Enhancement of Vaccine-Induced T-Cell Responses by PD-L1 Blockade in Calves
Abstract
1. Introduction
2. Materials and Methods
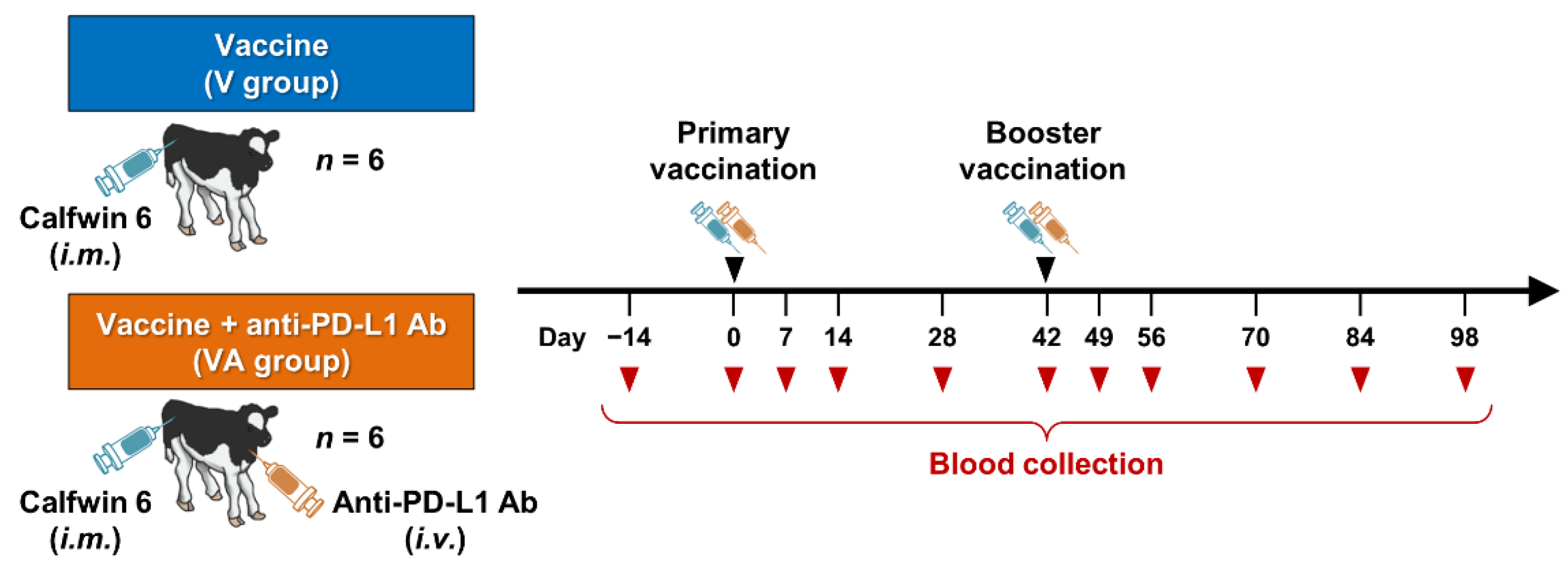
2.1. Animals, Vaccination, and Sample Collection
2.2. Flow Cytometric Analysis of PD-1 Expression
2.3. PBMC Cultivation Assay
2.4. Statistical Analyses
3. Results
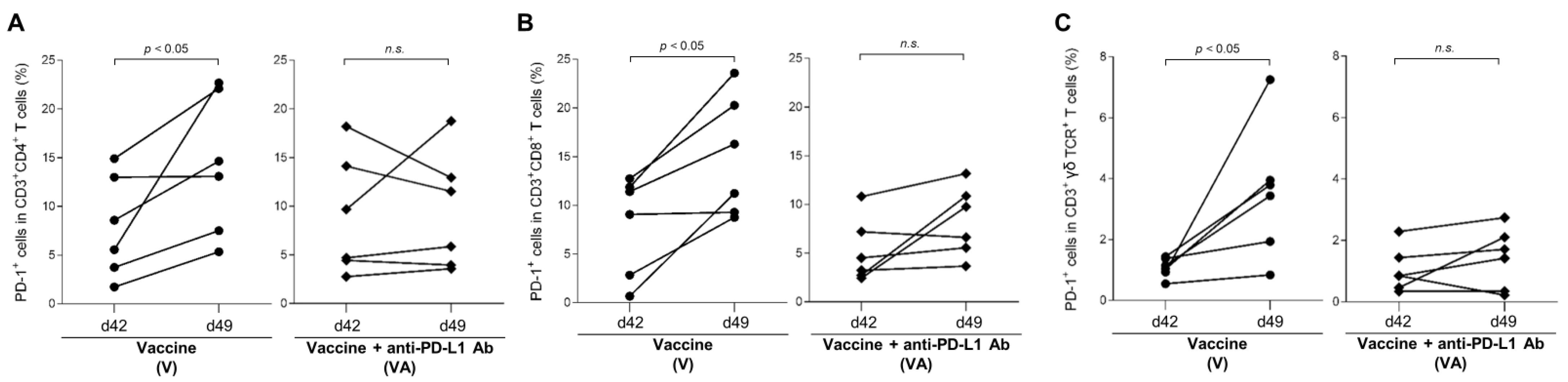
3.1. Kinetics of PD-1 Expression by T Cells in Vaccinated Calves
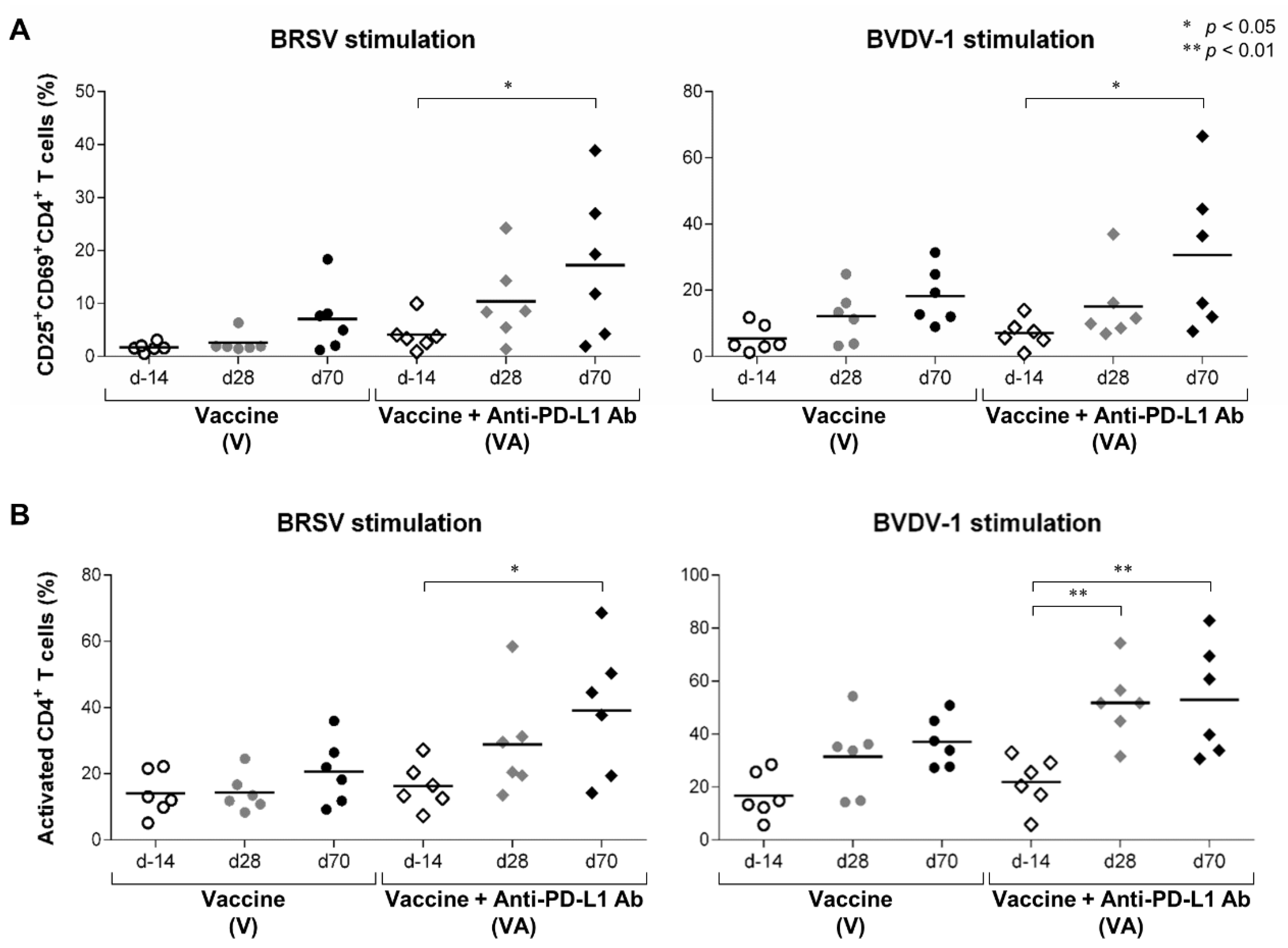
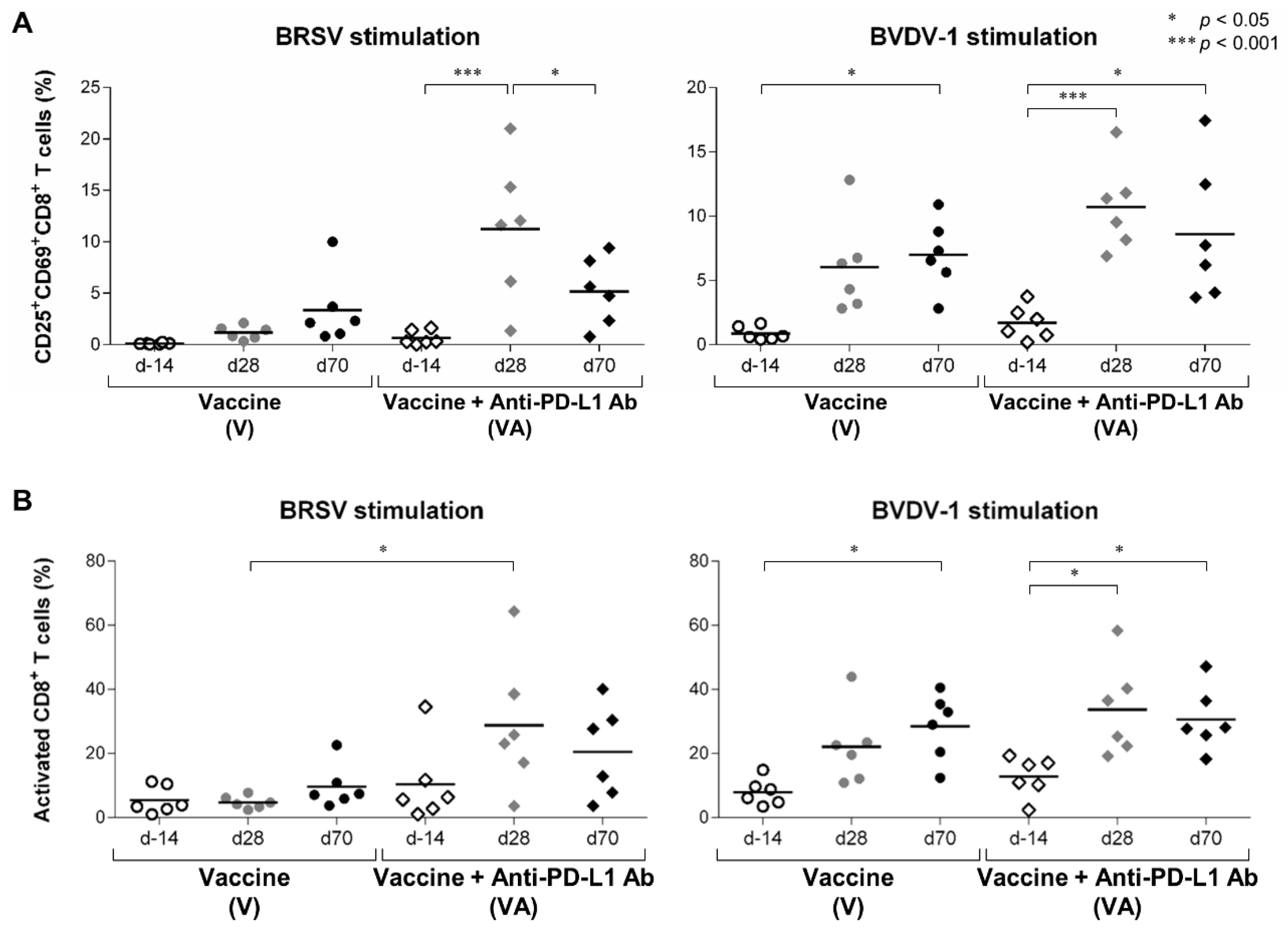
3.2. Activation of T Cells by the Combination of Vaccination and PD-L1 Blockade in Calves
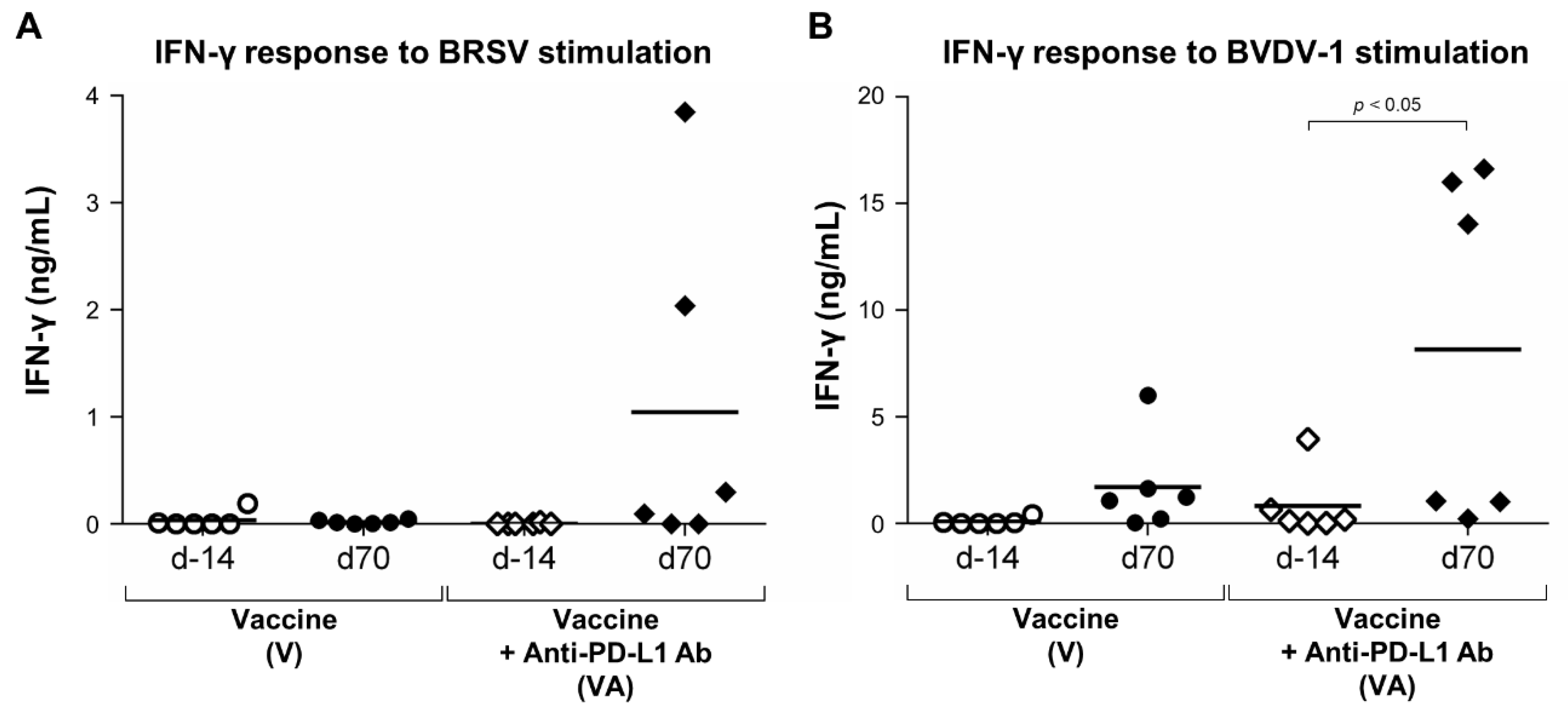
3.3. Enhancement of IFN-γ Response by the Combination of Vaccination and PD-L1 Blockade in Calves
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seder, R.A.; Hill, A.V.S. Vaccines against Intracellular Infections Requiring Cellular Immunity. Nature 2000, 406, 793–798. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T Cell Exhaustion by Multiple Inhibitory Receptors during Chronic Viral Infection. Nat. Immunol. 2009, 10, 29–37. [Google Scholar] [CrossRef]
- Khaitan, A.; Unutmaz, D. Revisiting Immune Exhaustion during HIV Infection. Curr. HIV/AIDS Rep. 2011, 8, 4–11. [Google Scholar] [CrossRef]
- Wherry, E.J. T Cell Exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Ikebuchi, R.; Konnai, S.; Shirai, T.; Sunden, Y.; Murata, S.; Onuma, M.; Ohashi, K. Increase of Cells Expressing PD-L1 in Bovine Leukemia Virus Infection and Enhancement of Anti-Viral Immune Responses in Vitro via PD-L1 Blockade. Vet. Res. 2011, 42, 103. [Google Scholar] [CrossRef] [PubMed]
- Ikebuchi, R.; Konnai, S.; Okagawa, T.; Yokoyama, K.; Nakajima, C.; Suzuki, Y.; Murata, S.; Ohashi, K. Blockade of Bovine PD-1 Increases T Cell Function and Inhibits Bovine Leukemia Virus Expression in B Cells in Vitro. Vet. Res. 2013, 44, 59. [Google Scholar] [CrossRef] [PubMed]
- Okagawa, T.; Konnai, S.; Nishimori, A.; Ikebuchi, R.; Mizorogi, S.; Nagata, R.; Kawaji, S.; Tanaka, S.; Kagawa, Y.; Murata, S.; et al. Bovine Immunoinhibitory Receptors Contribute to the Suppression of Mycobacterium avium subsp. paratuberculosis-Specific T-Cell Responses. Infect. Immun. 2016, 84, 77–89. [Google Scholar] [CrossRef]
- Okagawa, T.; Konnai, S.; Deringer, J.R.; Ueti, M.W.; Scoles, G.A.; Murata, S.; Ohashi, K.; Brown, W.C. Cooperation of PD-1 and LAG-3 Contributes to T-Cell Exhaustion in Anaplasma marginale-Infected Cattle. Infect. Immun. 2016, 84, 2779–2790. [Google Scholar] [CrossRef]
- Ikebuchi, R.; Konnai, S.; Okagawa, T.; Yokoyama, K.; Nakajima, C.; Suzuki, Y.; Murata, S.; Ohashi, K. Influence of PD-L1 Cross-Linking on Cell Death in PD-L1-Expressing Cell Lines and Bovine Lymphocytes. Immunology 2014, 142, 551–561. [Google Scholar] [CrossRef]
- Nishimori, A.; Konnai, S.; Okagawa, T.; Maekawa, N.; Ikebuchi, R.; Goto, S.; Sajiki, Y.; Suzuki, Y.; Kohara, J.; Ogasawara, S.; et al. In Vitro and in Vivo Antivirus Activity of an Anti-Programmed Death-Ligand 1 (PD-L1) Rat-Bovine Chimeric Antibody against Bovine Leukemia Virus Infection. PLoS ONE 2017, 12, e0174916. [Google Scholar] [CrossRef]
- Goto, S.; Konnai, S.; Hirano, Y.; Kohara, J.; Okagawa, T.; Maekawa, N.; Sajiki, Y.; Watari, K.; Minato, E.; Kobayashi, A.; et al. Clinical Efficacy of the Combined Treatment of Anti-PD-L1 Rat-Bovine Chimeric Antibody with a Cox-2 Inhibitor in Calves Infected with Mycoplasma bovis. Jpn. J. Vet. Res. 2020, 68, 77–90. [Google Scholar] [CrossRef]
- Sajiki, Y.; Konnai, S.; Nagata, R.; Kawaji, S.; Nakamura, H.; Fujisawa, S.; Okagawa, T.; Maekawa, N.; Kato, Y.; Suzuki, Y.; et al. The Enhancement of Th1 Immune Response by Anti-PD-L1 Antibody in Cattle Infected with Mycobacterium avium subsp. paratuberculosis. J. Vet. Med. Sci. 2021, 83, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Kurachi, M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-J.; Mueller, S.N.; Wherry, E.J.; Barber, D.L.; Aubert, R.D.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Enhancing Therapeutic Vaccination by Blocking PD-1-Mediated Inhibitory Signals during Chronic Infection. J. Exp. Med. 2008, 205, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Park, S.; Nam, H.J.; Choi, D.; Sung, Y. Enhancement of Vaccine-Induced Primary and Memory CD8(+) T-Cell Responses by Soluble PD-1. J. Immunother. 2011, 34, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Finnefrock, A.C.; Tang, A.; Li, F.; Freed, D.C.; Feng, M.; Cox, K.S.; Sykes, K.J.; Guare, J.P.; Miller, M.D.; Olsen, D.B.; et al. PD-1 Blockade in Rhesus Macaques: Impact on Chronic Infection and Prophylactic Vaccination. J. Immunol. 2009, 182, 980–987. [Google Scholar] [CrossRef]
- Bowyer, G.; Rampling, T.; Powlson, J.; Morter, R.; Wright, D.; Hill, A.V.S.; Ewer, K.J. Activation-Induce Markers Detect Vaccine-Specific CD4+ T Cell Responses Not Measured by Assays Conventionally Used in Clinical Trials. Vaccines 2018, 6, 50. [Google Scholar] [CrossRef]
- Baldwin, C.L.; Telfer, J.C. The Bovine Model for Elucidating the Role of Γδ T Cells in Controlling Infectious Diseases of Importance to Cattle and Humans. Mol. Immunol. 2015, 66, 34–47. [Google Scholar] [CrossRef]
- Naiman, B.M.; Alt, D.; Bolin, C.A.; Zuerner, R.; Baldwin, C.L. Protective Killed Leptospira borgpetersenii Vaccine Induces Potent Th1 Immunity Comprising Responses by CD4 and γδ T Lymphocytes. Infect. Immun. 2001, 69, 7550–7558. [Google Scholar] [CrossRef]
- Naiman, B.M.; Blumerman, S.; Alt, D.; Bolin, C.A.; Brown, R.; Zuerner, R.; Baldwin, C.L. Evaluation of Type 1 Immune Response in Naïve and Vaccinated Animals Following Challenge with Leptospira borgpetersenii Serovar Hardjo: Involvement of WC1+ γδ and CD4 T Cells. Infect. Immun. 2002, 70, 6147–6157. [Google Scholar] [CrossRef]
- Guerra-Maupome, M.; McGill, J.L. Characterization of Local and Circulating Bovine γδ T Cell Responses to Respiratory BCG Vaccination. Sci. Rep. 2019, 9, 15996. [Google Scholar] [CrossRef]
- Ames, T.R. Dairy Calf Pneumonia: The Disease and Its Impact. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 379–391. [Google Scholar] [CrossRef]
- Valdez, J.R.; Gonzalez-Avalos, R.; Avila-Cisneros, R.; Peña-Revuelta, B.; Reyes-Romero, A. Economic Impact of Mortality and Morbidity from Diseases in Dairy Calves. Abanico. Vet. 2019, 9, 209. [Google Scholar]
- Fulton, R.W. Viral Diseases of the Bovine Respiratory Tract. In Food Animal Practice; Elsevier: Amsterdam, The Netherlands, 2009; pp. 171–191. [Google Scholar]
- Gershwin, L.J. Immunology of Bovine Respiratory Syncytial Virus Infection of Cattle. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F. Immunology of BVDV Vaccines. Biologicals 2013, 41, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Griebel, P.J. BVDV Vaccination in North America: Risks versus Benefits. Anim. Health Res. Rev. 2015, 16, 27–32. [Google Scholar] [CrossRef]
- Kolb, E.A.; Buterbaugh, R.E.; Rinehart, C.L.; Ensley, D.; Perry, G.A.; Abdelsalam, K.W.; Chase, C.C.L. Protection against Bovine Respiratory Syncytial Virus in Calves Vaccinated with Adjuvanted Modified Live Vaccine Administered in the Face of Maternal Antibody. Vaccine 2020, 38, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.A.; Newcomer, B.; Passler, T.; Chamorro, M.F. Efficacy of Bovine Respiratory Syncytial Virus Vaccines to Reduce Morbidity and Mortality in Calves within Experimental Infection Models: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 2022, 9, 906636. [Google Scholar] [CrossRef]
- Rodning, S.P.; Marley, M.S.D.; Zhang, Y.; Eason, A.B.; Nunley, C.L.; Walz, P.H.; Riddell, K.P.; Galik, P.K.; Brodersen, B.W.; Givens, M.D. Comparison of Three Commercial Vaccines for Preventing Persistent Infection with Bovine Viral Diarrhea Virus. Theriogenology 2010, 73, 1154–1163. [Google Scholar] [CrossRef]
- Fulton, R.W.; Cook, B.J.; Payton, M.E.; Burge, L.J.; Step, D.L. Immune Response to Bovine Viral Diarrhea Virus (BVDV) Vaccines Detecting Antibodies to BVDV Subtypes 1a, 1b, 2a, and 2c. Vaccine 2020, 38, 4032–4037. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A Canine Chimeric Monoclonal Antibody Targeting PD-L1 and Its Clinical Efficacy in Canine Oral Malignant Melanoma or Undifferentiated Sarcoma. Sci. Rep. 2017, 7, 8951. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Nishimura, M.; Kagawa, Y.; Takagi, S.; Hosoya, K.; Ohta, H. PD-L1 Immunohistochemistry for Canine Cancers and Clinical Benefit of Anti-PD-L1 Antibody in Dogs with Pulmonary Metastatic Oral Malignant Melanoma. npj Precis. Oncol. 2021, 5, 10. [Google Scholar] [CrossRef]
- Okagawa, T.; Konnai, S.; Nishimori, A.; Maekawa, N.; Ikebuchi, R.; Goto, S.; Nakajima, C.; Kohara, J.; Ogasawara, S.; Kato, Y.; et al. Anti-Bovine Programmed Death-1 Rat-Bovine Chimeric Antibody for Immunotherapy of Bovine Leukemia Virus Infection in Cattle. Front. Immunol. 2017, 8, 650. [Google Scholar] [CrossRef]
- Sajiki, Y.; Konnai, S.; Okagawa, T.; Nishimori, A.; Maekawa, N.; Goto, S.; Watari, K.; Minato, E.; Kobayashi, A.; Kohara, J.; et al. Prostaglandin E2–Induced Immune Exhaustion and Enhancement of Antiviral Effects by Anti–PD-L1 Antibody Combined with COX-2 Inhibitor in Bovine Leukemia Virus Infection. J. Immunol. 2019, 203, 1313–1324. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer Vaccines as Promising Immuno-Therapeutics: Platforms and Current Progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Reiss, S.; Baxter, A.E.; Cirelli, K.M.; Dan, J.M.; Morou, A.; Daigneault, A.; Brassard, N.; Silvestri, G.; Routy, J.P.; Havenar-Daughton, C.; et al. Comparative Analysis of Activation Induced Marker (AIM) Assays for Sensitive Identification of Antigen-Specific CD4 T Cells. PLoS ONE 2017, 12, e0186998. [Google Scholar] [CrossRef]
- Taylor, G.; Thomas, L.H.; Wyld, S.G.; Furze, J.; Sopp, P.; Howard, C.J. Role of T-Lymphocyte Subsets in Recovery from Respiratory Syncytial Virus Infection in Calves. J. Virol. 1995, 69, 6658–6664. [Google Scholar] [CrossRef]
- Hussell, T.; Openshaw, P.J.M. Intracellular IFN-γ Expression in Natural Killer Cells Precedes Lung CD8+ T Cell Recruitment during Respiratory Syncytial Virus Infection. J. Gen. Virol. 1998, 79, 2593–2601. [Google Scholar] [CrossRef]
- Woolums, A.R.; Singer, R.S.; Boyle, G.A.; Gershwin, L.J. Interferon Gamma Production during Bovine Respiratory Syncytial Virus (BRSV) Infection Is Diminished in Calves Vaccinated with Formalin-Inactivated BRSV. Vaccine 1999, 17, 1293–1297. [Google Scholar] [CrossRef]
- Durbin, J.E.; Johnson, T.R.; Durbin, R.K.; Mertz, S.E.; Morotti, R.A.; Peebles, R.S.; Graham, B.S. The Role of IFN in Respiratory Syncytial Virus Pathogenesis. J. Immunol. 2002, 168, 2944–2952. [Google Scholar] [CrossRef]
- Ostler, T.; Davidson, W.; Ehl, S. Virus Clearance and Immunopathology by CD8+ T Cells during Infection with Respiratory Syncytial Virus Are Mediated by IFN-γ. Eur. J. Immunol. 2002, 32, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Wolf, G.; Pichler, J.; Wolfmeyer, A.; Kaaden, O.R. Cytotoxic T-Lymphocyte Responses in Cattle Infected with Bovine Viral Diarrhea Virus. Vet. Microbiol. 1997, 58, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Collen, T.; Morrison, W.I. CD4(+) T-Cell Responses to Bovine Viral Diarrhoea Virus in Cattle. Virus Res. 2000, 67, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Endsley, J.J.; Roth, J.A.; Ridpath, J.; Neill, J. Maternal Antibody Blocks Humoral but Not T Cell Responses to BVDV. Biologicals 2003, 31, 123–125. [Google Scholar] [CrossRef]
- Endsley, J.J.; Ridpath, J.F.; Neill, J.D.; Sandbulte, M.R.; Roth, J.A. Induction of T Lymphocytes Specific for Bovine Viral Diarrhea Virus in Calves with Maternal Antibody. Viral Immunol. 2004, 17, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Ikebuchi, R.; Okagawa, T.; Adachi, M.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; Murata, S.; et al. Expression of PD-L1 on Canine Tumor Cells and Enhancement of IFN-γ Production from Tumor-Infiltrating Cells by PD-L1 Blockade. PLoS ONE 2014, 9, e98415. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; et al. Immunohistochemical Analysis of PD-L1 Expression in Canine Malignant Cancers and PD-1 Expression on Lymphocytes in Canine Oral Melanoma. PLoS ONE 2016, 11, e0157176. [Google Scholar] [CrossRef]
- Nascimento, C.; Urbano, A.C.; Gameiro, A.; Ferreira, J.; Correia, J. Serum PD-1 / PD-L1 Levels, Tumor Expression and PD-L1 Somatic Mutations in HER2-Positive and Triple Negative Normal-Like Feline Mammary Carcinoma Subtypes. Cancers 2020, 12, 1386. [Google Scholar] [CrossRef]
- Maekawa, N.; Konnai, S.; Asano, Y.; Otsuka, T.; Aoki, E.; Takeuchi, H.; Kato, Y.; Kaneko, M.K.; Yamada, S.; Kagawa, Y.; et al. Molecular Characterization of Feline Immune Checkpoint Molecules and Establishment of PD-L1 Immunohistochemistry for Feline Tumors. PLoS ONE 2023, 18, e0281143. [Google Scholar] [CrossRef]
- Ganbaatar, O.; Konnai, S.; Okagawa, T.; Nojima, Y.; Maekawa, N.; Minato, E.; Kobayashi, A.; Ando, R.; Sasaki, N.; Miyakoshi, D.; et al. PD-L1 Expression in Equine Malignant Melanoma and Functional Effects of PD-L1 Blockade. PLoS ONE 2020, 15, e0234218. [Google Scholar] [CrossRef]
- Jeon, D.-H.; Oh, K.; Oh, B.C.; Nam, D.H.; Kim, C.H.; Park, H.-B.; Cho, J.; Lee, J.R.; Lee, D.-S.; Lee, G. Porcine PD-L1: Cloning, Characterization, and Implications during Xenotransplantation. Xenotransplantation 2007, 14, 236–242. [Google Scholar] [CrossRef]
- Ganbaatar, O.; Konnai, S.; Okagawa, T.; Nojima, Y.; Maekawa, N.; Ichikawa, Y.; Kobayashi, A.; Shibahara, T.; Yanagawa, Y.; Higuchi, H.; et al. Programmed Death-ligand 1 Expression in Swine Chronic Infections and Enhancement of Interleukin-2 Production via Programmed Death-1/Programmed Death-ligand 1 Blockade. Immun. Inflamm. Dis. 2021, 9, 1573–1583. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okagawa, T.; Konnai, S.; Nakamura, H.; Ganbaatar, O.; Sajiki, Y.; Watari, K.; Noda, H.; Honma, M.; Kato, Y.; Suzuki, Y.; et al. Enhancement of Vaccine-Induced T-Cell Responses by PD-L1 Blockade in Calves. Vaccines 2023, 11, 559. https://doi.org/10.3390/vaccines11030559
Okagawa T, Konnai S, Nakamura H, Ganbaatar O, Sajiki Y, Watari K, Noda H, Honma M, Kato Y, Suzuki Y, et al. Enhancement of Vaccine-Induced T-Cell Responses by PD-L1 Blockade in Calves. Vaccines. 2023; 11(3):559. https://doi.org/10.3390/vaccines11030559
Chicago/Turabian StyleOkagawa, Tomohiro, Satoru Konnai, Hayato Nakamura, Otgontuya Ganbaatar, Yamato Sajiki, Kei Watari, Haruka Noda, Mitsuru Honma, Yukinari Kato, Yasuhiko Suzuki, and et al. 2023. "Enhancement of Vaccine-Induced T-Cell Responses by PD-L1 Blockade in Calves" Vaccines 11, no. 3: 559. https://doi.org/10.3390/vaccines11030559
APA StyleOkagawa, T., Konnai, S., Nakamura, H., Ganbaatar, O., Sajiki, Y., Watari, K., Noda, H., Honma, M., Kato, Y., Suzuki, Y., Maekawa, N., Murata, S., & Ohashi, K. (2023). Enhancement of Vaccine-Induced T-Cell Responses by PD-L1 Blockade in Calves. Vaccines, 11(3), 559. https://doi.org/10.3390/vaccines11030559






