A Perspective on Oral Immunotherapeutic Tools and Strategies for Autoimmune Disorders
Abstract
1. Introduction
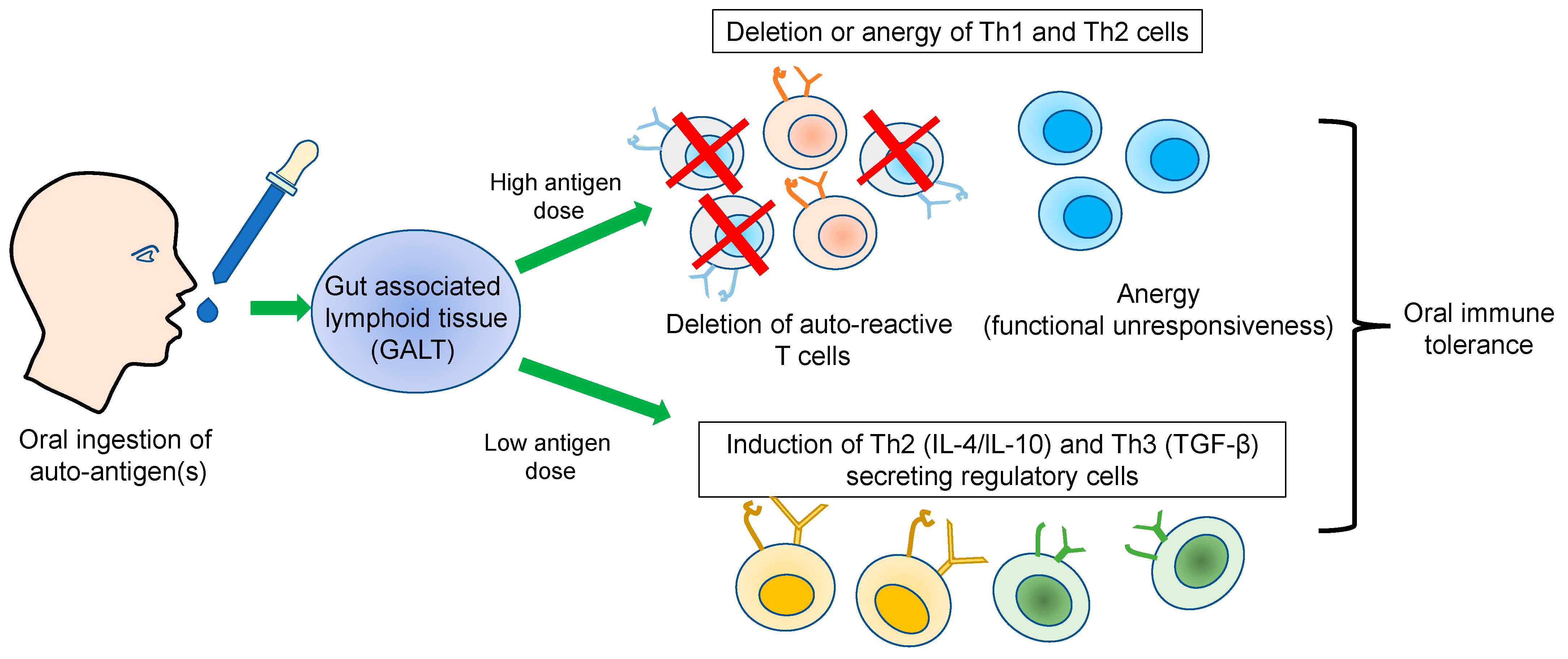
2. Oral Immune Tolerance
3. Immunological Basis of Oral Tolerance
3.1. Dose Effect
3.2. Bystander Effect
4. Tools and Approaches for Antigen-Specific Oral Immune Tolerance
4.1. Synthetic Nanoparticles
4.2. Plant-Based Antigen Delivery
5. Antigen Nonspecific Oral Immune Tolerance
5.1. Therapeutics
5.2. Probiotics
5.3. Plant-Based Products
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Aaron Lerner, P.J.; Matthias, T. The World Incidence and Prevalence of Autoimmune Diseases is Increasing. Int. J. Celiac Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef][Green Version]
- Meier, F.M.; Frerix, M.; Hermann, W.; Muller-Ladner, U. Current immunotherapy in rheumatoid arthritis. Immunotherapy 2013, 5, 955–974. [Google Scholar] [CrossRef][Green Version]
- Fujita, H.; Soyka, M.B.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy. Clin. Transl. Allergy 2012, 2, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelson, H.S. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016, 37, 268–272. [Google Scholar] [CrossRef]
- Anderson, R.P.; Jabri, B. Vaccine against autoimmune disease: Antigen-specific immunotherapy. Curr. Opin. Immunol. 2013, 25, 410–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korn, T.; Bettelli, E.; Gao, W.; Awasthi, A.; Jager, A.; Strom, T.B.; Oukka, M.; Kuchroo, V.K. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007, 448, 484–487. [Google Scholar] [CrossRef][Green Version]
- Schaffert, H.; Pelz, A.; Saxena, A.; Losen, M.; Meisel, A.; Thiel, A.; Kohler, S. IL-17-producing CD4(+) T cells contribute to the loss of B-cell tolerance in experimental autoimmune myasthenia gravis. Eur. J. Immunol. 2015, 45, 1339–1347. [Google Scholar] [CrossRef]
- Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 2017, 17, 281–294. [Google Scholar] [CrossRef]
- Mostarica-Stojkovic, M. Mechanisms of the induction of autoimmunity. Srp. Arh. Celok. Lek. 2005, 133 (Suppl. 1), 9–15. [Google Scholar] [CrossRef]
- Fang, Q.; Li, T.; Chen, P.; Wu, Y.; Wang, T.; Mo, L.; Ou, J.; Nandakumar, K.S. Comparative Analysis on Abnormal Methylome of Differentially Expressed Genes and Disease Pathways in the Immune Cells of RA and SLE. Front. Immunol. 2021, 12, 668007. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.C.; Hafler, D.A. Antigen-specific therapy for autoimmune disease. Curr. Opin. Immunol. 2000, 12, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Peakman, M.; Dayan, C.M. Antigen-specific immunotherapy for autoimmune disease: Fighting fire with fire? Immunology 2001, 104, 361–366. [Google Scholar] [CrossRef]
- Levich, J.D.; Parks, D.E.; Weigle, W.O. Tolerance induction in antigen-specific helper T cell clones and lines in vitro. J. Immunol. 1985, 135, 873–878. [Google Scholar] [CrossRef]
- Falb, D.; Briner, T.J.; Sunshine, G.H.; Bourque, C.R.; Luqman, M.; Gefter, M.L.; Kamradt, T. Peripheral tolerance in T cell receptor-transgenic mice: Evidence for T cell anergy. Eur. J. Immunol. 1996, 26, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Auphan, N.; Schonrich, G.; Malissen, M.; Barad, M.; Hammerling, G.; Arnold, B.; Malissen, B.; Schmitt-Verhulst, A.M. Influence of antigen density on degree of clonal deletion in T cell receptor transgenic mice. Int. Immunol. 1992, 4, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Liblau, R.S.; Tisch, R.; Shokat, K.; Yang, X.; Dumont, N.; Goodnow, C.C.; McDevitt, H.O. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 3031–3036. [Google Scholar] [CrossRef][Green Version]
- Sytwu, H.K.; Liblau, R.S.; McDevitt, H.O. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity 1996, 5, 17–30. [Google Scholar] [CrossRef][Green Version]
- Sakaguchi, S.; Wing, K.; Miyara, M. Regulatory T cells-a brief history and perspective. Eur. J. Immunol. 2007, 37 (Suppl. 1), S116–S123. [Google Scholar] [CrossRef]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef][Green Version]
- Miyara, M.; Wing, K.; Sakaguchi, S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J. Allergy Clin. Immunol. 2009, 123, 749–755. [Google Scholar] [CrossRef]
- Bettini, M.; Vignali, D.A. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr. Opin. Immunol. 2009, 21, 612–618. [Google Scholar] [CrossRef][Green Version]
- Luczynski, W.; Stasiak-Barmuta, A.; Urban, R.; Urban, M.; Florys, B.; Hryszko, M. Lower percentages of T regulatory cells in children with type 1 diabetes-preliminary report. Pediatr. Endocrinol. Diabetes Metab. 2009, 15, 34–38. [Google Scholar]
- Michel, L.; Berthelot, L.; Pettre, S.; Wiertlewski, S.; Lefrere, F.; Braudeau, C.; Brouard, S.; Soulillou, J.P.; Laplaud, D.A. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J. Clin. Investig. 2008, 118, 3411–3419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wing, K.; Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010, 11, 7–13. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T. Regulatory T cells: How do they suppress immune responses? Int. Immunol. 2009, 21, 1105–1111. [Google Scholar] [CrossRef][Green Version]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Mustafa, S.; Hussain, M.F.; Latif, M.; Ijaz, M.; Asif, M.; Hassan, M.; Faisal, M.; Iqbal, F. A Missense Mutation (c.1037 G > C, p. R346P) in PAPSS2 Gene Results in Autosomal Recessive form of Brachyolmia Type 1 (Hobaek Form) in A Consanguineous Family. Genes 2022, 13, 2096. [Google Scholar] [CrossRef] [PubMed]
- Mangan, P.R.; Harrington, L.E.; O’Quinn, D.B.; Helms, W.S.; Bullard, D.C.; Elson, C.O.; Hatton, R.D.; Wahl, S.M.; Schoeb, T.R.; Weaver, C.T. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006, 441, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. The role of transforming growth factor beta in T helper 17 differentiation. Immunology 2018, 155, 24–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kehrl, J.H.; Wakefield, L.M.; Roberts, A.B.; Jakowlew, S.; Alvarez-Mon, M.; Derynck, R.; Sporn, M.B.; Fauci, A.S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 1986, 163, 1037–1050. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Kuchroo, V.K.; Inobe, J.; Hafler, D.A.; Weiner, H.L. Regulatory T cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science 1994, 265, 1237–1240. [Google Scholar] [CrossRef]
- Kaufman, D.L.; Clare-Salzler, M.; Tian, J.; Forsthuber, T.; Ting, G.S.; Robinson, P.; Atkinson, M.A.; Sercarz, E.E.; Tobin, A.J.; Lehmann, P.V. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 1993, 366, 69–72. [Google Scholar] [CrossRef]
- Carambia, A.; Freund, B.; Schwinge, D.; Heine, M.; Laschtowitz, A.; Huber, S.; Wraith, D.C.; Korn, T.; Schramm, C.; Lohse, A.W.; et al. TGF-beta-dependent induction of CD4(+)CD25(+)Foxp3(+) Tregs by liver sinusoidal endothelial cells. J. Hepatol. 2014, 61, 594–599. [Google Scholar] [CrossRef]
- Umeshappa, C.S.; Singha, S.; Blanco, J.; Shao, K.; Nanjundappa, R.H.; Yamanouchi, J.; Pares, A.; Serra, P.; Yang, Y.; Santamaria, P. Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines. Nat. Commun. 2019, 10, 2150. [Google Scholar] [CrossRef][Green Version]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef][Green Version]
- Zhu, G.; Xu, Y.; Cen, X.; Nandakumar, K.S.; Liu, S.; Cheng, K. Targeting pattern-recognition receptors to discover new small molecule immune modulators. Eur. J. Med. Chem. 2018, 144, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, R.A.; Martins, A.J.; Angermann, B.R.; Dutta, B.; Ng, C.E.; Uderhardt, S.; Tsang, J.S.; Fraser, I.D.; Meier-Schellersheim, M.; Germain, R.N. Distinct NF-kappaB and MAPK Activation Thresholds Uncouple Steady-State Microbe Sensing from Anti-pathogen Inflammatory Responses. Cell. Syst. 2016, 2, 378–390. [Google Scholar] [CrossRef][Green Version]
- Clark, M.A.; Jepson, M.A.; Hirst, B.H. Exploiting M cells for drug and vaccine delivery. Adv. Drug. Deliv. Rev. 2001, 50, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Bland, P.W.; Warren, L.G. Antigen presentation by epithelial cells of the rat small intestine. II. Selective induction of suppressor T cells. Immunology 1986, 58, 9–14. [Google Scholar] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef]
- Campbell, D.J.; Debes, G.F.; Johnston, B.; Wilson, E.; Butcher, E.C. Targeting T cell responses by selective chemokine receptor expression. Semin. Immunol. 2003, 15, 277–286. [Google Scholar] [CrossRef]
- Miller, A.; Lider, O.; Roberts, A.B.; Sporn, M.B.; Weiner, H.L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc. Natl. Acad. Sci. USA 1992, 89, 421–425. [Google Scholar] [CrossRef][Green Version]
- Whitacre, C.C.; Gienapp, I.E.; Orosz, C.G.; Bitar, D.M. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J. Immunol. 1991, 147, 2155–2163. [Google Scholar] [CrossRef]
- Chambers, C.A.; Sullivan, T.J.; Truong, T.; Allison, J.P. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur. J. Immunol. 1998, 28, 3137–3143. [Google Scholar] [CrossRef]
- Lider, O.; Santos, L.M.; Lee, C.S.; Higgins, P.J.; Weiner, H.L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J. Immunol. 1989, 142, 748–752. [Google Scholar] [CrossRef]
- Barone, K.S.; Herms, B.; Karlosky, L.; Murray, S.; Qualls, J. Effect of in vivo administration of anti-CTLA-4 monoclonal antibody and IL-12 on the induction of low-dose oral tolerance. Clin. Exp. Immunol. 2002, 130, 196–203. [Google Scholar]
- Wilkes, D.S.; Chew, T.; Flaherty, K.R.; Frye, S.; Gibson, K.F.; Kaminski, N.; Klemsz, M.J.; Lange, W.; Noth, I.; Rothhaar, K. Oral immunotherapy with type V collagen in idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1393–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, A.; Lider, O.; Weiner, H.L. Antigen-driven bystander suppression after oral administration of antigens. J. Exp. Med. 1991, 174, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jin, W.; Cook, M.; Weiner, H.L.; Wahl, S.M. Oral delivery of group A streptococcal cell walls augments circulating TGF-beta and suppresses streptococcal cell wall arthritis. J. Immunol. 1998, 161, 6297–6304. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.N.; Gibson, C.; Ryden, A.K.; Perdue, N.; Boursalian, T.E.; Pagni, P.P.; Coppieters, K.; Skonberg, C.; Porsgaard, T.; von Herrath, M.; et al. Oral insulin (human, murine, or porcine) does not prevent diabetes in the non-obese diabetic mouse. Clin. Immunol. 2016, 164, 28–33. [Google Scholar] [CrossRef]
- Kim, W.U.; Lee, W.K.; Ryoo, J.W.; Kim, S.H.; Kim, J.; Youn, J.; Min, S.Y.; Bae, E.Y.; Hwang, S.Y.; Park, S.H.; et al. Suppression of collagen-induced arthritis by single administration of poly(lactic-co-glycolic acid) nanoparticles entrapping type II collagen: A novel treatment strategy for induction of oral tolerance. Arthritis Rheum. 2002, 46, 1109–1120. [Google Scholar] [CrossRef]
- Haggag, Y.; Abdel-Wahab, Y.; Ojo, O.; Osman, M.; El-Gizawy, S.; El-Tanani, M.; Faheem, A.; McCarron, P. Preparation and in vivo evaluation of insulin-loaded biodegradable nanoparticles prepared from diblock copolymers of PLGA and PEG. Int. J. Pharm. 2016, 499, 236–246. [Google Scholar] [CrossRef][Green Version]
- Salvioni, L.; Fiandra, L.; Del Curto, M.D.; Mazzucchelli, S.; Allevi, R.; Truffi, M.; Sorrentino, L.; Santini, B.; Cerea, M.; Palugan, L.; et al. Oral delivery of insulin via polyethylene imine-based nanoparticles for colonic release allows glycemic control in diabetic rats. Pharmacol. Res. 2016, 110, 122–130. [Google Scholar] [CrossRef]
- Jabri, T.R.T.; Razzak, A.; Aziz, S.; Imran, M.; Sikandar, B.; Elhissi, A.; Shafiullah Aslam, S.M.; RazaShah, M. Fabrication of hesperidin hybrid lecithin-folic acid silver nanoparticles and its evaluation as anti-arthritis formulation in autoimmune arthritic rat model. J. Mol. Struct. 2023, 1276, 134722. [Google Scholar] [CrossRef]
- Raimondi, G.Z.Y.; Hou, S.; Wang, J.; Calderon-Colon, X.; Tiburzi, O.; Igleasias Lozano, M.; Patrone, J. Prevention of Type 1 Diabetes via a Lipid Nanoparticle-Based Oral Immunotherapy that Targets Selective Lymphoid Tissues. J. Immunol. 2022, 208, 174.12. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Y.; Yin, Z.; Menassa, R.; Brandle, J.E.; Jevnikar, A.M. Induction of oral tolerance to prevent diabetes with transgenic plants requires glutamic acid decarboxylase (GAD) and IL-4. Proc. Natl. Acad. Sci. USA 2004, 101, 5680–5685. [Google Scholar] [CrossRef][Green Version]
- Ma, S.W.; Zhao, D.L.; Yin, Z.Q.; Mukherjee, R.; Singh, B.; Qin, H.Y.; Stiller, C.R.; Jevnikar, A.M. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat. Med. 1997, 3, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Ruhlman, T.; Ahangari, R.; Devine, A.; Samsam, M.; Daniell, H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts--oral administration protects against development of insulitis in non-obese diabetic mice. Plant. Biotechnol. J. 2007, 5, 495–510. [Google Scholar] [CrossRef][Green Version]
- Iizuka, M.; Wakasa, Y.; Tsuboi, H.; Asashima, H.; Hirota, T.; Kondo, Y.; Matsumoto, I.; Sumida, T.; Takaiwa, F. Prophylactic effect of the oral administration of transgenic rice seeds containing altered peptide ligands of type II collagen on rheumatoid arthritis. Biosci. Biotechnol. Biochem. 2014, 78, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Hansson, C.; Schon, K.; Kalbina, I.; Strid, A.; Andersson, S.; Bokarewa, M.I.; Lycke, N.Y. Feeding transgenic plants that express a tolerogenic fusion protein effectively protects against arthritis. Plant. Biotechnol. J. 2016, 14, 1106–1115. [Google Scholar] [CrossRef][Green Version]
- Dittel, L.J.; Dittel, B.N.; Brod, S.A. Ingested ACTH blocks Th17 production by inhibiting GALT IL-6. J. Neurol. Sci. 2020, 409, 116602. [Google Scholar] [CrossRef]
- Dittel, L.J.; Dittel, B.N.; Brod, S.A. Ingested (Oral) Adrenocorticotropic Hormone Inhibits IL-17 in the Central Nervous System in the Mouse Model of Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Immunohorizons 2022, 6, 497–506. [Google Scholar] [CrossRef]
- Brod, S.A.; Bauer, V.L. Ingested (oral) tocilizumab inhibits EAE. Cytokine 2014, 68, 86–93. [Google Scholar] [CrossRef]
- Brod, S.A. Ingested (oral) anti-IL-12/23 inhibits EAE. J. Neurol. Sci. 2016, 361, 19–25. [Google Scholar] [CrossRef]
- Fang, X.; Liu, C.; Zhang, K.; Yang, W.; Wu, Z.; Shen, S.; Ma, Y.; Lu, X.; Chen, Y.; Lu, T.; et al. Discovery of orally active 1,4,5,6,8-pentaazaacenaphthylens as novel, selective, and potent covalent BTK inhibitors for the treatment of rheumatoid arthritis. Eur. J. Med. Chem. 2023, 246, 114940. [Google Scholar] [CrossRef] [PubMed]
- Salehipour, Z.; Haghmorad, D.; Sankian, M.; Rastin, M.; Nosratabadi, R.; Soltan Dallal, M.M.; Tabasi, N.; Khazaee, M.; Nasiraii, L.R.; Mahmoudi, M. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed. Pharmacother. 2017, 95, 1535–1548. [Google Scholar] [CrossRef]
- Ibrahim, H.I.M.; Sheikh, A.; Khalil, H.E.; Khalifa, A. Bacillus amyloliquifaciens-Supplemented Camel Milk Suppresses Neuroinflammation of Autoimmune Encephalomyelitis in a Mouse Model by Regulating Inflammatory Markers. Nutrients 2023, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, D.; Ortiz-Velez, L.C.; Perry, J.L.; Pennington, M.W.; Hyser, J.M.; Britton, R.A.; Beeton, C. A bioengineered probiotic for the oral delivery of a peptide Kv1.3 channel blocker to treat rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2023, 120, e2211977120. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, M.A.F.; Pinheiro-Rosa, N.; Oliveira, R.P.; Aguiar, S.L.F.; Miranda, M.C.G.; Lemos, L.; Souza, A.L.; Dos Reis, D.S.; Medeiros, S.R.; Goncalves, W.A.; et al. Hsp65-producing Lactococcus lactis inhibits experimental autoimmune encephalomyelitis by preventing cell migration into spinal cord. Cell. Immunol. 2023, 384, 104661. [Google Scholar] [CrossRef]
- Ying, S.; Yang, H.; Gu, Q.; Wu, Z.; Zou, N.; Wang, C.Z.; Wan, C.; Yuan, C.S. The Small-Molecule compound baicalein alleviates experimental autoimmune encephalomyelitis by suppressing pathogenetic CXCR6(+) CD4 cells. Int. Immunopharmacol. 2023, 114, 109562. [Google Scholar] [CrossRef] [PubMed]
- Que, W.; Lin, H.; Li, X.; Zhang, B.; Liu, M.; Hu, X.; Fu, J.; Cheng, Y.; Qiu, H. Koumine ameliorates concanavalin A-induced autoimmune hepatitis in mice: Involvement of the Nrf2, NF-kappaB pathways, and gut microbiota. Int. Immunopharmacol. 2023, 114, 109573. [Google Scholar] [CrossRef] [PubMed]
- Nevius, E.; Srivastava, P.K.; Basu, S. Oral ingestion of Capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunol. 2012, 5, 76–86. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakya, A.K.; Mallick, B.; Nandakumar, K.S. A Perspective on Oral Immunotherapeutic Tools and Strategies for Autoimmune Disorders. Vaccines 2023, 11, 1031. https://doi.org/10.3390/vaccines11061031
Shakya AK, Mallick B, Nandakumar KS. A Perspective on Oral Immunotherapeutic Tools and Strategies for Autoimmune Disorders. Vaccines. 2023; 11(6):1031. https://doi.org/10.3390/vaccines11061031
Chicago/Turabian StyleShakya, Akhilesh Kumar, Buddhadev Mallick, and Kutty Selva Nandakumar. 2023. "A Perspective on Oral Immunotherapeutic Tools and Strategies for Autoimmune Disorders" Vaccines 11, no. 6: 1031. https://doi.org/10.3390/vaccines11061031
APA StyleShakya, A. K., Mallick, B., & Nandakumar, K. S. (2023). A Perspective on Oral Immunotherapeutic Tools and Strategies for Autoimmune Disorders. Vaccines, 11(6), 1031. https://doi.org/10.3390/vaccines11061031






