Vaccination and Factors Related to the Clinical Outcome of COVID-19 in Healthcare Workers—A Romanian Front-Line Hospital’s Experience
Abstract
1. Introduction
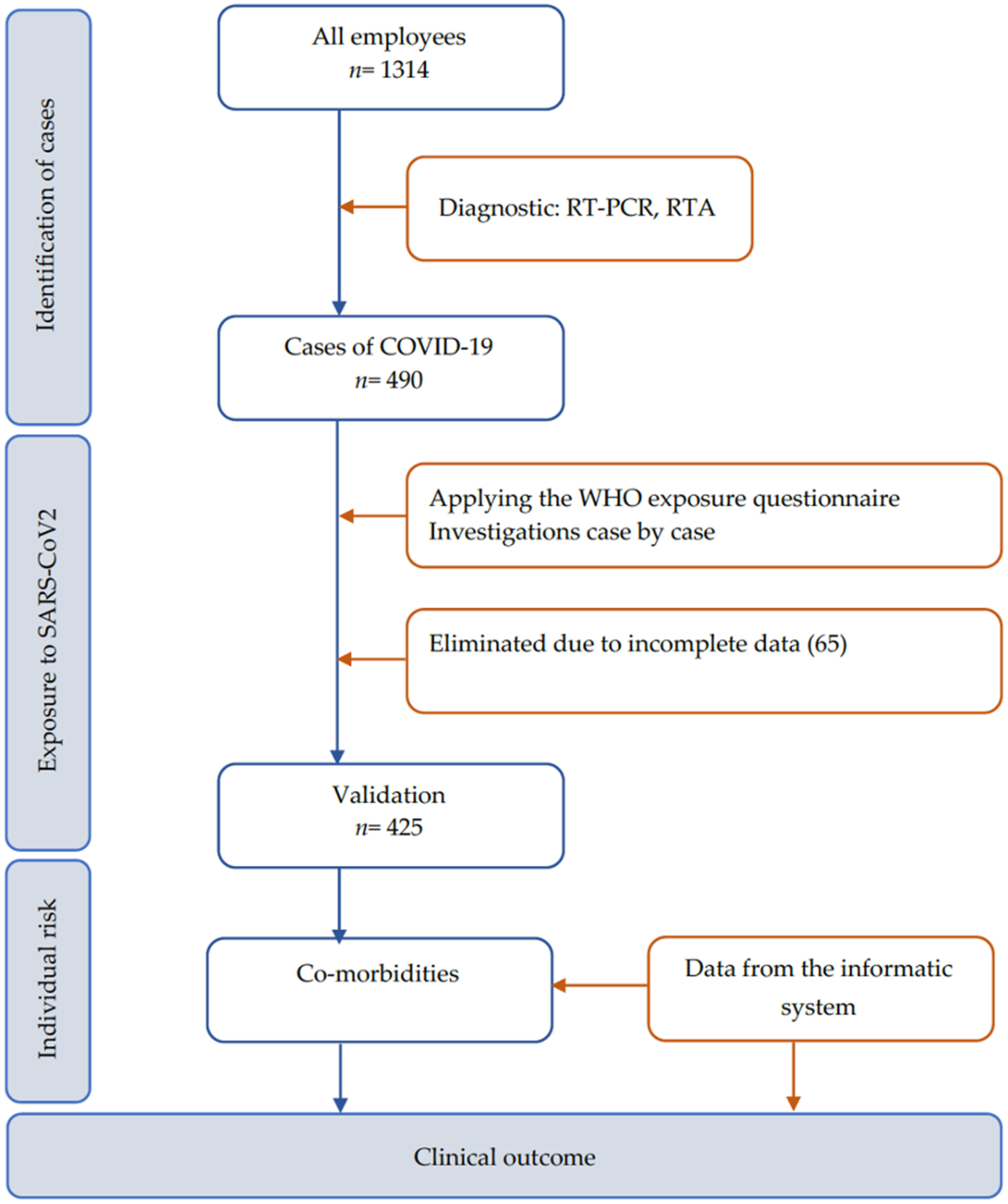
2. Materials and Methods
2.1. Hospital Setting and Participants
2.2. Definition and Data Collection
2.3. Statistical Analysis
2.4. Ethics Approval
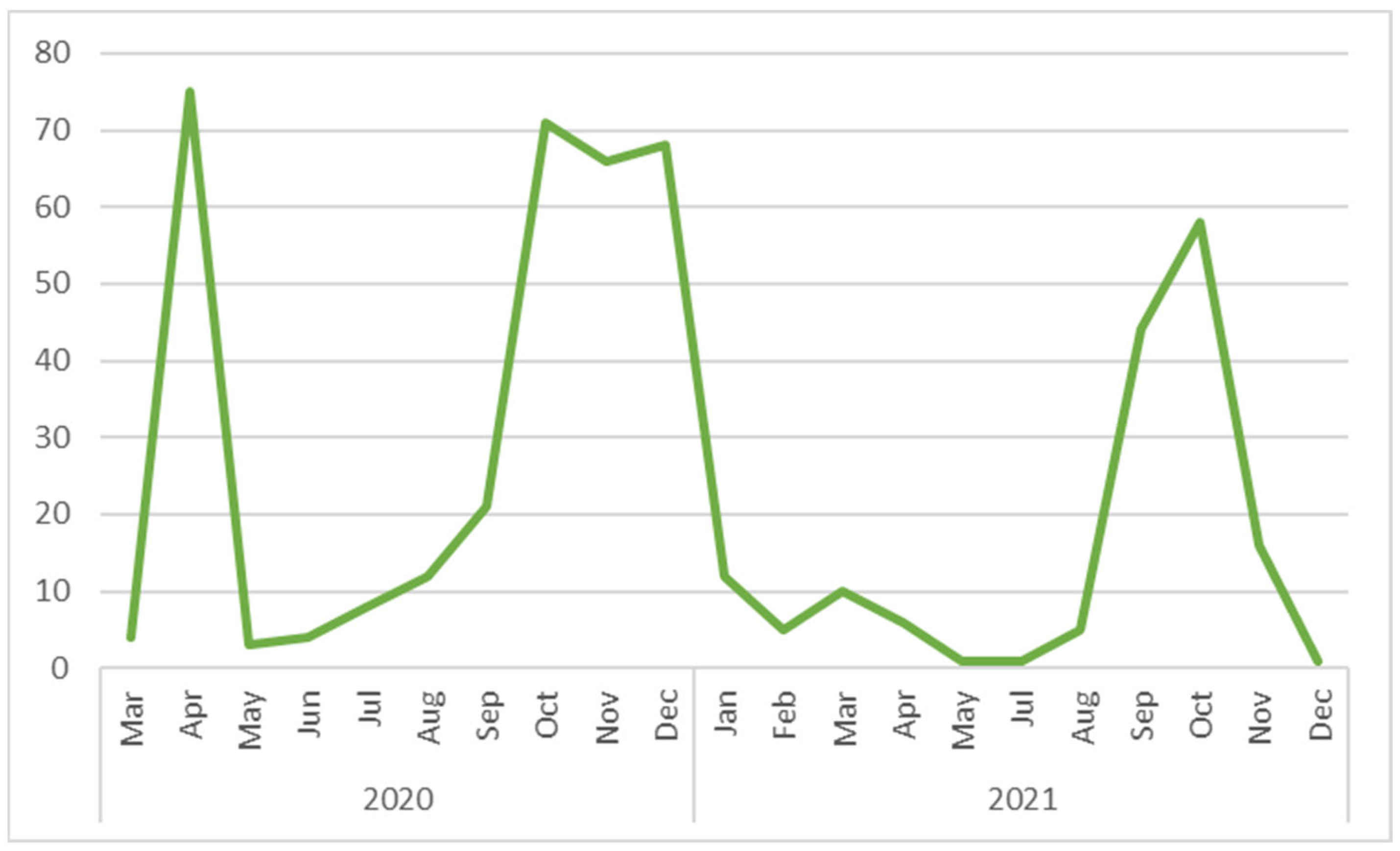
3. Results
3.1. Vaccination Status
3.2. Co-Morbidities
3.3. Exposure
3.4. Logistic Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. COVID-19 Detailed Surveillance Data Dashboard. Available online: https://covid19.who.int/ (accessed on 11 April 2023).
- WHO. The Impact of COVID-19 on Health and Care Workers: A Closer Look at Deaths. Available online: https://apps.who.int/iris/handle/10665/345300 (accessed on 11 April 2023).
- National Center for Surveillance and Control of Communicable Disease. COVID-19 Confirmed Cases Analysis. Available online: https://www.cnscbt.ro/index.php/analiza-cazuri-confirmate-covid19 (accessed on 15 March 2023).
- WHO. Infection Prevention and Control during Health Care when Novel Coronavirus (nCoV) Infection is Suspected. Available online: https://www.who.int/publications/i/item/10665-331495 (accessed on 22 March 2023).
- WHO. Prevention, Identification and Management of Health Worker Infection in the Context of COVID-19. Available online: https://www.who.int/publications/i/item/10665-336265 (accessed on 22 March 2023).
- WHO. Infection Prevention and Control and Preparedness for COVID-19 in Healthcare Settings—Sixth Update. Available online: https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings (accessed on 22 March 2023).
- European Centre for Disease Prevention and Control. COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (accessed on 11 April 2023).
- Enciu, B.G.; Pițigoi, D.; Zaharia, A.; Popescu, R.; Niculcea, A.; Crăciun, M.-D.; Pistol, A. COVID-19 Vaccination in Romania and the Benefits of the National Electronic Registry of Vaccinations. Vaccines 2023, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Xu, J.; Chen, W.; Yang, Z.; Xu, X.; Liu, L.; Chen, R.; Xie, J.; Liu, M.; Wu, J.; et al. Anemia is associated with severe illness in COVID-19: A retrospective cohort study. J. Med. Virol. 2020, 19, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; de Andreis, F.B.; Aronico, N.; Lenti, M.V.; Barteselli, C.; Merli, S.; Pellegrino, I.; Coppola, L.; Cremonte, E.M.; Croce, G.; et al. Anemia in patients with COVID-19: Pathogenesis and clinical significance. Clin. Exp. Med. 2021, 21, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Jha, M.; Tak, M.; Gupta, R.; Sharma, P.; Rajpurohit, V.; Mathur, P. Relationship of anemia with COVID-19 deaths: A retrospective cross-sectional study. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 115. [Google Scholar] [CrossRef] [PubMed]
- Jugulete, G.; Pacurar, D.; Pavelescu, M.L.; Safta, M.; Gheorghe, E.; Borcoș, B.; Pavelescu, C.; Oros, M.; Merișescu, M. Clinical and Evolutionary Features of SARS-CoV-2 Infection (COVID-19) in Children, a Romanian Perspective. Children 2022, 9, 1282. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- CDC. Obesity, Race/Ethnicity, and COVID-19|Overweight & Obesity|. Available online: https://www.cdc.gov/obesity/data/obesity-and-covid-19.html (accessed on 11 April 2023).
- Ministry of Health-MS-Romania. Order 550/2020. Available online: https://www.cnscbt.ro/index.php/lex/1753-ordinul-nr-555-2020-privind-aprobarea-planului-de-masuri-pentru-pregatirea-spitalelor-in-contextul-epidemiei-de-coronavirus-covid-19-a-listei-spitalelor-care-asigura-asistenta-medicala-pacientilor-tes/file (accessed on 15 March 2023).
- Piţigoi, D.; Săndulescu, O.; Ionescu, T.; Niţescu, B.; Streinu-Cercel, A. Assessment of knowledge, attitudes and perceptions regarding Ebola disease in healthcare workers from a tertiary care hospital in Romania. Public Health 2018, 164, 7–15. [Google Scholar] [CrossRef] [PubMed]
- National Center for Surveillance and Control of Communicable Disease. Methodologies for Surveillance COVID-19. Available online: https://www.cnscbt.ro/index.php/metodologii/infectia-2019-cu-ncov (accessed on 15 March 2023).
- National Center for Surveillance and Control of Communicable Disease. Methodologies for Surveillance COVID-19-04-01-2021. Available online: https://www.cnscbt.ro/index.php/metodologii/infectia-2019-cu-ncov/2199-metodologia-de-supraveghere-a-covid-19-actualizare-04-01-2021-1 (accessed on 15 March 2023).
- WHO. Risk Assessment and Management of Exposure of Health Care Workers in the Context of COVID-19: Interim Guidance, 19 March 2020. Available online: https://apps.who.int/iris/handle/10665/331496 (accessed on 12 March 2023).
- WHO. Clinical Management of COVID-19: Living Guideline, 15 September 2022. Available online: https://apps.who.int/iris/handle/10665/362783 (accessed on 16 March 2023).
- Riley, R.D.; Ensor, J.; Snell, K.I.; Harrell, F.E.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, M.; Wu, F.; Xiong, N.; Ma, Y.; Wang, Z.; Duan, L.; Chen, L.; Ouyang, H.; Jin, Y. Factors associated with asymptomatic infection in health-care workers with severe acute respiratory syndrome coronavirus 2 infection in Wuhan, China: A multicentre retrospective cohort study. Clin. Microbiol. Infect. 2020, 26, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Misra-Hebert, A.D.; Jehi, L.; Ji, X.; Nowacki, A.S.; Gordon, S.; Terpeluk, P.; Chung, M.K.; Mehra, R.; Dell, K.M.; Pennell, N.; et al. Impact of the COVID-19 Pandemic on Healthcare Workers’ Risk of Infection and Outcomes in a Large, Integrated Health System. J. Gen. Intern. Med. 2020, 35, 3293–3301. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Healthcare Workers: A Living Systematic Review and Meta-analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2020, 190, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Pan, D.; Melbourne, C.; Teece, L.; Aujayeb, A.; Baggaley, R.F.; Bryant, L.; Carr, S.; Gregary, B.; Gupta, A.; et al. Risk factors associated with SARS-CoV-2 infection in a multiethnic cohort of United Kingdom healthcare workers (UK-REACH): A cross-sectional analysis. PLoS Med. 2022, 19, e1004015. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Wong, D.J.N.; Owen, R.; Neuman, M.D.; Pocock, S.; Carlisle, J.B.; Johnstone, C.; Andruszkiewicz, P.; Baker, P.A.; Biccard, B.M.; et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: A prospective international multicentre cohort study. Anaesthesia 2020, 75, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Fawad, I.; Shadan, S.; Rowaiee, R.; Ghanem, H.; Khamis, A.H.; Ho, S.B. COVID-19 and healthcare workers: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Nasa, P.; Modi, P.; Setubal, G.; Puspha, A.; Upadhyay, S.; Talal, S.H. Demographic and risk characteristics of healthcare workers infected with SARS-CoV-2 from two tertiary care hospitals in the United Arab Emirates. World J. Virol. 2023, 12, 122–131. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All HCWs n = 1314 | Laboratory Confirmed Cases n = 490 (37.3%) | Uninfected n = 824 (62.7%) | p-Value | |
|---|---|---|---|---|---|
| Gender | Female | 1104 (84.0%) | 417 (85.1%) | 687 (83.4%) | p = 0.43 a, χ2(1) = 0.62 |
| Male | 210 (16.0%) | 73 (14.9%) | 137 (16.6%) | p = 0.42 a, χ2(1) = 0.66 | |
| Age | Mean (SD) | 41.92 (10.5) | 43.98 (9.5) | 40.70 (10.9) | pb, SE = 0.59 |
| Median age (years) | 42, IQR 33–50 | 45, IQR 37–51 | 41, IQR 30–49 | - | |
| ≤29 | 232 (17.7%) | 51 (10.4%) | 181 (22.0%) | pa, χ2(1) = 28.39 | |
| 30–39 | 316 (24.0%) | 110 (22.4%) | 206 (25.0%) | p = 0.28 a, χ2(1) = 1.14 | |
| 40–49 | 427 (32.5%) | 181 (36.9%) | 246 (29.9%) | p = 0.0088 a, χ2(1) = 6.85 | |
| ≥50 | 339 (25.8%) | 148 (30.2%) | 191 (23.2%) | p = 0.0051 a, χ2(1) = 7.86 | |
| Job category | Nurses | 467 (35.5%) | 186 (38.0%) | 281 (34.1%) | p = 0.15 a, χ2(1) = 1.99 |
| Physicians | 319 (24.3%) | 96 (19.6%) | 223 (27.1%) | p = 0.0023 a, χ2(1) = 9.32 | |
| Healthcare auxiliary activities | 359 (27.3%) | 148 (30.2%) | 211 (25.6%) | p = 0.07 a, χ2(1) = 3.27 | |
| Other categories | 169 (12.9%) | 60 (12.2%) | 109 (13.2%) | p = 0.60 a, χ2(1) = 0.26 | |
| Characteristics | All COVID-19 Cases in HCWs n = 425(%) | Non-Severe Cases n = 279(64.7%) | Moderate-to-Severe Cases n = 146(34.3%) | p-Value | |
|---|---|---|---|---|---|
| Gender | Female | 362 (85.2%) | 237 (84.9%) | 125 (85.6%) | p = 0.85 a, χ2(1) = 0.034 |
| Male | 63 (14.8%) | 42 (15.1%) | 21 (14.4%) | p = 0.85 a, χ2(1) = 0.034 | |
| Age | Mean (SD) | 44.2 (9.41) | 43.2 (9.45) | 46.0 (9.08) | p = 0.0035 b, SE = 0.95 |
| Median age (years), IQR | 45, IQR 37–51 | 44, IQR 36–50 | 47, IQR 40–52 | ||
| ≤29 | 42 (9.9%) | 33 (11.8%) | 9 (6.2%) | p = 0.06 a, χ2(1) = 3.452 | |
| 30–39 | 92 (21.6%) | 67 (24.0%) | 25 (17.1%) | p = 0.10 a, χ2(1) = 2.677 | |
| 40–49 | 161 (37.9%) | 101 (36.2%) | 60 (41.1%) | p = 0.32 a, χ2(1) = 0.976 | |
| ≥50 | 130 (30.6%) | 78 (28.0%) | 52 (35.6%) | p = 0.10 a, χ2(1) = 2.64 | |
| Job category | Nurses | 167 (39.3%) | 107 (38.3%) | 60 (41.1%) | p = 0.58 a, χ2(1) = 0.303 |
| Physicians | 85 (20.0%) | 54 (19.4%) | 31 (21.2%) | p = 0.64 a, χ2(1) = 0.211 | |
| Healthcare auxiliary activities | 123 (28.9%) | 81 (29.0%) | 42 (28.8%) | p = 0.96 a, χ2(1) = 0.003 | |
| Other categories | 50 (11.8%) | 37 (13.3%) | 13 (8.9%) | p = 0.18 a, χ2(1) = 1.751 | |
| Department | High risk (ICU, ER, COVID-19 wards) | 336 (79.1%) | 206 (73.8%) | 130 (89.0%) | p = 0.0003 a, χ2(1) = 13.345 |
| Low risk (other departments) | 89 (20.9%) | 73 (26.2%) | 16 (11.0%) | ||
| Exposure | Yes | 268 (63.1%) | 159 (57.0%) | 109 (74.7%) | p = 0.0003 a, χ2(1) = 12.816 |
| No | 157 (36.9%) | 120 (43.0%) | 37 (25.3%) | ||
| Risk categorization after exposure c | High risk | 29 (6.8%) | 24 (8.6%) | 5 (3.4%) | p = 0.0445 a, χ2(1) = 4.04 |
| Low risk | 396 (93.2%) | 255 (91.4%) | 141 (96.6%) | ||
| Vaccination | Yes | 85 (20.0%) | 70 (25.1%) | 15 (10.3%) | p = 0.0003 a, χ2(1) = 13.126 |
| No | 340 (80.0%) | 209 (74.9%) | 131 (89.7%) | ||
| Co-morbidities | Yes | 107 (25.2%) | 37 (13.3%) | 70 (47.9%) | p < 0.0001 a, χ2(1) = 61.082 |
| No | 318 (74.8%) | 242 (86.7%) | 76 (52.1%) | ||
| Exposure | All COVID-19 Cases in HCWs n = 425 (%) | Non-Severe Cases n = 279 (65.7%) | Moderate-to-Severe Cases n = 146 (34.3%) | p-Value |
|---|---|---|---|---|
| Direct care to a confirmed COVID-19 patient (yes) | 253 (59.5%) | 147 (52.7%) | 106 (72.6%) | p = 0.0001 χ2(1) = 15.748 |
| Face-to-face contact (yes) | 259 (60.9%) | 153 (54.8%) | 106 (72.6%) | p = 0.0004, χ2(1) = 15.748 |
| Aerosol-generating procedures (yes) | 74 (17.4%) | 45 (16.1%) | 29 (19.9%) | p = 0.3348, χ2(1) = 12.676 |
| Direct contact with the environment of COVID-19 patient (yes) | 246 (57.9%) | 139 (49.8%) | 107 (73.3%) | p < 0.0001, χ2(1) = 21.6 |
| Factors | Multivariable OR (95%) | p Value |
|---|---|---|
| Age (years) | 1.037 (1.012–1.062) | 0.004 |
| Obesity | 4.941 (2.462–9.913) | <0.001 |
| Anemia | 5.821 (2.402–14.112) | <0.001 |
| Exposure (yes) | 2.652 (1.632–4.31) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chivu, C.-D.; Crăciun, M.-D.; Pițigoi, D.; Aramă, V.; Luminos, M.L.; Jugulete, G.; Apostolescu, C.G.; Streinu Cercel, A. Vaccination and Factors Related to the Clinical Outcome of COVID-19 in Healthcare Workers—A Romanian Front-Line Hospital’s Experience. Vaccines 2023, 11, 899. https://doi.org/10.3390/vaccines11050899
Chivu C-D, Crăciun M-D, Pițigoi D, Aramă V, Luminos ML, Jugulete G, Apostolescu CG, Streinu Cercel A. Vaccination and Factors Related to the Clinical Outcome of COVID-19 in Healthcare Workers—A Romanian Front-Line Hospital’s Experience. Vaccines. 2023; 11(5):899. https://doi.org/10.3390/vaccines11050899
Chicago/Turabian StyleChivu, Carmen-Daniela, Maria-Dorina Crăciun, Daniela Pițigoi, Victoria Aramă, Monica Luminița Luminos, Gheorghiță Jugulete, Cătălin Gabriel Apostolescu, and Adrian Streinu Cercel. 2023. "Vaccination and Factors Related to the Clinical Outcome of COVID-19 in Healthcare Workers—A Romanian Front-Line Hospital’s Experience" Vaccines 11, no. 5: 899. https://doi.org/10.3390/vaccines11050899
APA StyleChivu, C.-D., Crăciun, M.-D., Pițigoi, D., Aramă, V., Luminos, M. L., Jugulete, G., Apostolescu, C. G., & Streinu Cercel, A. (2023). Vaccination and Factors Related to the Clinical Outcome of COVID-19 in Healthcare Workers—A Romanian Front-Line Hospital’s Experience. Vaccines, 11(5), 899. https://doi.org/10.3390/vaccines11050899





