Development of Effective PEDV Vaccine Candidates Based on Viral Culture and Protease Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Isolation of PED Virus
2.3. Live Attenuated PEDV
2.4. Titration of PEDV
2.5. Immunofluorescence Assay
2.6. Evaluation of Pathogenicity
2.7. Quantitative Real-Time RT-PCR
2.8. Histopathology and Immunochemistry
2.9. Next-Generation Sequencing (NGS)
2.10. Phylogenetic Tree
3. Results
3.1. Isolation of PEDV from Porcine Intestines
3.2. Development of Live Attenuated Vaccine Candidates
3.3. Growth Characteristics of CKT-7 Strains
3.4. Growth Kinetics of CKT-7 Strains
3.5. Evaluation for Pathogenicity of CKT-7 Strains
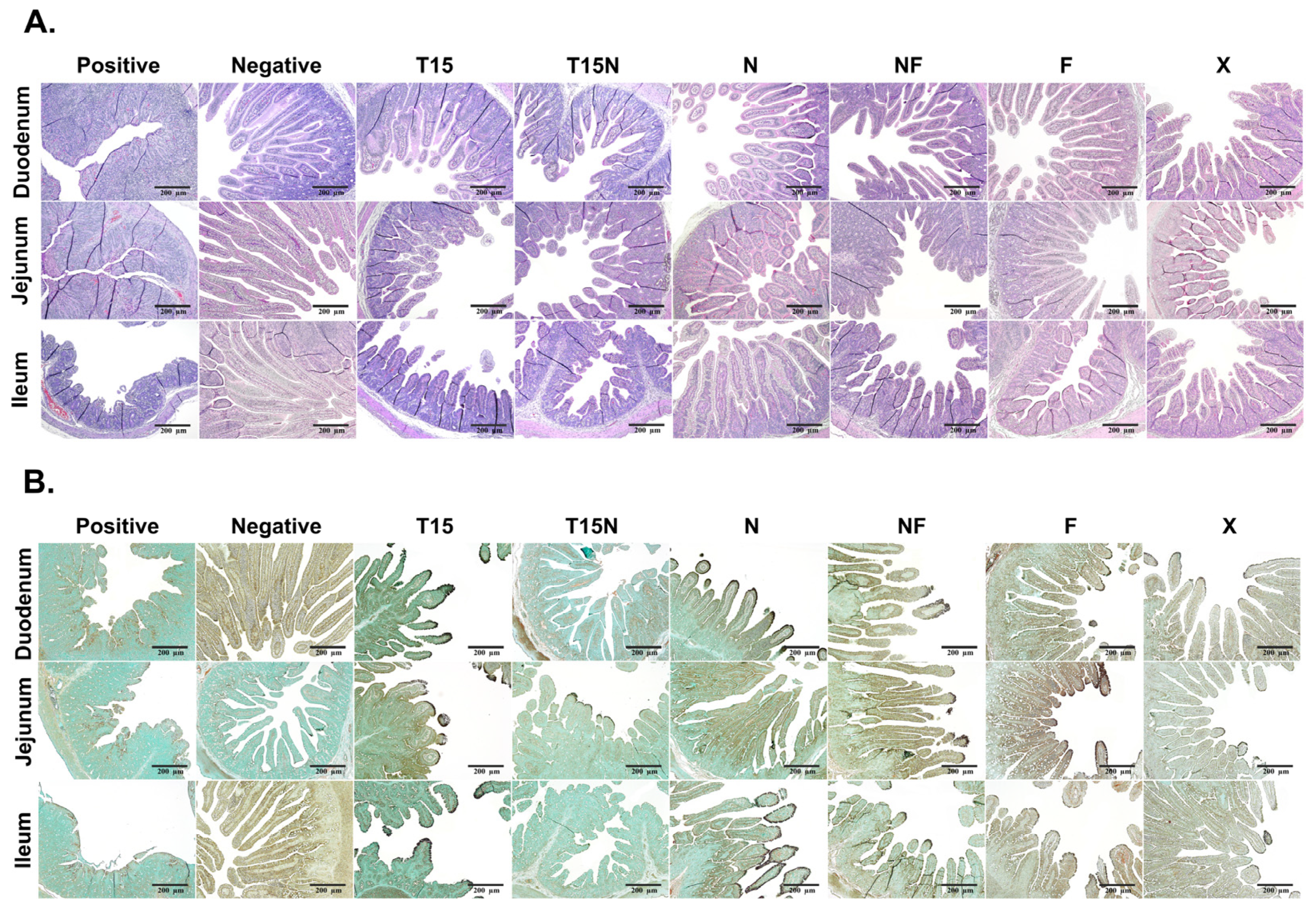
3.6. Analysis of Histopathology and Immunohistochemistry (IHC)
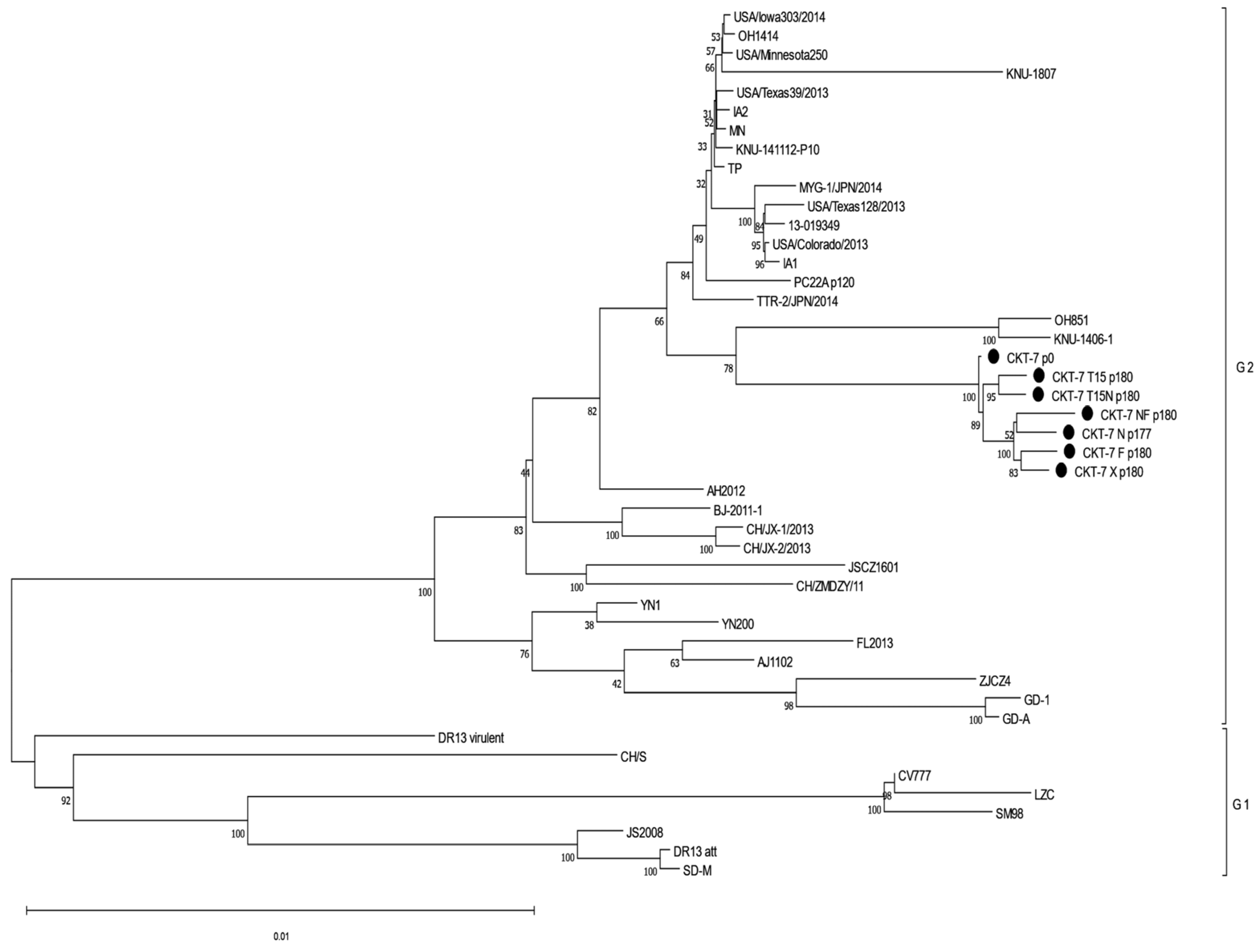
3.7. Analysis of the Phylogenetic Relationship
3.8. Comparison of Whole-Genome Sequence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, G.; Singh, P.; Pillatzki, A.; Nelson, E.; Webb, B.; Dillberger-Lawson, S.; Ramamoorthy, S. A Minimally Replicative Vaccine Protects Vaccinated Piglets Against Challenge With the Porcine Epidemic Diarrhea Virus. Front. Vet. Sci. 2019, 6, 347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, G.; Stasko, J.; Thomas, J.T.; Stensland, W.R.; Pillatzki, A.E.; Gauger, P.C.; Schwartz, K.J.; Madson, D.; Yoon, K.J.; et al. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014, 52, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, W.; Zhou, Q.; Li, Q.; Xu, Z.; Shen, H.; Chen, F. Characterization and pathogenicity of Vero cell-attenuated porcine epidemic diarrhea virus CT strain. Virol. J. 2019, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Dervas, E.; Hepojoki, J.; Laimbacher, A.; Romero-Palomo, F.; Jelinek, C.; Keller, S.; Smura, T.; Hepojoki, S.; Kipar, A.; Hetzel, U. Nidovirus-Associated Proliferative Pneumonia in the Green Tree Python (Morelia viridis). J. Virol. 2017, 91, e00718-17. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, K.; Yoo, D. Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology 2016, 489, 252–268. [Google Scholar] [CrossRef]
- Lee, S.H.; Yang, D.K.; Kim, H.H.; Cho, I.S. Efficacy of inactivated variant porcine epidemic diarrhea virus vaccines in growing pigs. Clin. Exp. Vaccine Res. 2018, 7, 61–69. [Google Scholar] [CrossRef]
- Gerdts, V.; Zakhartchouk, A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet. Microbiol. 2017, 206, 45–51. [Google Scholar] [CrossRef]
- Park, J.E.; Shin, H.J. Porcine epidemic diarrhea vaccine efficacy evaluation by vaccination timing and frequencies. Vaccine 2018, 36, 2760–2763. [Google Scholar] [CrossRef]
- Lin, C.N.; Chung, W.B.; Chang, S.W.; Wen, C.C.; Liu, H.; Chien, C.H.; Chiou, M.T. US-like strain of porcine epidemic diarrhea virus outbreaks in Taiwan, 2013–2014. J. Vet. Med. Sci. 2014, 76, 1297–1299. [Google Scholar] [CrossRef]
- Baek, P.S.; Choi, H.W.; Lee, S.; Yoon, I.J.; Lee, Y.J.; Lee du, S.; Lee, S.; Lee, C. Efficacy of an inactivated genotype 2b porcine epidemic diarrhea virus vaccine in neonatal piglets. Vet. Immunol. Immunopathol. 2016, 174, 45–49. [Google Scholar] [CrossRef]
- Lee, S.; Son, K.Y.; Noh, Y.H.; Lee, S.C.; Choi, H.W.; Yoon, I.J.; Lee, C. Genetic characteristics, pathogenicity, and immunogenicity associated with cell adaptation of a virulent genotype 2b porcine epidemic diarrhea virus. Vet. Microbiol. 2017, 207, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ke, H.; Kim, J.; Yoo, D.; Su, Y.; Boley, P.; Chepngeno, J.; Vlasova, A.N.; Saif, L.J.; Wang, Q. Engineering a Live Attenuated Porcine Epidemic Diarrhea Virus Vaccine Candidate via Inactivation of the Viral 2′-O-Methyltransferase and the Endocytosis Signal of the Spike Protein. J. Virol. 2019, 93, e00406-19. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Song, D.; Huang, T.; Peng, Q.; Chen, Y.; Li, A.; Zhang, F.; Wu, Q.; Ye, Y.; et al. Comparison and evaluation of conventional RT-PCR, SYBR green I and TaqMan real-time RT-PCR assays for the detection of porcine epidemic diarrhea virus. Mol. Cell. Probes 2017, 33, 36–41. [Google Scholar] [CrossRef]
- Chrzastek, K.; Lee, D.H.; Smith, D.; Sharma, P.; Suarez, D.L.; Pantin-Jackwood, M.; Kapczynski, D.R. Use of Sequence-Independent, Single-Primer-Amplification (SISPA) for rapid detection, identification, and characterization of avian RNA viruses. Virology 2017, 509, 159–166. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Wang, L.; Byrum, B.; Zhang, Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014, 20, 917–919. [Google Scholar] [CrossRef]
- Fan, B.; Jiao, D.; Zhao, X.; Pang, F.; Xiao, Q.; Yu, Z.; Mao, A.; Guo, R.; Yuan, W.; Zhao, P.; et al. Characterization of Chinese Porcine Epidemic Diarrhea Virus with Novel Insertions and Deletions in Genome. Sci. Rep. 2017, 7, 44209. [Google Scholar] [CrossRef]
- Shirato, K.; Maejima, M.; Matsuyama, S.; Ujike, M.; Miyazaki, A.; Takeyama, N.; Ikeda, H.; Taguchi, F. Mutation in the cytoplasmic retrieval signal of porcine epidemic diarrhea virus spike (S) protein is responsible for enhanced fusion activity. Virus Res. 2011, 161, 188–193. [Google Scholar] [CrossRef]
- Hou, Y.; Meulia, T.; Gao, X.; Saif, L.J.; Wang, Q. Deletion of both the Tyrosine-Based Endocytosis Signal and the Endoplasmic Reticulum Retrieval Signal in the Cytoplasmic Tail of Spike Protein Attenuates Porcine Epidemic Diarrhea Virus in Pigs. J. Virol. 2019, 93, e01758-18. [Google Scholar] [CrossRef]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takeyama, N.; Katsumata, A.; Tuchiya, K.; Kodama, T.; Kusanagi, K. Mutations in the spike gene of porcine epidemic diarrhea virus associated with growth adaptation in vitro and attenuation of virulence in vivo. Virus Genes 2011, 43, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, Q. Emerging Highly Virulent Porcine Epidemic Diarrhea Virus: Molecular Mechanisms of Attenuation and Rational Design of Live Attenuated Vaccines. Int. J. Mol. Sci. 2019, 20, 5478. [Google Scholar] [CrossRef] [PubMed]
- Rott, R.; Klenk, H.D.; Nagai, Y.; Tashiro, M. Influenza viruses, cell enzymes, and pathogenicity. Am. J. Respir. Crit. Care Med. 1995, 152, S16–S19. [Google Scholar] [CrossRef] [PubMed]
- de Haan, C.A.; Stadler, K.; Godeke, G.J.; Bosch, B.J.; Rottier, P.J. Cleavage inhibition of the murine coronavirus spike protein by a furin-like enzyme affects cell-cell but not virus-cell fusion. J. Virol. 2004, 78, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- Chandran, K.; Sullivan, N.J.; Felbor, U.; Whelan, S.P.; Cunningham, J.M. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 2005, 308, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Matsuyama, S.; Ujike, M.; Taguchi, F. Role of proteases in the release of porcine epidemic diarrhea virus from infected cells. J. Virol. 2011, 85, 7872–7880. [Google Scholar] [CrossRef]
- Zamolodchikova, T.S. Serine proteases of small intestine mucosa--localization, functional properties, and physiological role. Biochem. Biokhimiia 2012, 77, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Wicht, O.; Li, W.; Willems, L.; Meuleman, T.J.; Wubbolts, R.W.; van Kuppeveld, F.J.; Rottier, P.J.; Bosch, B.J. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture. J. Virol. 2014, 88, 7952–7961. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, C.; Shivanna, V.; Hesse, R.A.; Chang, K.O. Trypsin-independent porcine epidemic diarrhea virus US strain with altered virus entry mechanism. BMC Vet. Res. 2017, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Kim, Y.; Saif, L.J.; Green, K.Y. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. USA 2004, 101, 8733–8738. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.O.; George, D.W. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J. Virol. 2007, 81, 9633–9640. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Boyer, J.L. Bile salt transporters: Molecular characterization, function, and regulation. Physiol. Rev. 2003, 83, 633–671. [Google Scholar] [CrossRef]
- Deng, X.; van Geelen, A.; Buckley, A.C.; O’Brien, A.; Pillatzki, A.; Lager, K.M.; Faaberg, K.S.; Baker, S.C. Coronavirus Endoribonuclease Activity in Porcine Epidemic Diarrhea Virus Suppresses Type I and Type III Interferon Responses. J. Virol. 2019, 93, e02000-18. [Google Scholar] [CrossRef]
- Hou, Y.; Lin, C.M.; Yokoyama, M.; Yount, B.L.; Marthaler, D.; Douglas, A.L.; Ghimire, S.; Qin, Y.; Baric, R.S.; Saif, L.J.; et al. Deletion of a 197-Amino-Acid Region in the N-Terminal Domain of Spike Protein Attenuates Porcine Epidemic Diarrhea Virus in Piglets. J. Virol. 2017, 91, e00227-17. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Hou, Y.; Marthaler, D.G.; Gao, X.; Liu, X.; Zheng, L.; Saif, L.J.; Wang, Q. Attenuation of an original US porcine epidemic diarrhea virus strain PC22A via serial cell culture passage. Vet. Microbiol. 2017, 201, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016, 226, 93–107. [Google Scholar] [CrossRef] [PubMed]
| Strains | Passage Number | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin | 1 | 2 | 3 | …**** | 9 | 10 | 11 | … | 32 | 33 | 34 | … | 39 | 40 | 41 | 42 | … | 51 | 52 | 53 | 54 | … | 60 | 61 | 62 | 63 | 64 | 65 | 66 | … | 70 | 71 | 72 | … | 180 |
| T15 * | 1 | 2 | 3 | … | 9 | 10 | 11 | … | 32 | 33 | 34 | … | 39 | 40 | 41 | 42 | … | 51 | 52 | 53 | 54 | … | 60 | 61 | 62 | 63 | 64 | 65 | 66 | … | 70 | 71 | 72 | … | 180 |
| T15N ** | 1 | 2 | 3 | … | 24 | 25 | 26 | … | 31 | 32 | 33 | 34 | … | 43 | 44 | 45 | 46 | … | 52 | 53 | 54 | 55 | 56 | 57 | 58 | … | 62 | 63 | 64 | … | 172 | ||||
| T10N | 1 | 2 | 3 | … | 8 | ||||||||||||||||||||||||||||||
| T5N | 1 | 2 | 3 | … | 12 | ||||||||||||||||||||||||||||||
| T2N | 1 | 2 | 3 | … | 9 | ||||||||||||||||||||||||||||||
| N | 1 | 2 | 3 | 4 | 5 | 6 | … | 10 | 11 | 12 | … | 120 | |||||||||||||||||||||||
| NF *** | 1 | 2 | 3 | … | 7 | 8 | 9 | … | 117 | ||||||||||||||||||||||||||
| X | 1 | 2 | 3 | … | 111 | ||||||||||||||||||||||||||||||
| F | 1 | 2 | … | 110 | |||||||||||||||||||||||||||||||
| T15 | T15N | N | NF | F | X | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Passage No. | Titer * | Passage No. | Titer | Passage No. | Titer | Passage No. | Titer | Passage No. | Titer | Passage No. | Titer |
| P10 | 3.50 ± 0.00 | P10 | 3.67 ± 0.14 | P10 | P10 | P10 | P10 | ||||
| P20 | 4.58 ± 0.14 | P20 | 4.75 ± 0.00 | P20 | P20 | P20 | P20 | ||||
| P30 | 5.42 ± 0.29 | P30 | 5.92 ± 0.14 | P30 | P30 | P30 | P30 | ||||
| P40 | 6.00 ± 0.25 | P40 | 5.42 ± 0.14 | P40 | P40 | P40 | P40 | ||||
| P50 | 6.17 ± 0.14 | P50 | 6.50 ± 0.25 | P50 | P50 | P50 | P50 | ||||
| P60 | 5.33 ± 0.29 | P60 | 5.33 ± 0.52 | P60 | P60 | P60 | P60 | ||||
| P70 | 6.08 ± 0.38 | P70 | 5.50 ± 0.00 | P70 | P70 | P70 | P70 | ||||
| P80 | 6.00 ± 0.00 | P80 | 5.85 ± 0.14 | P80 | 7.17 ± 0.14 | P80 | 6.67 ± 0.14 | P80 | 6.33 ± 0.14 | P80 | 6.75 ± 0.00 |
| P90 | 6.33 ± 0.14 | P90 | 5.75 ± 0.43 | P90 | 7.08 ± 0.29 | P90 | 7.00 ± 0.43 | P90 | 6.83 ± 0.38 | P90 | 6.92 ± 0.38 |
| P100 | 5.50 ± 0.00 | P100 | 4.83 ± 0.14 | P100 | 7.25 ± 0.43 | P100 | 5.92 ± 0.14 | P100 | 6.83 ± 0.14 | P100 | 6.75 ± 0.25 |
| P110 | 6.58 ± 0.14 | P110 | 7.08 ± 0.14 | P110 | 7.42 ± 0.38 | P110 | 6.67 ± 0.52 | P110 | 6.75 ± 0.00 | P110 | 6.42 ± 0.29 |
| P120 | 6.33 ± 0.14 | P120 | 6.92 ± 0.38 | P120 | 7.83 ± 0.29 | P120 | 7.83 ± 0.29 | P120 | 7.00 ± 0.00 | P120 | 7.17 ± 0.52 |
| P130 | 6.58 ± 0.14 | P130 | 6.42 ± 0.29 | P130 | 8.25 ± 0.25 | P130 | 7.08 ± 0.38 | P130 | 7.08 ± 0.29 | P130 | 6.83 ± 0.38 |
| P140 | 7.42 ± 0.14 | P140 | 7.67 ± 0.29 | P140 | 8.67 ± 0.29 | P140 | 7.28 ± 0.29 | P140 | 7.33 ± 0.14 | P140 | 7.25 ± 0.25 |
| P150 | 8.30 ± 0.14 | P150 | 8.50 ± 0.00 | P150 | 8.50 ± 0.25 | P150 | 7.08 ± 0.14 | P150 | 7.17 ± 0.14 | P150 | 7.42 ± 0.52 |
| P160 | 7.33 ± 0.38 | P160 | 7.08 ± 0.29 | P160 | 8.25 ± 0.66 | P160 | 8.33 ± 0.14 | P160 | 7.92 ± 0.14 | P160 | 7.83 ± 0.14 |
| P170 | 7.25 ± 0.25 | P170 | 7.50 ± 0.25 | P170 | 8.00 ± 0.00 | P170 | 7.67 ± 0.14 | P170 | 7.00 ± 0.25 | P170 | 6.83 ± 0.29 |
| P180 | 7.00 ± 0.00 | P180 | 7.83 ± 0.14 | P180 | 8.25 ± 0.25 | P180 | 7.92 ± 0.14 | P180 | 7.25 ± 0.25 | P180 | 6.92 ± 0.14 |
| Group | Inoculum | Route | No. of Pigs | Mortality Rate * [% (No/Total)] | Severe Diarrhea Rate [% (No/Total)] | Clinical Symptoms | Virus Shedding | Peak Fecal Virus Shedding Titer [log10 Copies/μL], dpi |
|---|---|---|---|---|---|---|---|---|
| 1 | Positive control | Oral | 3 | 100 (3/3) | 100 (3/3) | NA ** (>4) | Started at 1 dpi | 5.05 ± 0.24, 2 |
| 2 | Negative control | 3 | 0 (0/3) | 0 (0/3) | No diarrhea | N/D *** | NA | |
| 3 | CKT-7 T15 | 3 | 0 (0/3) | 0 (0/3) | Mild | Limited | NA | |
| 4 | CKT-7 T15N | 3 | 0 (0/3) | 0 (0/3) | Mild | Started at 4 dpi | 2.48 ± 0.13, 5 | |
| 5 | CKT-7 N | 3 | 0 (0/3) | 0 (0/3) | No diarrhea | N/D | NA | |
| 6 | CKT-7 NF | 3 | 0 (0/3) | 0 (0/3) | Mild | Limited | NA | |
| 7 | CKT-7 F | 3 | 0 (0/3) | 0 (0/3) | No diarrhea | N/D | NA | |
| 8 | CKT-7 X | 3 | 0 (0/3) | 0 (0/3) | No diarrhea | Started at 2 dpi | 2.68 ± 0.49, 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-M.; Moon, S.-H.; Kim, S.-C.; Cho, H.-S.; Tark, D. Development of Effective PEDV Vaccine Candidates Based on Viral Culture and Protease Activity. Vaccines 2023, 11, 923. https://doi.org/10.3390/vaccines11050923
Kim D-M, Moon S-H, Kim S-C, Cho H-S, Tark D. Development of Effective PEDV Vaccine Candidates Based on Viral Culture and Protease Activity. Vaccines. 2023; 11(5):923. https://doi.org/10.3390/vaccines11050923
Chicago/Turabian StyleKim, Dae-Min, Sung-Hyun Moon, Seung-Chai Kim, Ho-Seong Cho, and Dongseob Tark. 2023. "Development of Effective PEDV Vaccine Candidates Based on Viral Culture and Protease Activity" Vaccines 11, no. 5: 923. https://doi.org/10.3390/vaccines11050923
APA StyleKim, D.-M., Moon, S.-H., Kim, S.-C., Cho, H.-S., & Tark, D. (2023). Development of Effective PEDV Vaccine Candidates Based on Viral Culture and Protease Activity. Vaccines, 11(5), 923. https://doi.org/10.3390/vaccines11050923






