Return on Investment (ROI) of Three Vaccination Programmes in Italy: HPV at 12 Years, Herpes Zoster in Adults, and Influenza in the Elderly
Abstract
1. Introduction
2. Methods
- the universal (male and female) HPV vaccination with nono-valent vaccine for 12-year-olds, actively offered since 2017 in all regions;
- the herpes zoster (HZ) vaccination according to the current indications of the PNPV 2017–2019: the cohort of subjects = 65 years of age, and subjects aged >50 years at increased risk (diabetes, chronic obstructive pulmonary disease (COPD), heart failure, subjects on immunosuppressive therapy);
- annual influenza vaccination in persons ≥ 65 years of age.
2.1. The HPV Model
2.1.1. Epidemiological and Vaccine Efficacy Data
| HPV-Related Condition | Efficacy HPV9 from RCP | HPV9 Correlation (B) | Efficacy (Calculated) | |
|---|---|---|---|---|
| (A) | HPV Fraction Compared to Total Disease (De Martel) [21] | HPV9 Fraction (Harwig) [22] | (A × B) | |
| CIN2+ | 97.1% (83.5–99.9) | - | 82.3% | 79.9% |
| Cervical cancer (CC) | 97.4% (85.0–99.9) | 100% | 89.1% [87.7–90.4] | 86.8% |
| 89.1% | ||||
| NIVs | 100% (55.5–100) | - | 94.4% [91.0–96.9] | 94.4% |
| Vaginal cancer | 97.4% (85.0–99.9) | 78% | 87.1% [78.8–92.6] | 66.2% |
| 67.9% | ||||
| Vulvar cancer | 97.4% (85.0–99.9) | 48% | 94.3% [89.1–97.5] | 44% |
| 45.3% | ||||
| Penile cancer | 100% (−52.1–100.0) ~50% | 51% | 90.7% [84.1–95.3] | 23.15% |
| 46.3% | ||||
| Anal cancer | 74.9% (8.8–95.4) | 100% | 94.4% [89.2–97.5] | 70.7% |
| 94.4% | ||||
| Oropharyngeal cancer | 77.5% (39.6–93.3) | 24% | 97.5% [93.7–99.3] | 18.11% |
| 23.4% | ||||
| Genital condylomas | 99% (96.2–99.9) | - | 90% | 89.1% |
2.1.2. Cost Parameters
2.2. The Herpes Zoster Model
- For the live attenuated vaccine (ZVL), a non-vaccination scenario was compared with vaccination of the cohort of subjects = 65 years of age at current coverage rates (according to OSMeD data and some regional reports it was estimated to be 20% of the routine cohort) and target coverage (the only cohort for which a target of 50% is set in the PNPV 2017–2019) and of the cohort of subjects aged 50–64 years with risk conditions (current estimated coverage: 5%; assumed target coverage: 20%);
- For the RZV, in addition to the previous two immunization groups, the cohort of immunocompromised individuals between 18 and 49 years of age with current coverage of 0% and an assumed target coverage of 20% was considered.
2.2.1. Epidemiological and Vaccine Efficacy Parameters
2.2.2. Cost Parameters
2.3. The Influenza Model
2.3.1. Epidemiological and Vaccine Efficacy Data
2.3.2. Cost Parameters
3. Results
3.1. HPV Model Results
3.2. HZ Model Results
3.3. Influenza Model Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloom, D.E. The value of vaccination. Adv. Exp. Med. Biol. 2011, 697, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.; Chevat, C.; Lothgren, M. Do we fully understand the economic value of vaccines? Vaccine 2007, 25, 5945–5957. [Google Scholar] [CrossRef] [PubMed]
- Jit, M.; Hutubessy, R.; Png, M.E.; Sundaram, N.; Audimulam, J.; Salim, S.; Yoong, J. The broader economic impact of vaccination: Reviewing and appraising the strength of evidence. BMC Med. 2015, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Luyten, J.; Beutels, P. The Social Value of Vaccination Programs: Beyond Cost-Effectiveness. Health Aff. 2016, 35, 212–218. [Google Scholar] [CrossRef] [PubMed]
- GAVI.org. New Evidence Shows Investments in Vaccination Produce Even Greater Returns Than Previously Thought. Available online: https://www.gavi.org/vaccineswork/new-evidence-shows-investments-vaccination-produce-even-greater-returns-previously (accessed on 1 December 2021).
- IVAC. The Value of Vaccine Programmes. Available online: https://www.jhsph.edu/ivac/wp-content/uploads/2021/02/FINAL-DRAFT-Vaccine-ROI-Policy-Brief_feb-2021.pdf (accessed on 1 December 2021).
- DOvE. The Value of Vaccine Programmes. Available online: Vaccineroi.org (accessed on 1 December 2021).
- Masters, R.; Anwar, E.; Collins, B.; Cookson, R.; Capewell, S. Return on investment of public health interventions: A systematic review. J. Epidemiol. Community Health 2017, 71, 827–834. [Google Scholar] [CrossRef]
- Brassel, S.; Steuten, L. The Broader Value of Vaccines—The Return on Investment from a Governmental Perspective. OHE Consulting Report, London: Office of Health Economics. 2020. Available online: https://www.ohe.org/publications/broader-value-vaccines-return-investment-governmentalperspective (accessed on 1 December 2021).
- Resch, S.; Menzies, N.; Portnoy, A.; Clarke-Deelder, E.; O’Keeffe, L.; Suharlim, C.; Brenzel, L. How to Cost Immunisation Programmes: A Practical Guide on Primary Data Collection and Analysis; Harvard T.H. Chan: Cambridge, MA, USA, 2020. [Google Scholar]
- Demography in Figures. Available online: https://demo.istat.it/index.php (accessed on 1 December 2021).
- National Vaccination Prevention Plan 2017–2019. Available online: https://www.salute.gov.it/portale/vaccinazioni/dettaglioContenutiVaccinazioni.jsp?lingua=italiano&id=4828&area=vaccinazioni&menu=vuoto (accessed on 1 December 2021).
- Koutsky, L. Epidemiology of genital human papillomavirus infection. Am. J. Med. 1997, 102, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Baseman, J.G.; Koutsky, L.A. The epidemiology of human papillomavirus infections. J. Clin. Virol. 2005, 32 (Suppl. 1), S16–S24. [Google Scholar] [CrossRef]
- Marcellusi, A.; Mennini, F.S.; Sciattella, P.; Favato, G. Human papillomavirus in Italy: Retrospective cohort analysis and preliminary vaccination effect from real-world data. Eur. J. Health Econ. 2021, 22, 1371–1379. [Google Scholar] [CrossRef]
- Papilloma Virus (HPV) Vaccination—Vaccination Coverage as of 31 December 2019. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=27 (accessed on 1 December 2021).
- Mortality Tables. Available online: http://dati.istat.it/index.aspx?queryid=7283 (accessed on 1 December 2021).
- RCP EMA Gardasil. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/gardasil (accessed on 1 December 2021).
- RCP EMA Gardasil9. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/gardasil-9 (accessed on 1 December 2021).
- FDA. Label Database for Gardasil9. 21 August 2020 Update. Available online: https://www.fda.gov/media/90064/download (accessed on 1 December 2021).
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Hartwig, S.; St Guily, J.L.; Dominiak-Felden, G.; Alemany, L.; de Sanjosé, S. Estimation of the overall burden of cancers, precancerous lesions, and genital warts attributable to 9-valent HPV vaccine types in women and men in Europe. Infect. Agent Cancer 2017, 12, 19. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale. Remuneration of Acute Hospital Care, Rehabilitation and Post-Acute Long-Term Hospital Care and Outpatient Specialist Care. GU Serie Generale n. 23 del 28-01-2013—Suppl. Ordinario n. 8. Official Document of Ministry of Health. Available online: https://www.gazzettaufficiale.it (accessed on 10 March 2022).
- Baio, G.; Capone, A.; Marcellusi, A.; Mennini, F.S.; Favato, G. Economic burden of human papillomavirus-related diseases in Italy. PLoS ONE 2012, 7, e49699, Erratum in PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Ricciardi, A.; Largeron, N.; Giorgi Rossi, P.; Raffaele, M.; Cohet, C.; Federici, A.; Palazzo, F. Incidence of invasive cervical cancer and direct costs associated with its management in Italy. Tumori 2009, 95, 146–152. [Google Scholar] [CrossRef]
- FARMADATI. Available online: https://www.farmadati.it/ (accessed on 1 December 2021).
- Abstract Presented at ISPOR Italy Rome Chapter, Mennini et al., ‘HPV9-Related Diseases: The Economic Burden on the Social Security System in Italy’. 2018. Available online: https://tools.ispor.org/research_pdfs/60/pdffiles/PIN11.pdf (accessed on 1 December 2021).
- Boutry, C.; Hastie, A.; Diez-Domingo, J.; Tinoco, J.C.; Yu, C.-J.; Andrews, C.; Beytout, J.; Caso, C.; Cheng, H.-S.; Cheong, H.J.; et al. The Adjuvanted Recombinant Zoster Vaccine Confers Long-term Protection Against Herpes Zoster: Interim Results of an Extension Study of the Pivotal Phase III Clinical Trials (ZOE-50 and ZOE-70). Clin. Infect Dis. 2021, 20, ciab629. [Google Scholar] [CrossRef]
- ISTAT MULTISCOPE. Available online: https://www.istat.it/it/archivio/129916 (accessed on 1 December 2021).
- SER. Epidemiological Report on Chronic Diseases in Veneto Data Year 2018. Available online: https://www.ser-veneto.it/public/Rapporto_epidemiologico_malattie_croniche_Veneto_2018.pdf (accessed on 1 December 2021).
- Schmader, K.E.; Levin, M.J.; Gnann, J.W.; McNeil, S.A.; Vesikari, T.; Betts, R.F.; Keay, S.; Stek, J.E.; Bundick, N.D.; Su, S.-C.; et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin. Infect Dis. 2012, 54, 922–928. [Google Scholar] [CrossRef]
- Willer, D.O.; Oostvogels, L.; Cunningham, A.L.; Gervais, P.; Gorfinkel, I.; Kim, J.H.; Talarico, C.; Wascotte, V.; Zahaf, T.; Colindres, R.; et al. Efficacy of the adjuvanted recombinant zoster vaccine (RZV) by sex, geographic region, and geographic ancestry/ethnicity: A post-hoc analysis of the ZOE-50 and ZOE-70 randomised trials. Vaccine 2019, 37, 6262–6267. [Google Scholar] [CrossRef]
- Alicino, C.; Trucchi, C.; Paganino, C.; Barberis, I.; Boccalini, S.; Martinelli, D.; Pellizzari, B.; Bechini, A.; Orsi, A.; Bonanni, P.; et al. Incidence of herpes zoster and post-herpetic neuralgia in Italy: Results from a 3-years population-based study. Hum. Vaccines Immunother. 2017, 13, 399–404. [Google Scholar] [CrossRef]
- Panatto, D.; Bragazzi, N.; Rizzitelli, E.; Bonanni, P.; Boccalini, S.; Icardi, G.; Gasparini, R.; Amicizia, D. Evaluation of the economic burden of Herpes Zoster (HZ) infection. Hum. Vaccines Immunother. 2015, 11, 245–262. [Google Scholar] [CrossRef]
- Ruggeri, M.; Di Brino, E.; Cicchetti, A. Estimating the fiscal impact of three vaccination strategies in Italy. Int. J. Technol. Assess. Health Care 2020, 36, 133–138. [Google Scholar] [CrossRef]
- Coretti, S.; Codella, C.; Romano, F.; Ruggeri, M.; Cicchetti, A. Cost-effectiveness analysis of Herpes Zoster Vaccination in italian elderly persons. Int. J. Technol. Assess. Health Care 2016, 32, 233–240. [Google Scholar] [CrossRef]
- La Sorveglianza Passi d’Argento. Available online: https://www.epicentro.iss.it/passi-argento (accessed on 1 December 2021).
- Capri, S.; Barbieri, M.; de Waure, C.; Boccalini, S.; Panatto, D. Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly population. Hum. Vaccines Immunother. 2018, 14, 1331–1341. [Google Scholar] [CrossRef]
- Meier, C.R.; Napalkov, P.N.; Wegmüller, Y.; Jefferson, T.; Jick, H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur. J. Clin. Microbiol. Infect Dis. 2000, 19, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, S. Pharmacoeconomic evaluation of the MF59--adjuvanted influenza vaccine in the elderly population in Italy. J. Prev. Med. Hyg. 2011, 52, 1–8. [Google Scholar] [PubMed]
- Nomenclatore Tariffario Delle Prestazioni Aggiuntive, Accordo Collettivo Nazionale Medici Generici, 23 Marzo 2005. Available online: http://www.sisac.info/aree/www.sisac.info/resources/MEDICINA_GENERALE/ACN_testo_integrato.pdf (accessed on 1 December 2021).
- Carrat, F.; Vergu, E.; Ferguson, N.M.; Lemaitre, M.; Cauchemez, S.; Leach, S.; Valleron, A.-J. Time lines of infection and disease in human influenza: A review of volunteer challenge studies. Am. J. Epidemiol. 2008, 167, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Kuehnel, U.M.; Colombo, G.L.; Esposito, S.; Principi, N. Cost-effectiveness of adjuvanted influenza vaccination of healthy children 6 to 60 months of age. Hum. Vaccines 2007, 3, 14–22. [Google Scholar] [CrossRef]
- Sessa, A.; Costa, B.; Bamfi, F.; Bettoncelli, G.; D’Ambrosio, G. The incidence, natural history and associated outcomes of influenza-like illness and clinical influenza in Italy. Fam. Pract. 2001, 18, 629–634. [Google Scholar] [CrossRef]
- Esposito, S.; Cantarutti, L.; Molteni, C.G.; Daleno, C.; Scala, A.; Tagliabue, C.; Pelucchi, C.; Giaquinto, C.; Principi, N. Clinical manifestations and socio-economic impact of influenza among healthy children in the community. J. Infect. 2011, 62, 379–387. [Google Scholar] [CrossRef]
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Beyer, W.; Palache, A.; Boulfich, M.; Osterhaus, A. Rationale for two influenza B lineages in seasonal vaccines: A meta-regression study on immunogenicity and controlled field trials. Vaccine 2017, 35, 4167–4176. [Google Scholar] [CrossRef]
| Category of Costs | Total Direct Cost (EUR) (Females) [24] | Total Direct Costs (EUR) (Males) [24] | Social Security Costs (EUR) [27] |
|---|---|---|---|
| CIN1 | 452 | - | Not applicable (na) |
| CIN2 | 1485 | - | 4265 |
| CIN3 | 1972 | - | 4265 |
| Cervical cancer, local disease | 20,653 | - | na |
| Cervical cancer, regional disease | 35,930 | - | 9131 |
| Cervical cancer, distant disease | 34,575 | - | 9131 |
| Vaginal cancer, local disease | 7703 | - | na |
| Vaginal cancer, regional disease | 19,836 | - | 8150 |
| Vaginal cancer, distant disease | 29,647 | - | 8150 |
| Vulvar cancer, local disease | 7184 | - | na |
| Vulvar cancer, regional disease | 16,033 | - | 8150 |
| Vulvar cancer, distant disease | 20,365 | - | 8150 |
| Penile cancer | - | 10,498 | 8218 |
| Anal cancer, local disease | 9812 | 9812 | na |
| Anal cancer, regional disease | 18,480 | 18,480 | 9136 |
| Anal cancer, distant disease | 11,994 | 11,994 | 9136 |
| Head & Neck, local disease | 10,082 | 10,082 | na |
| Head & Neck, regional and distant disease | 28,572 | 28,572 | 9308 |
| Genital warts | 700 | 495 | na |
| Recurrent respiratory papillomatosis | 195,815 | 195,815 | 9308 |
| Immunocompromised Adults | Adults with Risk Conditions | Routine Cohort | Notes | ||||
|---|---|---|---|---|---|---|---|
| 18–49 Years | 50–64 Years | 65 Years | |||||
| Healthy people | - | - | - | - | 38.7% | 240,903 | Healthy people from multiscope 2019 |
| With at least one risk condition and immunocompetent | - | - | 32.1% | 4,364,595 | 45.0% | 280,120 | Data from Veneto Region [29,30] |
| Immunocompromised | 1.4% | 334,455 | 4.3% | 587,611 | 16.3% | 101,466 | Veneto Region ACG system report: 18–49 y (4.34%)–50–64 (4.32%) > 65 (16.3%) [29,30] |
| HZ Incidence | 0.01596 | RR Immunocompromessi = 3 | 0.006916 | RR a rischio = 1.3 | 0.6070% | Alicino 2017 [33] | |
| PHN incidence | 0.002676492 | 0.001159813 | 0.001465298 | ||||
| Current coverage rate | 0% | 5% | 20% | Assumptions from Osmed data | |||
| Target coverage rate | 20% | 20% | 50% | Assumption | |||
| VE ZVL (1 dose) | na | HZ: 64% PHN: 63% | HZ: 64% PHN: 66% | RCP ZVL and STIKO report | |||
| VE RZV (2 dose) | HZ: 71.80% PHN: 89% | At risk: HZ: 96.6%, PHN: 100% Immunocompromised HZ: 67.3%, PHN: 88% | healthy: HZ: 97.4%, PHN: 100%; At risk: HZ: 96.6%, PHN: 100% Immunocompromised HZ: 67.3%, PHN: 88% | RCP EMA RZV and STIKO report | |||
| Cost Category | Value (EUR) | Source |
|---|---|---|
| ZVL acquisition cost | 87.00 | Farmadati [26] |
| RZV acquisition cost | 166.10 per dose (2 doses) | Farmadati [26] |
| Administration cost | 6.64 | Coretti et al., 2016 [36] |
| HZ treated as outpatient | 145.01 | Panatto et al., 2015 [34] |
| HZ hospitalised | 3063 | Panatto et al., 2015 [34] |
| PHN treated as outpatient | 661.92 | Panatto et al., 2015 [34] |
| PHN hospitalised | 3316 | Panatto et al., 2015 [34] |
| Daily productivity loss | 129.60 | Calculated from Ruggeri et al., 2020 [35] |
| Other parameters | Value | |
| Percentage of hospitalised HZ | 1.65% | Panatto et al., 2015 [34] |
| Percentage of hospitalised PHN | 4.06% | Panatto et al., 2015 [34] |
| Productivity loss HZ (days) | 6 | Ruggeri et al., 2020 [35] |
| Productivity loss PHN (days) | 10 | Ruggeri et al., 2020 [35] |
| Percentage of workers (65 years or more) Percentage of workers (50–64 years) | 30% 61.2% | Assumption ISTAT DEMO [10] |
| Cost Category | Cost (EUR) | Notes |
|---|---|---|
| QIV acquisition cost | 11.08 | Ex-factory price |
| Administration cost | 6.16 | Nomenclatore tariffario delle prestazioni aggiuntive, Accordo Collettivo Nazionale medici generici, 23 marzo 2005 [41] |
| GP visit | 20.66 | Tariffario prestazioni ambulatoriali 2013, Ministero della Salute [21] |
| Bronchitis (hospitalisation) | 1832 | DRG 097, bronchitis and asthma, age > 17 years without CC [21] |
| Pneumonia (hospitalisation) | 3558 | DRG 089 simple pneumonia, age > 17 years with CC [21] |
| Unspecified respiratory tract infection | 4422 | DRG 080, respiratory infections and inflammations, age > 17 years without CC |
| Cardiac complications | 3544 | Iannazzo, 2011 [40] |
| Renal complications | 3734 | DRG 316, renal failure [21] |
| Central nervous system complications | 3507 | Iannazzo, 2011 [40] |
| Otitis media | 1247 | DRG 069, otitis media and upper respiratory tract infections, age > 17 years without CC [21] |
| Gastrointestinal haemorrhage | 2091 | DRG 175, gastrointestinal haemorrhage without CC [21] |
| Productivity loss due to influenza | 4.7 days | Ruggeri et al., 2020 [35] |
| Daily wage | 103.03 | Ruggeri et al., 2020 [35] |
| Percentage of workers ≥ 65 years | 4.87% | Calculated from WHO report |
| No Vaccination | Current Vaccination | Target Vaccination | |
|---|---|---|---|
| Vaccine cost | EUR 0 | EUR 28,713,084 | EUR 73,560,963 |
| Direct costs of HPV-related diseases | EUR 154,078,832 | EUR 121,320,045 | EUR 74,483,857 |
| Indirect costs of HPV-related diseases | EUR 53,140,903 | EUR 43,111,943 | EUR 29,488,385 |
| Total costs | EUR 207,219,735 | EUR 193,145,073 | EUR 177,533,205 |
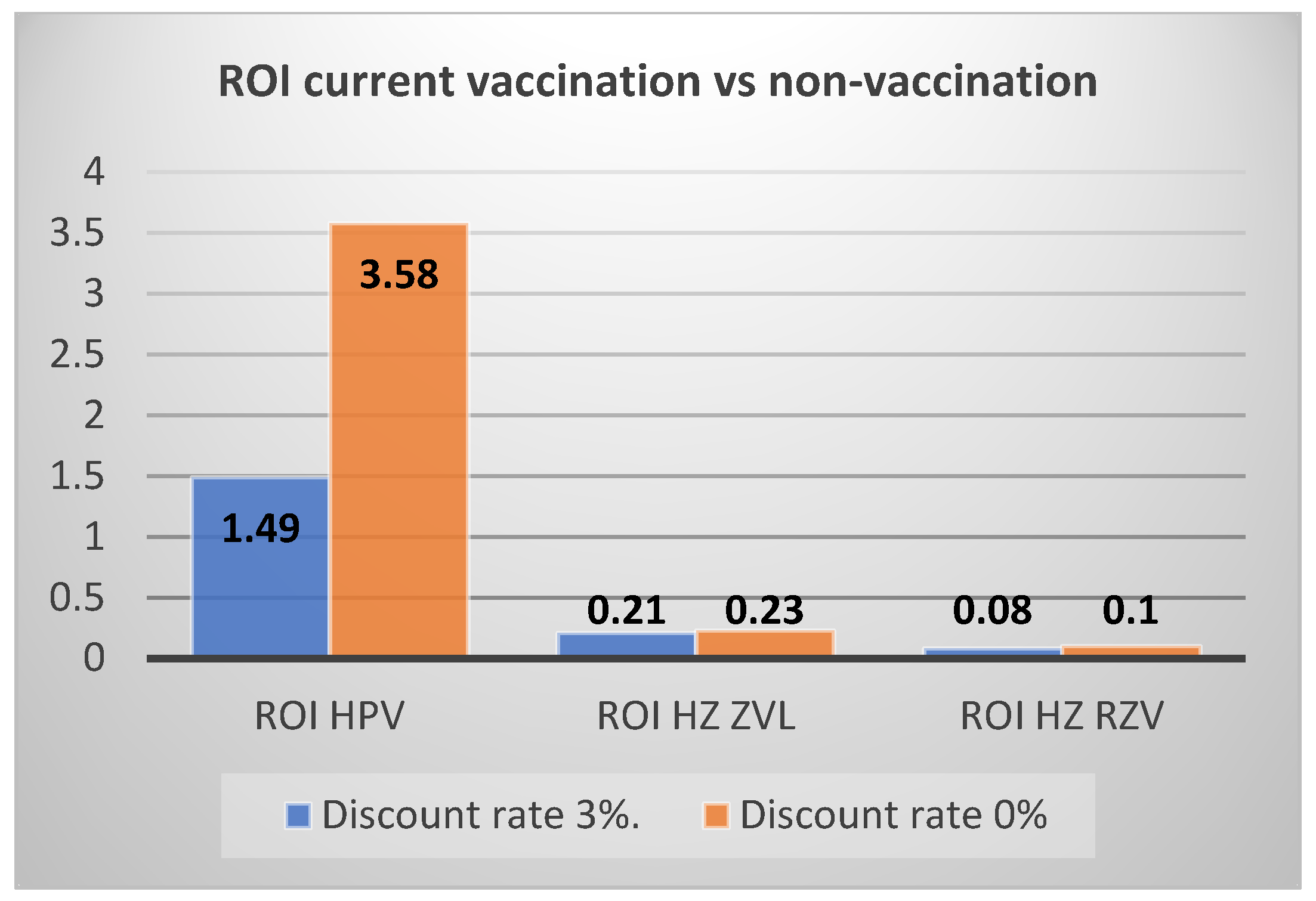
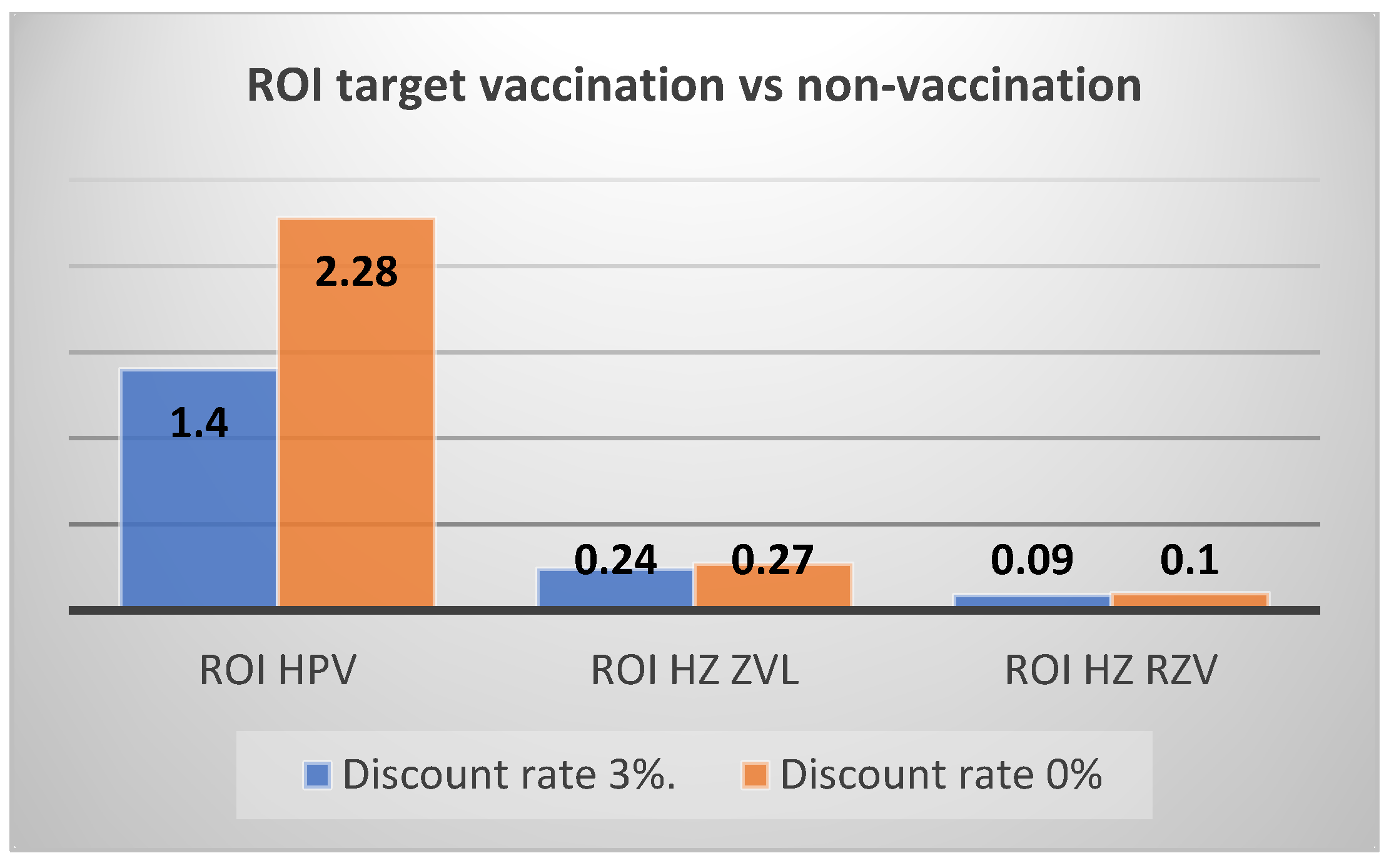
| ROI vs. no vaccination | 1.49 | 1.40 | |
| ROI vs. current vaccination | 1.35 |
| No Vaccination | Current Vaccination | Target Vaccination | |
|---|---|---|---|
| Vaccine costs | EUR 0 | EUR 19,831,988 | EUR 110,885,070 |
| Direct costs of HZ/PHN complications | EUR 56,517,312 | EUR 54,814,827 | EUR 47,064,976 |
| Indirect costs of HZ/PHN complications | EUR 134,518,156 | EUR 131,632,086 | EUR 114,466,618 |
| Total costs | EUR 191,035,467 | EUR 206,278,901 | EUR 272,416,664 |
| ROI vs. no vaccination | 0.21 | 0.24 | |
| ROI vs. current vaccination | 0.25 |
| No Vaccination | Current Vaccination | Target Vaccination | |
|---|---|---|---|
| Vaccine costs | EUR 0 | EUR 83,960,127 | EUR 484,266,536 |
| Direct costs of HZ/PHN complications | EUR 73,064,973 | EUR 70,438,608 | EUR 57,261,427 |
| Indirect costs of HZ/PHN complications | EUR 163,772,122 | EUR 159,232,906 | EUR 130,804,974 |
| Total costs | EUR 236,837,095 | EUR 313,631,641 | EUR 672,332,937 |
| ROI vs. no vaccination | 0.08 | 0.09 | |
| ROI vs. current vaccination | 0.09 |
| No Vaccination | Current Vaccination | Vaccination at 75% Target | Vaccination at 95% Target | |
|---|---|---|---|---|
| Vaccine costs | EUR 0.00 | EUR 133,166,249 | EUR 159,657,432 | EUR 178,118,186 |
| Direct costs of influenza (no complications) | EUR 7,862,407 | EUR 4,456,558 | EUR 4,435,542 | EUR 4,078,589 |
| Direct costs of complications | EUR 117,071,491 | EUR 64,209,048 | EUR 54,765,739 | EUR 46,307,002 |
| Indirect costs | EUR 23,250,293 | EUR 15,145,006 | EUR 12,473,782 | EUR 9,600,046 |
| Total costs | EUR 148,184,192 | EUR 216,976,863 | EUR 231,332,497 | EUR 238,103,824 |
| ROI vs. no vaccination | 0.48 | 0.48 | 0.495 | |
| ROI vs. current vaccination | 0.46 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, M.; Boccalini, S. Return on Investment (ROI) of Three Vaccination Programmes in Italy: HPV at 12 Years, Herpes Zoster in Adults, and Influenza in the Elderly. Vaccines 2023, 11, 924. https://doi.org/10.3390/vaccines11050924
Barbieri M, Boccalini S. Return on Investment (ROI) of Three Vaccination Programmes in Italy: HPV at 12 Years, Herpes Zoster in Adults, and Influenza in the Elderly. Vaccines. 2023; 11(5):924. https://doi.org/10.3390/vaccines11050924
Chicago/Turabian StyleBarbieri, Marco, and Sara Boccalini. 2023. "Return on Investment (ROI) of Three Vaccination Programmes in Italy: HPV at 12 Years, Herpes Zoster in Adults, and Influenza in the Elderly" Vaccines 11, no. 5: 924. https://doi.org/10.3390/vaccines11050924
APA StyleBarbieri, M., & Boccalini, S. (2023). Return on Investment (ROI) of Three Vaccination Programmes in Italy: HPV at 12 Years, Herpes Zoster in Adults, and Influenza in the Elderly. Vaccines, 11(5), 924. https://doi.org/10.3390/vaccines11050924






