, C.K.; Nagahawatte, A.; Tillekeratne, L.G.; Woods, C.W. Influenza Vaccination Implementation in Sri Lanka: A Cost-Effectiveness Analysis. Vaccines 2023, 11, 932. https://doi.org/10.3390/vaccines11050932
Neighbors CE, Myers ER, Weerasinghe NP, Wijayaratne GB, Bodinayake CK, Nagahawatte A, Tillekeratne LG, Woods CW. Influenza Vaccination Implementation in Sri Lanka: A Cost-Effectiveness Analysis. Vaccines. 2023; 11(5):932. https://doi.org/10.3390/vaccines11050932
Chicago/Turabian StyleNeighbors, Coralei E., Evan R. Myers, Nayani P. Weerasinghe, Gaya B. Wijayaratne, Champica K. Bodinayake, Ajith Nagahawatte, L. Gayani Tillekeratne, and Christopher W. Woods. 2023. "Influenza Vaccination Implementation in Sri Lanka: A Cost-Effectiveness Analysis" Vaccines 11, no. 5: 932. https://doi.org/10.3390/vaccines11050932
APA StyleNeighbors, C. E., Myers, E. R., Weerasinghe, N. P., Wijayaratne, G. B., Bodinayake, C. K., Nagahawatte, A., Tillekeratne, L. G., & Woods, C. W. (2023). Influenza Vaccination Implementation in Sri Lanka: A Cost-Effectiveness Analysis. Vaccines, 11(5), 932. https://doi.org/10.3390/vaccines11050932






