The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development
Abstract
1. Introduction
Necessity Is the Mother of All Inoculation
2. Stimulating the Immune System by Vaccination
2.1. Optimizing the Efficacy of Vaccines
2.2. Vaccine Platforms
2.2.1. Attenuated Vaccines
2.2.2. Inactivated Vaccines
2.2.3. Toxoid Vaccines
2.2.4. Subunit Vaccines
2.2.5. Gene Vaccines
2.3. Adjuvants
2.3.1. Aluminum Salts
2.3.2. Other Adjuvants
2.3.3. The Outcome of Formulating with Different Adjuvants
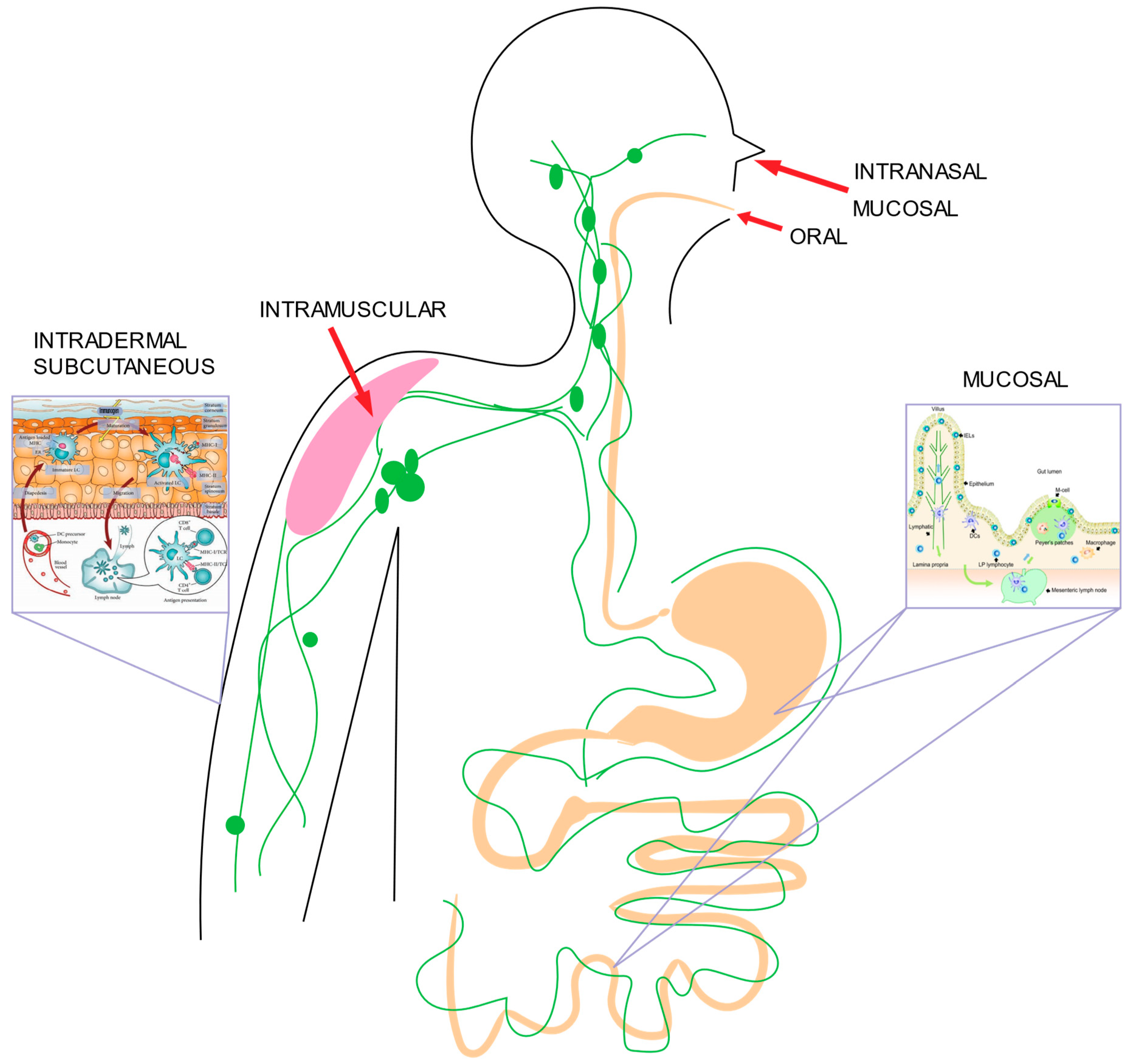
2.4. Delivery Routes
2.4.1. Mucosal Delivery
2.4.2. Oral Delivery
2.4.3. Intranasal Delivery
2.4.4. Subcutaneous and Intramuscular Delivery
2.4.5. Intradermal Delivery
2.4.6. Helium-Based Gene-Gun Delivery
2.4.7. Tattoo Gun via Electroporation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The use of viral vectors in vaccine development. Npj Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Korsman, S.N.J.; van Zyl, G.U.; Nutt, L.; Andersson, M.I.; Preiser, W. Immunotherapy and immunoprophylaxis–: Passive and active immunity. In Virology; Korsman, S.N.J., van Zyl, G.U., Nutt, L., Andersson, M.I., Preiser, W., Eds.; Churchill Livingstone: Edinburgh, UK, 2012; pp. 46–47. [Google Scholar]
- Jenner, E. On the Origin of the Vaccine Inoculation. Med. Phys. J. 1801, 5, 505–508. [Google Scholar] [PubMed]
- CDC. History of Smallpox. 2021. Available online: https://www.cdc.gov/smallpox/history/history.html (accessed on 18 January 2023).
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.A.; Moss, B. Smallpox and Vaccinia; Saunders: Philadephia, PA, USA, 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK7294/ (accessed on 18 January 2023).
- Hicks, D.J.; Fooks, A.R.; Johnson, N. Developments in rabies vaccines. Clin. Exp. Immunol. 2012, 169, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.L.; Gonzales, M.L.A.; Aldaba, J.G.; Nair, G.B. Killed oral cholera vaccines: History, development and implementation challenges. Ther. Adv. Vaccines 2014, 2, 123–136. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Stratton, K.R.; Howe, C.J.; Johnston, R.B., Jr. Diphtheria and Tetanus Toxoids. In Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Verma, A.; Verma, M.; Singh, A. Animal tissue culture principles and applications. In Animal Biotechnology; Academic Press: Cambridge, MA, USA, 2020; pp. 269–293. [Google Scholar] [CrossRef]
- Burnet, F.M. Influenza Virus Infections of the Chick Embryo Lung. Br. J. Exp. Pathol. 1940, 21, 147–153. [Google Scholar]
- Hannoun, C. The evolving history of influenza viruses and influenza vaccines. Expert Rev. Vaccines 2013, 12, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Maassab, H.F.; DeBorde, D.C. Development and characterization of cold-adapted viruses for use as live virus vaccines. Vaccine 1985, 3, 355–369. [Google Scholar] [CrossRef]
- Blume, S.; Geesink, I. A Brief History of Polio Vaccines. Science 2000, 288, 1593–1594. [Google Scholar] [CrossRef]
- Park, W.H.; Biggs, H.M. Vaccine Timeline—Before Jenner and after COVID-19. Available online: https://cpp-hov.netlify.app//history/vaccine-timeline/timeline (accessed on 19 February 2023).
- Tan, S.Y.; Ponstein, N. Jonas Salk (1914–1995): A vaccine against polio. Singapore Med. J. 2019, 60, 9–10. [Google Scholar] [CrossRef]
- Emini, E.A.; Ellis, R.W.; Miller, W.J.; McAleer, W.J.; Scolnick, E.M.; Gerety, R.J. Production and immunological analysis of recombinant hepatitis B vaccine. J. Infect. 1986, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M. First recombinant DNA vaccine for HBV. Nat. Res. 2020, 13 (Suppl. A), 3–9. [Google Scholar] [CrossRef]
- Ebertz, D.A. A Journey through The History of DNA Sequencing. The DNA Universe BLOG. 2 November 2020. Available online: https://the-dna-universe.com/2020/11/02/a-journey-through-the-history-of-dna-sequencing/ (accessed on 18 January 2023).
- Zhao, H.; Zhou, X.; Zhou, Y.-H. Hepatitis B vaccine development and implementation. Hum. Vaccines Immunother. 2020, 16, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel-Conrat, H.; Williams, R.C. Reconstitution of Active Tobacco Mosaic Virus From Its Inactive Protein and Nucleic Acid Components. Proc. Natl. Acad. Sci. USA 1955, 41, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-Like Particles: Revolutionary Platforms for Developing Vaccines Against Emerging Infectious Diseases. Front. Microbiol. 2022, 12, 4137. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2021.790121 (accessed on 18 January 2023). [CrossRef] [PubMed]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.T.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Ollmann Saphire, E. A Vaccine against Ebola Virus. Cell 2020, 181, 6. [Google Scholar] [CrossRef]
- Srivastava, I.K.; Liu, M.A. Gene vaccines. Ann. Intern. Med. 2003, 138, 550–559. [Google Scholar] [CrossRef]
- Alonso, P.L.; O’Brien, K.L. A Malaria Vaccine for Africa—An Important Step in a Century-Long Quest. N. Engl. J. Med. 2022, 386, 1005–1007. [Google Scholar] [CrossRef]
- Delamarre, L.; Holcombe, H.; Mellman, I. Presentation of Exogenous Antigens on Major Histocompatibility Complex (MHC) Class I and MHC Class II Molecules Is Differentially Regulated during Dendritic Cell Maturation. J. Exp. Med. 2003, 198, 111–122. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Fay, E.J.; Langlois, R.A. MicroRNA-Attenuated Virus Vaccines. Non-Coding RNA 2018, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Shen, Q.; Li, J.; Chen, L.; Shen, J.; Xiao, X.; Bai, H.; Feng, T.; Ye, A.Y.; Li, L.; et al. Generation of a live attenuated influenza A vaccine by proteolysis targeting. Nat. Biotechnol. 2022, 40, 1370–1377. [Google Scholar] [CrossRef]
- Giesker, K.; Hensel, M. Bacterial Vaccines. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated Viral Vaccines. In Vaccine Analysis: Strategies, Principles, and Control; Springer: Berlin/Heidelberg, Germany, 2014; pp. 45–80. [Google Scholar] [CrossRef]
- Khoshnood, S.; Arshadi, M.; Akrami, S.; Koupaei, M.; Ghahramanpour, H.; Shariati, A.; Sadeghifard, N.; Heidary, M. An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. J. Clin. Lab. Anal. 2022, 36, e24418. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Lowe, R.S.; Lanier, B.Q. Adverse Reactions to Tetanus Toxoid. JAMA 1982, 247, 40–42. [Google Scholar] [CrossRef] [PubMed]
- About Pneumococcal Vaccine: For Providers|CDC. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html (accessed on 19 January 2023).
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.-Y. Role of the IgM Fc Receptor in Immunity and Tolerance. Front. Immunol. 2019, 10, 529. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00529 (accessed on 14 March 2023). [CrossRef] [PubMed]
- Ouchida, R.; Mori, H.; Hase, K.; Takatsu, H.; Kurosaki, T.; Tokuhisa, T.; Ohno, H.; Wang, J.-Y. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc. Natl. Acad. Sci. USA 2012, 109, E2699–E2706. [Google Scholar] [CrossRef]
- Khan, S. Conjugate vaccines and polysaccharide response. CMAJ Can. Med. Assoc. J. 2006, 174, 976–977. [Google Scholar] [CrossRef]
- Mandell, L.A. 297—Streptococcus Pneumoniae Infections. In Goldman’s Cecil Medicine, 24th ed.; Goldman, L., Schafer, A.I., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 1820–1823. [Google Scholar]
- Plotkin, S.A.; Plotkin, S.L. The development of vaccines: How the past led to the future. Nat. Rev. Microbiol. 2011, 9, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Golos, M.; Eliakim-Raz, N.; Stern, A.; Leibovici, L.; Paul, M. Conjugated pneumococcal vaccine versus polysaccharide pneumococcal vaccine for prevention of pneumonia and invasive pneumococcal disease in immunocompetent and immunocompromised adults and children. Cochrane Database Syst. Rev. 2019, 2019, CD012306. [Google Scholar] [CrossRef]
- What are protein subunit vaccines and how could they be used against COVID-19? 2022. Available online: https://www.gavi.org/vaccineswork/what-are-protein-subunit-vaccines-and-how-could-they-be-used-against-covid-19 (accessed on 19 January 2023).
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
- Mallapaty, S. India’s DNA COVID Vaccine Is a World First—More Are Coming. 2021. Available online: https://www.nature.com/articles/d41586-021-02385-x (accessed on 20 January 2023).
- de Apostólico, J.S.; Lunardelli, V.A.S.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, Modus Operandi, and Licensing. J. Immunol. Res. 2016, 2016, e1459394. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Pasquale, A.D.; Preiss, S.; Silva, F.T.D.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Alving, C.R.; Peachman, K.K.; Rao, M.; Reed, S.G. Adjuvants for Human Vaccines. Curr. Opin. Immunol. 2012, 24, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2016, 13, 19–33. [Google Scholar] [CrossRef]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-H.; Hora, M. The Adjuvant MF59: A 10-Year Perspective Gary Ott, Ramachandran Radhakrishnan. In Vaccine Adjuvants. Methods in Molecular Medicine; O’Hagan, D.T., Ed.; Springer: Totowa, NJ, USA, 2000; Available online: https://experiments.springernature.com/articles/10.1385/1-59259-083-7:211 (accessed on 20 January 2023).
- Villareal, R.; Casale, T. Commonly Used Adjuvant Human Vaccines: Advantages and Side Effects—ClinicalKey. 2020. Available online: https://www-clinicalkey-com.medjournal.hmc.psu.edu:2200/#!/content/journal/1-s2.0-S2213219820304025?scrollTo=%23bib7 (accessed on 20 January 2023).
- Coccia, M.; Collignon, C.; Hervé, C.; Chalon, A.; Welsby, I.; Detienne, S.; van Helden, M.J.; Dutta, S.; Genito, C.J.; Waters, N.C.; et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. Npj Vaccines 2017, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.M.; Ogbe, A.; Pedroza-Pacheco, I.; Doeleman, S.E.; Chen, Y.; Silk, S.E.; Barrett, J.R.; Elias, S.C.; Miura, K.; Diouf, A.; et al. Protein/AS01B vaccination elicits stronger, more Th2-skewed antigen-specific human T follicular helper cell responses than heterologous viral vectors. Cell Rep. Med. 2021, 2, 100207. [Google Scholar] [CrossRef]
- Krieg, A.M.; Yi, A.K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef]
- Lee, S.; Nguyen, M.T. Recent Advances of Vaccine Adjuvants for Infectious Diseases. Immune Netw. 2015, 15, 51–57. [Google Scholar] [CrossRef]
- Mousavi, T.; Sattari Saravi, S.; Valadan, R.; Haghshenas, M.R.; Rafiei, A.; Jafarpour, H.; Shamshirian, A. Different types of adjuvants in prophylactic and therapeutic human papillomavirus vaccines in laboratory animals: A systematic review. Arch. Virol. 2020, 165, 263–284. [Google Scholar] [CrossRef]
- Merck & Co. Gardasil 9 Prescribing Information: Summary of Prduct Characteristics. 2021. Available online: https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Package-Insert---Gardasil.pdf (accessed on 18 January 2023).
- McKeage, K.; Romanowski, B. AS04-adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix®): A review of its use in the prevention of premalignant cervical lesions and cervical cancer causally related to certain oncogenic HPV types. Drugs 2011, 71, 465–488. [Google Scholar] [CrossRef]
- Xu, H.; Alzhrani, R.F.; Warnken, Z.N.; Thakkar, S.G.; Zeng, M.; Smyth, H.D.C.; Williams, R.O.I.; Cui, Z. Immunogenicity of Antigen Adjuvanted with AS04 and Its Deposition in the Upper Respiratory Tract after Intranasal Administration. Mol. Pharm. 2020, 17, 3259–3269. [Google Scholar] [CrossRef]
- Sagonowsky, E. GSK Exits U.S Market with Its HPV Vaccine Cervarix. Fierce Pharma. 2016. Available online: https://www.fiercepharma.com/pharma/gsk-exits-u-s-market-its-hpv-vaccine-cervarix (accessed on 18 January 2023).
- Pennycook, K.B.; McCready, T.A. Condyloma Acuminata. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK547667/ (accessed on 18 January 2023).
- Draper, E.; Bissett, S.L.; Howell-Jones, R.; Waight, P.; Soldan, K.; Jit, M.; Andrews, N.; Miller, E.; Beddows, S. A Randomized, Observer-Blinded Immunogenicity Trial of Cervarix® and Gardasil® Human Papillomavirus Vaccines in 12-15 Year Old Girls. PLoS ONE 2013, 8, e61825. [Google Scholar] [CrossRef]
- Einstein, M.H.; Baron, M.; Levin, M.J.; Chatterjee, A.; Fox, B.; Scholar, S.; Rosen, J.; Chakhtoura, N.; Lebacq, M.; van der Most, R.; et al. Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum. Vaccin. 2011, 7, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Toft, L.; Tolstrup, M.; Müller, M.; Sehr, P.; Bonde, J.; Storgaard, M.; Østergaard, L.; Søgaard, O.S. Comparison of the immunogenicity of Cervarix® and Gardasil® human papillomavirus vaccines for oncogenic non-vaccine serotypes HPV-31, HPV-33, and HPV-45 in HIV-infected adults. Hum. Vaccines Immunother. 2014, 10, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Peiser, M. Role of Th17 Cells in Skin Inflammation of Allergic Contact Dermatits. J. Immunol. Res. 2013, 2013, e261037. [Google Scholar] [CrossRef]
- Wu, R.-Q.; Zhang, D.-F.; Tu, E.; Chen, Q.-M.; Chen, W. The mucosal immune system in the oral cavity—An orchestra of T cell diversity. Int. J. Oral Sci. 2014, 6, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Chowdhury, M.Y.E.; Tao, W.; Gill, H.S. Mucosal Vaccine Delivery: Current State and a Pediatric Perspective. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 394–413. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.E.P.; Palache, A.M.; de Jong, J.C.; Osterhaus, A.D.M.E. Cold-adapted live influenza vaccine versus inactivated vaccine: Systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine 2002, 20, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 vaccines: From bench to bed. EBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef] [PubMed]
- CDC. Vaccine Administration Route and Site. 2023. Available online: https://www.cdc.gov/vaccines/hcp/admin/administer-vaccines.html (accessed on 18 January 2023).
- Zuckerman, J.N. The importance of injecting vaccines into muscle. BMJ 2000, 321, 1237–1238. [Google Scholar] [CrossRef]
- Geddes, L. The Point of It: Why Do Vaccine Delivery Methods Vary? GAVI. 2021. Available online: https://www.gavi.org/vaccineswork/point-it-why-do-vaccine-delivery-methods-vary (accessed on 18 January 2023).
- Ols, S.; Yang, L.; Thompson, E.A.; Pushparaj, P.; Tran, K.; Liang, F.; Lin, A.; Eriksson, B.; Karlsson Hedestam, G.B.; Wyatt, R.T.; et al. Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity. Cell Rep. 2020, 30, 3964–3971.e7. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Tchitchek, N.; Joly, C.; Rodriguez Pozo, A.; Stimmer, L.; Langlois, S.; Hocini, H.; Gosse, L.; Pejoski, D.; Cosma, A.; et al. Vaccine Inoculation Route Modulates Early Immunity and Consequently Antigen-Specific Immune Response. Front. Immunol. 2021, 12, 645210. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.645210 (accessed on 18 January 2023). [CrossRef]
- Okafor, C.N.; Rewane, A.; Momodu, I.I. Bacillus Calmette Guerin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538185/ (accessed on 20 January 2023).
- BCG Vaccine for Tuberculosis (TB) Overview. 2019. Available online: https://www.nhs.uk/conditions/vaccinations/bcg-tuberculosis-tb-vaccine/ (accessed on 19 January 2023).
- Carlsen, W. Did Modern Medicine Spread an Epidemic?/After Decades, and Millions of Injections, Scientists Are Asking the Chilling Question. SF Chronicle. 2001. Available online: https://web.stanford.edu/class/stat30/web1/aids2.html (accessed on 15 March 2023).
- Donnelly, R.F. Vaccine delivery systems. Hum. Vaccines Immunother. 2017, 13, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, J.M.; Kesselring, J.R.; Frizzell, H.; Bagley, K.C.; Kulis, M.D. Induction of food-specific IgG by Gene Gun-delivered DNA vaccines. Front. Allergy 2022, 3, 969337. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, C.; Zhang, L.; Li, J.; Huang, Z.; Lu, S. The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine 2008, 26, 2100–2110. [Google Scholar] [CrossRef]
- Roy, M.J.; Wu, M.S.; Barr, L.J.; Fuller, J.T.; Tussey, L.G.; Speller, S.; Culp, J.; Burkholder, J.K.; Swain, W.F.; Dixon, R.M.; et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 2000, 19, 764–778. [Google Scholar] [CrossRef]
- Kikkert, J.R.; Vidal, J.R.; Reisch, B.I. Stable transformation of plant cells by particle bombardment/biolistics. In Transgenic Plants: Methods and Protocols; Methods in Molecular Biology; Humana Press: Clifton, NJ, USA, 2005; Volume 286, pp. 61–78. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.S.; Leitner, W.W. Vaccination Using Gene-Gun Technology. In Malaria Vaccines; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1325, pp. 289–302. [Google Scholar] [CrossRef]
- Loehr, B.I.; Willson, P.; Babiuk, L.A.; van Drunen Littel-van den Hurk, S. Gene Gun-Mediated DNA Immunization Primes Development of Mucosal Immunity against Bovine Herpesvirus 1 in Cattle. J. Virol. 2000, 74, 6077–6086. [Google Scholar] [CrossRef]
- Murakami, T.; Sunada, Y. Plasmid DNA gene therapy by electroporation: Principles and recent advances. Curr. Gene Ther. 2011, 11, 447–456. [Google Scholar] [CrossRef]
- Pokorna, D.; Rubio, I.; Müller, M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet. Vaccines Ther. 2008, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, K.; Ehrenfeld, E.; Wimmer, E.; Agol, V.I. Vaccination against polio should not be stopped. Nat. Rev. Microbiol. 2007, 5, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Hawken, J.; Troy, S.B. Adjuvants and inactivated polio vaccine: A systematic review. Vaccine 2012, 30, 6971–6979. [Google Scholar] [CrossRef] [PubMed]
- Hickling, J.; Jones, R.; Nundy, N. Improving the Affordability of Inactivated Poliovirus Vaccines (IPV) for Use in Low- and Middle-Income Countries: An Economic Analysis of Strategies to Reduce the Cost of Routine IPV Immunization. PATH. 2010. Available online: https://www.path.org/resources/improving-the-affordability-of-inactivated-poliovirus-vaccines-ipv-for-use-in-low-and-middle-income-countries-an-economic-analysis-of-strategies-to-reduce-the-cost-of-routine-ipv-immunization/ (accessed on 18 January 2023).
| Vaccine | Advantages | Disadvantages | Examples |
|---|---|---|---|
| Attenuated | Preservation of native antigen; mimicking natural infection, well-established technology, robust B and T cell response | Potential to cause infection, almost all given via syringe IM, cold chain storage, not suitable for immunocompromised | Measles, Mumps, Polio (Sabin), Rotavirus, Yellow Fever, Bacillus Calmette–Guérin (BCG), Rubella, Varicella |
| Inactivated | Strong immune response with B cell more than T cell, waning immunity; safer than live attenuated virus—incapable of regaining pathogenicity; stable, relatively easy to scale manufacturing and distribution | Potential epitope alteration by inactivation process | Typhoid, Cholera, Hepatitis A virus, Plague, Rabies, Influenza, Polio (Salk) |
| Toxoid | Non-virulent, stable, and long lasting in storage | Typically not robustly immunogenic, require regular booster doses, local site reactions, given by injection | Diphtheria, Tetanus |
| Subunit | Readily modifiable, generally safe for immunocompromised, stable in storage and scalable in production. | Relatively less immunogenic, often require adjuvant or conjugate. Development and manufacture are typically time-consuming | Pertussis, Influenza, Streptococcus pneumoniae, Haemophilus influenzae type b |
| Virus Like Particles (VLPs) | Safe and well-tolerated; mimicking native virus conformation; unable to replicate; scalable and combinable with adjuvants | Relatively complicated manufacturing process; lower stability, difficult downstream processing, high production costs, and sensitivity to environmental conditions | Hepatitis B virus, Human Papillomavirus |
| Viral vector | Strong immune response; preservation of native antigen; mimicking natural infection | Relatively complicated manufacturing process; risk of genomic integration; response dampened by pre-existing immunity against vector | Ebola virus |
| DNA/RNA | Safe and well-tolerated; highly adaptable to new pathogen; native antigen expression | Requirement of low temperature storage for RNA vaccine and transportation; potential risk of RNA-induced interferon response, risk of genomic integration for DNA vaccine. Cells do not easily take up large and polar nucleic acids. | SARS-CoV-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, M.; Hu, J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines 2023, 11, 695. https://doi.org/10.3390/vaccines11030695
Kozak M, Hu J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines. 2023; 11(3):695. https://doi.org/10.3390/vaccines11030695
Chicago/Turabian StyleKozak, Michael, and Jiafen Hu. 2023. "The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development" Vaccines 11, no. 3: 695. https://doi.org/10.3390/vaccines11030695
APA StyleKozak, M., & Hu, J. (2023). The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines, 11(3), 695. https://doi.org/10.3390/vaccines11030695