Crosstalk between COVID-19 Infection and Kidney Diseases: A Review on the Metabolomic Approaches
Abstract
1. Introduction
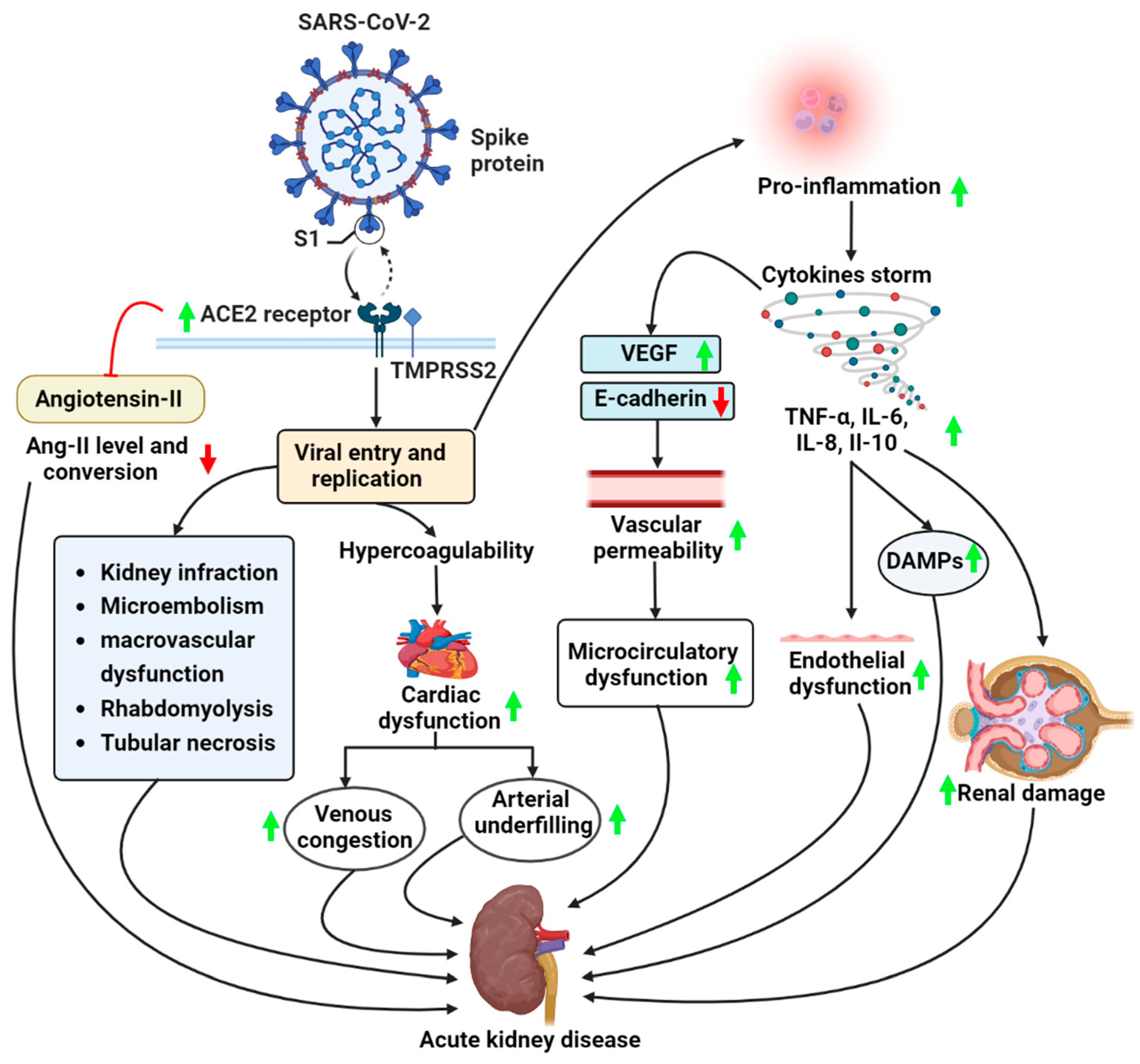
2. COVID-19 and AKI
2.1. Pathogenesis of AKI in COVID-19 Patients
2.1.1. Overactivation of Angiotensin II Pathway
2.1.2. Dysregulated Immune Responses in COVID-19
2.1.3. Rhabdomyolysis
2.1.4. Sepsis
2.2. Pathophysiology of COVID-19 and AKI
2.2.1. Tubular Injury
2.2.2. Endothelial Activation and Microvascular Injury
2.2.3. Podocyte Injury
2.3. Inflammatory Responses
2.4. Cytokine Storm Syndrome
3. COVID-19 and CKD
4. Metabolomics, COVID-19, and Kidney Injury
4.1. Untargeted Metabolomics in COVID-19 and Other Metabolomics Technologies
4.2. Antiviral Drug Efficacy in COVID-19
4.3. Metabolites Involved in the Diagnosis and Prognosis of COVID-19
4.4. Alteration of Plasma Metabolomics in COVID-19
5. Integrated Genomics and Metabolomics in Nephrology
5.1. CKD
5.2. Diabetic Nephropathy
6. Therapeutic Management of Renal Disorder Patients with COVID-19
6.1. Vitamins
6.2. Metal Supplements
6.3. Melatonin
6.4. Renal Replacement Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Tan, W. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Veesler, D. Structural insights into coronavirus entry. Adv. Virus Res. 2019, 105, 93–116. [Google Scholar] [PubMed]
- Chiu, M.-C. Suggested management of immunocompromized kidney patients suffering from SARS. Pediatr. Nephrol. 2003, 18, 1204–1205. [Google Scholar] [CrossRef]
- Kumar, D.; Tellier, R.; Draker, R.; Levy, G.; Humar, A. Severe Acute Respiratory Syndrome (SARS) in a Liver Transplant Recipient and Guidelines for Donor SARS Screening. Wiley Online Libr. 2003, 3, 977–981. [Google Scholar] [CrossRef]
- AlGhamdi, M.; Mushtaq, F.; Awn, N.; Shalhoub, S. MERS CoV Infection in Two Renal Transplant Recipients: Case Report. Wiley Online Libr. 2015, 15, 1101–1104. [Google Scholar] [CrossRef]
- Li, Z.; Wu, M.; Yao, J.; Guo, J.; Liao, X.; Song, S.; Li, J.; Duan, G.; Zhou, Y.; Wu, X. Caution on kidney dysfunctions of COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Krüger, N.; Müller, M.; Drosten, C.; Pöhlmann, S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv 2020. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; Ding, Y.; Lu, W.; Wang, J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Damage After 2019-nCoV Infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Santos, R.A.; Ferreira, A.J.; Verano-Braga, T.; Bader, M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: New players of the renin-angiotensin system. J. Endocrinol. 2013, 216, R1–R17. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ye, Q. Kidney involvement in COVID-19 and its treatments. Wiley Online Libr. 2021, 93, 1387–1395. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, H.; Yan, S.; Li, D.; Li, Y.; Gong, Z. Coronavirus disease 19 infection does not result in acute kidney injury: An analysis of 116 hospitalized patients from Wuhan, China. Am. J. Nephrol. 2020, 51, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Findling, M.G.; Blendon, R.J.; Benson, J.M. Delayed Care with Harmful Health Consequences—Reported Experiences from National Surveys During Coronavirus Disease 2019. JAMA Health Forum 2020, 1, e201463. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Iwashyna, T.J.; Blackwood, B.; Calandra, T.; Chlan, L.L.; Choong, K.; Connolly, B.; Dark, P.; Ferrucci, L.; Finfer, S.; et al. Understanding and Enhancing Sepsis Survivorship. Priorities for Research and Practice. ATS J. 2019, 200, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Puthumana, J.; Thiessen-Philbrook, H.; Xu, L.; Coca, S.G.; Garg, A.X.; Himmelfarb, J.; Bhatraju, P.K.; Ikizler, T.A.; Siew, E.D.; Ware, L.B.; et al. Biomarkers of inflammation and repair in kidney disease progression. J. Clin. Investig. 2021, 131, e139927. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Qiu, S.; Wang, X. Metabolomics insights into pathophysiological mechanisms of nephrology. Int. Urol. Nephrol. 2014, 46, 1025–1030. [Google Scholar] [CrossRef]
- Hasan, M.R.; Suleiman, M.; Pérez-López, A. Metabolomics in the Diagnosis and Prognosis of COVID-19. Front. Genet. 2021, 12, 721556. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef]
- Vincent, F.; Spillemaeker, H.; Kyheng, M.; Belin-Vincent, C.; Delhaye, C.; Piérache, A.; Denimal, T.; Verdier, B.; Debry, N.; Moussa, M.; et al. Ultrasound Guidance to Reduce Vascular and Bleeding Complications of Percutaneous Transfemoral Transcatheter Aortic Valve Replacement: A Propensity Score-Matched Comparison. J. Am. Heart Assoc. 2020, 9, e014916. [Google Scholar] [CrossRef]
- Pei, G.; Zhang, Z.; Peng, J.; Liu, L.; Zhang, C.; Yu, C.; Ma, Z.; Huang, Y.; Liu, W.; Yao, Y.; et al. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J. Am. Soc. Nephrol. JASN 2020, 31, 1157–1165. [Google Scholar] [CrossRef]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Robbins-Juarez, S.Y.; Qian, L.; King, K.L.; Stevens, J.S.; Husain, S.A.; Radhakrishnan, J.; Mohan, S. Outcomes for Patients With COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Kidney Int. Rep. 2020, 5, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Malik, A.; Zhang, Q.; Sadeghpour, A.; Zhu, Y.; Li, Q. Improving Cd risk managements of rice cropping system by integrating source-soil-rice-human chain for a typical intensive industrial and agricultural region. J. Clean. Prod. 2021, 313, 127883. [Google Scholar] [CrossRef]
- Gupta, S.; Coca, S.G.; Chan, L.; Melamed, M.L.; Brenner, S.K.; Hayek, S.S.; Sutherland, A.; Puri, S.; Srivastava, A.; Leonberg-Yoo, A.; et al. AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 161–176. [Google Scholar] [CrossRef]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Batlle, D.; Soler, M.J.; Sparks, M.A.; Hiremath, S.; South, A.M.; Welling, P.A.; Swaminathan, S. Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J. Am. Soc. Nephrol. 2020, 31, 1380–1383. [Google Scholar] [CrossRef]
- Kolhe, N.V.; Fluck, R.J.; Selby, N.M.; Taal, M.W. Acute kidney injury associated with COVID-19: A retrospective cohort study. PLoS Med. 2020, 17, e1003406. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Mehta, R.L.; Connor, M.J.; Liu, K.D.; Ostermann, M.; Rimmelé, T.; Zarbock, A.; Bell, S.; Bihorac, A.; et al. COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020, 16, 747–764. [Google Scholar] [CrossRef]
- Fan, J.; Jahed, V.; Klavins, K. Metabolomics in Bone Research. Metabolites 2021, 11, 434. [Google Scholar] [CrossRef]
- Sharma, P.; Uppal, N.N.; Wanchoo, R.; Shah, H.H.; Yang, Y.; Parikh, R.; Khanin, Y.; Madireddy, V.; Larsen, C.P.; Jhaveri, K.D.; et al. COVID-19-Associated Kidney Injury: A Case Series of Kidney Biopsy Findings. J. Am. Soc. Nephrol. 2020, 31, 1948–1958. [Google Scholar] [CrossRef] [PubMed]
- Golmai, P.; Larsen, C.P.; DeVita, M.V.; Wahl, S.J.; Weins, A.; Rennke, H.G.; Bijol, V.; Rosenstock, J.L. Histopathologic and Ultrastructural Findings in Postmortem Kidney Biopsy Material in 12 Patients with AKI and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1944–1947. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Brealey, J.K. Visualization of putative coronavirus in kidney. Kidney Int. 2020, 98, 231–232. [Google Scholar] [CrossRef]
- Mori, Y.; Fink, C.; Ichimura, T.; Sako, K.; Mori, M.; Lee, N.N.; Aschauer, P.; Das, K.M.P.; Hong, S.; Song, M. KIM-1/TIM-1 is a Receptor for SARS-CoV-2 in Lung and Kidney. medRxiv 2022. [Google Scholar] [CrossRef]
- Dargelos, M.; Couturier, A.; Ferlicot, S.; Goujon, J.M.; Roque-Afonso, A.M.; Gault, E.; Touchard, G.; Ory, C.; Kaaki, S.; Vilaine, E.; et al. Severe acute respiratory syndrome coronavirus 2 indirectly damages kidney structures. Clin. Kidney J. 2020, 13, 1101–1104. [Google Scholar] [CrossRef]
- Pan, X.W.; Xu, D.; Zhang, H.; Zhou, W.; Wang, L.H.; Cui, X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med. 2020, 46, 1114–1116. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Tomlinson, L.; Edmonston, D.; Hiremath, S.; Sparks, M.A. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020, 16, 305–307. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, J.; Zhang, Y.; Dong, M.; Wang, S.; Zhang, Q.; Liu, F.F.; Zhang, K.; Zhang, C. Angiotensin-converting enzyme 2 and angiotensin 1-7: Novel therapeutic targets. Nat. Rev. Cardiol. 2014, 11, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Groß, S.; Jahn, C.; Cushman, S.; Bär, C.; Thum, T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J. Mol. Cell Cardiol. 2020, 144, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.M.; Wysocki, J.; Batlle, D. Interaction of SARS-CoV-2 and Other Coronavirus With ACE (Angiotensin-Converting Enzyme)-2 as Their Main Receptor: Therapeutic Implications. Hypertension 2020, 76, 1339–1349. [Google Scholar] [CrossRef]
- Henry, B.M.; Benoit, J.L.; Berger, B.A.; Pulvino, C.; Lavie, C.J.; Lippi, G.; Benoit, S.W. Coronavirus disease 2019 is associated with low circulating plasma levels of angiotensin 1 and angiotensin 1,7. J. Med. Virol. 2021, 93, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Serfozo, P.; Wysocki, J.; Gulua, G.; Schulze, A.; Ye, M.; Liu, P.; Jin, J.; Bader, M.; Myöhänen, T.; García-Horsman, J.A.; et al. Ang II (Angiotensin II) Conversion to Angiotensin-(1-7) in the Circulation Is POP (Prolyloligopeptidase)-Dependent and ACE2 (Angiotensin-Converting Enzyme 2)-Independent. Hypertension 2020, 75, 173–182. [Google Scholar] [CrossRef]
- Villa, G.; Romagnoli, S.; De Rosa, S.; Greco, M.; Resta, M.; Pomarè Montin, D.; Prato, F.; Patera, F.; Ferrari, F.; Rotondo, G.; et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: A pilot study. Crit. Care 2020, 24, 605. [Google Scholar] [CrossRef]
- Leisman, D.E.; Deutschman, C.S.; Legrand, M. Facing COVID-19 in the ICU: Vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020, 46, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Lavie, C.J.; Henry, B.M.; Sanchis-Gomar, F. Do genetic polymorphisms in angiotensin converting enzyme 2 (ACE2) gene play a role in coronavirus disease 2019 (COVID-19)? Clin. Chem. Lab. Med. 2020, 58, 1415–1422. [Google Scholar] [CrossRef]
- Hall, A.; Busse, L.W.; Ostermann, M. Angiotensin in Critical Care. Crit. Care 2018, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Bokoch, M.P. The Yin and Yang of the Renin-Angiotensin-Aldosterone System in Acute Kidney Injury. Am. J. Respir. Crit. Care Med. 2021, 203, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Dudoignon, E.; Moreno, N.; Deniau, B.; Coutrot, M.; Longer, R.; Amiot, Q.; Mebazaa, A.; Pirracchio, R.; Depret, F.; Legrand, M. Activation of the renin-angiotensin-aldosterone system is associated with Acute Kidney Injury in COVID-19. Anaesth. Crit. Care Pain Med. 2020, 39, 453–455. [Google Scholar] [CrossRef]
- Ozkan, S.; Cakmak, F.; Konukoglu, D.; Biberoglu, S.; Ipekci, A.; Akdeniz, Y.S.; Bolayirli, I.M.; Balkan, I.I.; Dumanli, G.Y.; Ikizceli, I. Efficacy of Serum Angiotensin II Levels in Prognosis of Patients With Coronavirus Disease 2019. Crit. Care Med. 2021, 49, e613–e623. [Google Scholar] [CrossRef]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef] [PubMed]
- Casciola-Rosen, L.; Thiemann, D.R.; Andrade, F.; Trejo Zambrano, M.I.; Hooper, J.E.; Leonard, E.K.; Spangler, J.B.; Cox, A.L.; Machamer, C.E.; Sauer, L.; et al. IgM autoantibodies recognizing ACE2 are associated with severe COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Kugaevskaya, E.V.; Kolesanova, E.F.; Kozin, S.A.; Veselovsky, A.V.; Dedinsky, I.R.; Elisseeva, Y.E. Epitope mapping of the domains of human angiotensin converting enzyme. Biochim. Biophys. Acta 2006, 1760, 959–965. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Khamsi, R. Rogue antibodies could be driving severe COVID-19. Nature 2021, 590, 29–31. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Bawor, M.; Sairam, S.; Rozewicz, R.; Viegas, S.; Comninos, A.N.; Abbara, A. Rhabdomyolysis after COVID-19 Infection: A Case Report and Review of the Literature. Viruses 2022, 14, 2255. [Google Scholar] [CrossRef]
- de Oliveira, P.; Cunha, K.; Neves, P.; Muniz, M.; Gatto, G.; Salgado Filho, N.; Guedes, F.; Silva, G. Renal Morphology in Coronavirus Disease: A Literature Review. Medicina 2021, 57, 258. [Google Scholar] [CrossRef]
- Ahmadian, E.; Hosseiniyan Khatibi, S.M.; Razi Soofiyani, S.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Zununi Vahed, S. COVID-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021, 31, e2176. [Google Scholar] [CrossRef]
- Naicker, S.; Yang, C.W.; Hwang, S.J.; Liu, B.C.; Chen, J.H.; Jha, V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020, 97, 824–828. [Google Scholar] [CrossRef]
- Sharma, Y.; Nasr, S.H.; Larsen, C.P.; Kemper, A.; Ormsby, A.H.; Williamson, S.R. COVID-19-Associated Collapsing Focal Segmental Glomerulosclerosis: A Report of 2 Cases. Kidney Med. 2020, 2, 493–497. [Google Scholar] [CrossRef]
- Valente-Acosta, B.; Moreno-Sanchez, F.; Fueyo-Rodriguez, O.; Palomar-Lever, A. Rhabdomyolysis as an initial presentation in a patient diagnosed with COVID-19. BMJ Case Rep. 2020, 13, e236719. [Google Scholar] [CrossRef]
- Khosla, S.G.; Nylen, E.S.; Khosla, R. Rhabdomyolysis in Patients Hospitalized With COVID-19 Infection: Five Case Series. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620984603. [Google Scholar] [CrossRef] [PubMed]
- Chedid, N.R.; Udit, S.; Solhjou, Z.; Patanwala, M.Y.; Sheridan, A.M.; Barkoudah, E. COVID-19 and Rhabdomyolysis. J. Gen. Intern. Med. 2020, 35, 3087–3090. [Google Scholar] [CrossRef] [PubMed]
- Borku Uysal, B.; Ikitimur, H.; Yavuzer, S.; Islamoglu, M.S.; Cengiz, M. Case Report: A COVID-19 Patient Presenting with Mild Rhabdomyolysis. Am. J. Trop. Med. Hyg. 2020, 103, 847–850. [Google Scholar] [CrossRef]
- Solís, J.G.; Esquivel Pineda, A.; Alberti Minutti, P.; Albarrán Sánchez, A. Case Report: Rhabdomyolysis in a Patient with COVID-19: A Proposed Diagnostic-Therapeutic Algorithm. Am. J. Trop. Med. Hyg. 2020, 103, 1158–1161. [Google Scholar] [CrossRef]
- Jin, M.; Tong, Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg. Infect. Dis. 2020, 26, 1618. [Google Scholar] [CrossRef]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and acute kidney injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Smarz-Widelska, I.; Grywalska, E.; Morawska, I.; Forma, A.; Michalski, A.; Mertowski, S.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P.; Korona-Glowniak, I.; Parczewski, M.; et al. Pathophysiology and Clinical Manifestations of COVID-19-Related Acute Kidney Injury-The Current State of Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 7082. [Google Scholar] [CrossRef]
- Swanson, B.T. Rhabdomyolysis and COVID-19. 2021, Student Publications. 954. Available online: https://cupola.gettysburg.edu/student_scholarship/954 (accessed on 14 January 2023).
- Langenberg, C.; Wan, L.; Egi, M.; May, C.N.; Bellomo, R. Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Med. 2007, 33, 1614–1618. [Google Scholar] [CrossRef]
- Joannidis, M.; Truebsbach, S.; Bijuklic, K.; Schratzberger, P.; Dunzedorfer, S.; Wintersteiger, S.; Lhotta, K.; Mayer, G.; Wiedermann, C.J. Neutrophil transmigration in renal proximal tubular LLC-PK1 cells. Cell Physiol. Biochem. 2004, 14, 101–112. [Google Scholar] [CrossRef]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.-R.; Payen, D. Sepsis in European intensive care units: Results of the SOAP study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Dellepiane, S.; Marengo, M.; Cantaluppi, V. Detrimental cross-talk between sepsis and acute kidney injury: New pathogenic mechanisms, early biomarkers and targeted therapies. Crit. Care 2016, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santoriello, D.; Khairallah, P.; Bomback, A.S.; Xu, K.; Kudose, S.; Batal, I.; Barasch, J.; Radhakrishnan, J.; D’Agati, V.; Markowitz, G. Postmortem Kidney Pathology Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 2158–2167. [Google Scholar] [CrossRef]
- Schurink, B.; Roos, E.; Radonic, T.; Barbe, E.; Bouman, C.S.C.; de Boer, H.H.; de Bree, G.J.; Bulle, E.B.; Aronica, E.M.; Florquin, S.; et al. Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe 2020, 1, e290–e299. [Google Scholar] [CrossRef]
- Copur, S.; Berkkan, M.; Basile, C.; Tuttle, K.; Kanbay, M. Post-acute COVID-19 syndrome and kidney diseases: What do we know? J. Nephrol. 2022, 35, 795–805. [Google Scholar] [CrossRef]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Asakura, H.; Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021, 113, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-W.; Ilyas, I.; Weng, J.-P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2022, 1–15. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef]
- Reiser, J.; Sever, S. Podocyte biology and pathogenesis of kidney disease. Annu. Rev. Med. 2013, 64, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.; Jog, P.; Lee, J.H.; Blackler, D.; Wilmot, M.; D’Agati, V.; Markowitz, G.; Kopp, J.B.; Alper, S.L.; Pollak, M.R.; et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int. 2015, 87, 332–342. [Google Scholar] [CrossRef]
- Friedman, D.J.; Pollak, M.R. Apolipoprotein L1 and Kidney Disease in African Americans. Trends Endocrinol. Metab. 2016, 27, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, A.; Nast, C.C.; Bagga, A.; Wei, L.; Nurmamet, A.; Cohen, A.H.; Jordan, S.C.; Toyoda, M. Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int. 2001, 59, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.M.; Klotman, P.E.; D’Agati, V.D. HIV-associated nephropathy: Clinical presentation, pathology, and epidemiology in the era of antiretroviral therapy. Semin. Nephrol. 2008, 28, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Cantaluppi, V.; Quercia, A.D.; Dellepiane, S.; Ferrario, S.; Camussi, G.; Biancone, L. Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol. Dial. Transplant. 2014, 29, 2004–2011. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Assenzio, B.; Pasero, D.; Romanazzi, G.M.; Pacitti, A.; Lanfranco, G.; Puntorieri, V.; Martin, E.L.; Mascia, L.; Monti, G.; et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive Care Med. 2008, 34, 1638–1645. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Zhang, J.; Xia, J.; Mo, K.; Wang, J. Incorporating fish habitat requirements of the complete life cycle into ecological flow regime estimation of rivers. Ecohydrology 2020, 13, e2204. [Google Scholar] [CrossRef]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual Screening of Natural Products against Type II Transmembrane Serine Protease (TMPRSS2), the Priming Agent of Coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef]
- Dorjee, K.; Kim, H.; Bonomo, E.; Dolma, R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS ONE 2020, 15, e0243191. [Google Scholar] [CrossRef]
- Ji, W.; Huh, K.; Kang, M.; Hong, J.; Bae, G.H.; Lee, R.; Na, Y.; Choi, H.; Gong, S.Y.; Choi, Y.H.; et al. Effect of Underlying Comorbidities on the Infection and Severity of COVID-19 in Korea: A Nationwide Case-Control Study. J. Korean Med. Sci. 2020, 35, e237. [Google Scholar] [CrossRef] [PubMed]
- Jdiaa, S.S.; Mansour, R.; El Alayli, A.; Gautam, A.; Thomas, P.; Mustafa, R.A. COVID–19 and chronic kidney disease: An updated overview of reviews. J. Nephrol. 2022, 35, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Park, G.U.; Moon, Y.R.; Sung, K. Clinical Characteristics and Risk Factors for Fatality and Severity in Patients with Coronavirus Disease in Korea: A Nationwide Population-Based Retrospective Study Using the Korean Health Insurance Review and Assessment Service (HIRA) Database. Int. J. Environ. Res. Public Health 2020, 17, 8559. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cui, P.; Zeng, S.; Wang, S.; Feng, X.; Xu, S.; Li, R.; Gao, Y.; Yu, R.; Wang, Y.; et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: A multicenter, retrospective, cohort study. EClinicalMedicine 2020, 25, 100471. [Google Scholar] [CrossRef]
- Kim, S.R.; Nam, S.H.; Kim, Y.R. Risk Factors on the Progression to Clinical Outcomes of COVID-19 Patients in South Korea: Using National Data. Int. J. Environ. Res. Public Health 2020, 17, 8847. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Giannis, D.; Allen, S.L.; Tsang, J.; Flint, S.; Pinhasov, T.; Williams, S.; Tan, G.; Thakur, R.; Leung, C.; Snyder, M.; et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: The CORE-19 registry. Blood 2021, 137, 2838–2847. [Google Scholar] [CrossRef]
- Flythe, J.E.; Assimon, M.M.; Tugman, M.J.; Chang, E.H.; Gupta, S.; Shah, J.; Sosa, M.A.; Renaghan, A.D.; Melamed, M.L.; Wilson, F.P.; et al. Characteristics and Outcomes of Individuals With Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am. J. Kidney Dis. 2021, 77, 190–203.e191. [Google Scholar] [CrossRef]
- Mahalingasivam, V.; Su, G.; Iwagami, M.; Davids, M.R.; Wetmore, J.B.; Nitsch, D. COVID-19 and kidney disease: Insights from epidemiology to inform clinical practice. Nat. Rev. Nephrol. 2022, 18, 485–498. [Google Scholar] [CrossRef]
- Agur, T.; Ben-Dor, N.; Goldman, S.; Lichtenberg, S.; Herman-Edelstein, M.; Yahav, D.; Rozen-Zvi, B.; Zingerman, B. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients-A prospectivecohort study. Nephrol. Dial. Transplant. 2021, 36, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Oliver, S.G.J.M. The metabolome 18 years on: A concept comes of age. Metabolomics 2016, 12, 1–8. [Google Scholar] [CrossRef]
- López-Hernández, Y.; Monárrez-Espino, J.; Oostdam, A.H.; Delgado, J.E.C.; Zhang, L.; Zheng, J.; Valdez, J.J.O.; Mandal, R.; González, F.L.O.; Moreno, J.C.B.; et al. Targeted metabolomics identifies high performing diagnostic and prognostic biomarkers for COVID-19. Sci. Rep. 2021, 11, 14732. [Google Scholar] [CrossRef]
- Overmyer, K.A.; Shishkova, E.; Miller, I.J.; Balnis, J.; Bernstein, M.N.; Peters-Clarke, T.M.; Meyer, J.G.; Quan, Q.; Muehlbauer, L.K.; Trujillo, E.A.; et al. Large-Scale Multi-omic Analysis of COVID-19 Severity. Cell Syst. 2021, 12, 23–40.e27. [Google Scholar] [CrossRef]
- Collino, S.; Martin, F.P.J.; Rezzi, S.J. Clinical metabolomics paves the way towards future healthcare strategies. Br. J. Clin. Pharmacol. 2013, 75, 619–629. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.K.; Sullivan, P.F. The authorship network of genome-wide association studies. Nat. Genet. 2012, 44, 113. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.E.; Kosmicki, J.A.; Damask, A.; Sharma, D.; Roberts, G.H.L.; Justice, A.E.; Banerjee, N.; Coignet, M.V.; Yadav, A.; Leader, J.B.; et al. Genome-wide analysis provides genetic evidence that ACE2 influences COVID-19 risk and yields risk scores associated with severe disease. Nat. Genet. 2022, 54, 382–392. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef]
- Shelton, J.F.; Shastri, A.J.; Ye, C.; Weldon, C.H.; Filshtein-Sonmez, T.; Coker, D.; Symons, A.; Esparza-Gordillo, J.; Chubb, A.; Fitch, A.; et al. Trans-ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity. Nat. Genet. 2021, 53, 801–808. [Google Scholar] [CrossRef]
- Thibord, F.; Chan, M.V.; Chen, M.-H.; Johnson, A.D.J.M. A year of COVID-19 GWAS results from the GRASP portal reveals potential SARS-CoV-2 modifiers. medRxiv 2021. [Google Scholar] [CrossRef]
- Raines, N.H.; Cheung, M.D.; Wilson, L.S.; Edberg, J.C.; Erdmann, N.B.; Schmaier, A.A.; Berryhill, T.F.; Manickas-Hill, Z.; Li, J.Z.; Yu, X.G.; et al. Nicotinamide Adenine Dinucleotide Biosynthetic Impairment and Urinary Metabolomic Alterations Observed in Hospitalized Adults With COVID-19-Related Acute Kidney Injury. Kidney Int. Rep. 2021, 6, 3002–3013. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor Necrosis Factor-α Convertase (ADAM17) Mediates Regulated Ectodomain Shedding of the Severe-acute Respiratory Syndrome-Coronavirus (SARS-CoV) Receptor, Angiotensin-converting Enzyme-2 (ACE2) *. J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar]
- Palacios, Y.; Ruiz, A.; Ramón-Luing, L.A.; Ocaña-Guzman, R.; Barreto-Rodriguez, O.; Sánchez-Monciváis, A.; Tecuatzi-Cadena, B.; Regalado-García, A.G.; Pineda-Gudiño, R.D.; García-Martínez, A.; et al. Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality. Int. J. Mol. Sci. 2021, 22, 8423. [Google Scholar] [CrossRef] [PubMed]
- Zanol, J.F.; Niño, O.M.S.; da Costa, C.S.; Freitas-Lima, L.C.; Miranda-Alves, L.; Graceli, J.B. Tributyltin and high-refined carbohydrate diet lead to metabolic and reproductive abnormalities, exacerbating premature ovary failure features in the female rats. Reprod. Toxicol. 2021, 103, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Guo, D.; Chen, C.B.; Wang, W.; Schuster, M.; Loibner, H.; Penninger, J.M.; Scholey, J.W.; Kassiri, Z.; Oudit, G.Y. Prevention of Angiotensin II–Mediated Renal Oxidative Stress, Inflammation, and Fibrosis by Angiotensin-Converting Enzyme 2. Hypertension 2011, 57, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.; Wang, K.; Colombo, D.; Gheblawi, M.; Rasmuson, J.; Mandal, R.; Del Nonno, F.; Chiu, B.; Scholey, J.W.; Soler, M.J.; et al. Urinary angiotensin-converting enzyme 2 and metabolomics in COVID-19 mediated kidney injury. Clin. Kidney J. 2022, 16, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J. A Untargeted metabolomics strategies—Challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Raja, G.; Jang, Y.-K.; Suh, J.-S.; Kim, H.-S.; Ahn, S.H.; Kim, T.-J. Microcellular Environmental Regulation of Silver Nanoparticles in Cancer Therapy: A Critical Review. Cancers 2020, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- Raja, G.; Selvaraj, V.; Suk, M.; Suk, K.T.; Kim, T.-J. Metabolic phenotyping analysis of graphene oxide nanosheets exposures in breast cancer cells: Metabolomics profiling techniques. Process Biochem. 2021, 104, 39–45. [Google Scholar] [CrossRef]
- Raja, G.; Cao, S.; Kim, D.-H.; Kim, T.-J. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mater. Sci. Eng. C 2020, 107, 110303. [Google Scholar] [CrossRef]
- Chen, W.; Yao, M.; Chen, M.; Ou, Z.; Yang, Q.; He, Y.; Zhang, N.; Deng, M.; Wu, Y.; Chen, R.J. Using an untargeted metabolomics approach to analyze serum metabolites in COVID-19 patients with nucleic acid turning negative. Front. Pharmacol. 2022, 13, 964037. [Google Scholar] [CrossRef]
- Frampas, C.F.; Longman, K.; Spick, M.; Lewis, H.-M.; Costa, C.D.; Stewart, A.; Dunn-Walters, D.; Greener, D.; Evetts, G.; Skene, D. Untargeted saliva metabolomics by liquid chromatography—Mass spectrometry reveals markers of COVID-19 severity. PLoS ONE 2022, 17, e0274967. [Google Scholar] [CrossRef]
- Pozzi, C.; Levi, R.; Braga, D.; Carli, F.; Darwich, A.; Spadoni, I.; Oresta, B.; Dioguardi, C.C.; Peano, C.; Ubaldi, L.J. A ‘Multiomic’Approach of Saliva Metabolomics, Microbiota, and Serum Biomarkers to Assess the Need of Hospitalization in Coronavirus Disease 2019. Gastro Hep Adv. 2022, 1, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, C.; Li, D.; Huang, Q.; Liu, D.; Zhang, Y.; Ye, C.; Zhou, D.; Wang, Y.; Tan, Y.J. Metabolomic analyses reveal new stage-specific features of COVID-19. Eur. Respir. J. 2022, 59, 2100284. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell 2020, 183, 1479–1495.e1420. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B. Single-Cell Metabolomics by Mass Spectrometry. Methods Mol. Biol. 2020, 2064, 1–8. [Google Scholar] [PubMed]
- Palermo, A. Charting Metabolism Heterogeneity by Nanostructure Imaging Mass Spectrometry: From Biological Systems to Subcellular Functions. J. Am. Soc. Mass Spectrom. 2020, 31, 2392–2400. [Google Scholar] [CrossRef]
- Su, Y.; Ko, M.E.; Cheng, H.; Zhu, R.; Xue, M.; Wang, J.; Lee, J.W.; Frankiw, L.; Xu, A.; Wong, S.; et al. Multi-omic single-cell snapshots reveal multiple independent trajectories to drug tolerance in a melanoma cell line. Nat. Commun. 2020, 11, 2345. [Google Scholar] [CrossRef]
- Xue, M.; Wei, W.; Su, Y.; Johnson, D.; Heath, J.R. Supramolecular Probes for Assessing Glutamine Uptake Enable Semi-Quantitative Metabolic Models in Single Cells. J. Am. Chem. Soc. 2016, 138, 3085–3093. [Google Scholar] [CrossRef]
- Lee, D.; Du, J.; Yu, R.; Su, Y.; Heath, J.R.; Wei, L. Visualizing Subcellular Enrichment of Glycogen in Live Cancer Cells by Stimulated Raman Scattering. Anal. Chem. 2020, 92, 13182–13191. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Su, Y.; Qian, C.; Yuan, D.; Miao, K.; Lee, D.; Ng, A.H.C.; Wijker, R.S.; Ribas, A.; Levine, R.D.; et al. Raman-guided subcellular pharmaco-metabolomics for metastatic melanoma cells. Nat. Commun. 2020, 11, 4830. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, H.; Shao, S.; Lu, X.; Mo, L.; Tsang, J.; Zeng, P.; Guo, Z.; Wang, S.; Nathanson, D.A.; et al. Surface Immobilization of Redox-Labile Fluorescent Probes: Enabling Single-Cell Co-Profiling of Aerobic Glycolysis and Oncogenic Protein Signaling Activities. Angew. Chem. Int. Ed. Engl. 2018, 57, 11554–11558. [Google Scholar] [CrossRef]
- Hung, I.F.; Lung, K.C.; Tso, E.Y.; Liu, R.; Chung, T.W.; Chu, M.Y.; Ng, Y.Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Chen, F.; Chan, K.H.; Jiang, Y.; Kao, R.Y.; Lu, H.T.; Fan, K.W.; Cheng, V.C.; Tsui, W.H.; Hung, I.F.; Lee, T.S.; et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yao, Y.; Yeung, M.-L.; Deng, W.; Bao, L.; Jia, L.; Li, F.; Xiao, C.; Gao, H.; Yu, P.J.; et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015, 212, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, J.P.A.; Assis, L.C.; de Castro, A.A.; da Cunha, E.F.F.; Nepovimova, E.; Kuca, K.; de Castro Ramalho, T.; de Almeida La Porta, F. Effect of drug metabolism in the treatment of SARS-CoV-2 from an entirely computational perspective. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Migaud, M.; Gandotra, S.; Chand, H.S.; Gillespie, M.N.; Thannickal, V.J.; Langley, R.J. Metabolomics to Predict Antiviral Drug Efficacy in COVID-19. Am. J. Respir. Cell Mol. Biol. 2020, 63, 396–398. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Wang, L.; Zhu, X.; Zhang, M.; Zheng, J. Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis. Front. Pharmacol. 2022, 13, 833679. [Google Scholar] [CrossRef] [PubMed]
- Chouchana, L.; Preta, L.-H.; Tisseyre, M.; Terrier, B.; Treluyer, J.-M.; Montastruc, F. Kidney disorders as serious adverse drug reactions of remdesivir in coronavirus disease 2019: A retrospective case–noncase study. Kidney Int. 2021, 99, 1235–1236. [Google Scholar]
- Schneider, J.; Jaenigen, B.; Wagner, D.; Rieg, S.; Hornuss, D.; Biever, P.M.; Kern, W.V.; Walz, G. Therapy with lopinavir/ritonavir and hydroxychloroquine is associated with acute kidney injury in COVID-19 patients. PLoS ONE 2021, 16, e0249760. [Google Scholar] [CrossRef]
- Hanai, Y.; Yoshizawa, S.; Matsuo, K.; Uekusa, S.; Miyazaki, T.; Nishimura, K.; Mabuchi, T.; Ohashi, H.; Ishii, Y.; Tateda, K.; et al. Evaluation of risk factors for uric acid elevation in COVID-19 patients treated with favipiravir. Diagn. Microbiol. Infect. Dis. 2022, 102, 115640. [Google Scholar] [CrossRef]
- Hall, A.M.; Edwards, S.G.; Lapsley, M.; Connolly, J.O.; Chetty, K.; O’Farrell, S.; Unwin, R.J.; Williams, I.G. Subclinical Tubular Injury in HIV-Infected Individuals on Antiretroviral Therapy: A Cross-sectional Analysis. Am. J. Kidney Dis. 2009, 54, 1034–1042. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, A.U.; Kobori, H. Melatonin in chronic kidney disease: A promising chronotherapy targeting the intrarenal renin–angiotensin system. Hypertens. Res. 2019, 42, 920–923. [Google Scholar] [CrossRef]
- Personett, H.A.; Kayhart, B.M.; Barreto, E.F.; Tosh, P.; Dierkhising, R.; Mara, K.; Leung, N. Renal Recovery following Liposomal Amphotericin B-Induced Nephrotoxicity. Int. J. Nephrol. 2019, 2019, 8629891. [Google Scholar] [CrossRef]
- Hammock, B.D.; Wang, W.; Gilligan, M.M.; Panigrahy, D. Eicosanoids: The overlooked storm in coronavirus disease 2019 (COVID-19)? Am. J. Pathol. 2020, 190, 1782–1788. [Google Scholar] [CrossRef]
- Hoxha, M. What about COVID-19 and arachidonic acid pathway? Eur. J. Clin. Pharmacol. 2020, 76, 1501–1504. [Google Scholar] [CrossRef]
- Mehta, R.; Chekmeneva, E.; Jackson, H.; Sands, C.; Mills, E.; Arancon, D.; Li, H.K.; Arkell, P.; Rawson, T.M.; Hammond, R.J.M. Antiviral metabolite 3′-deoxy-3′, 4′-didehydro-cytidine is detectable in serum and identifies acute viral infections including COVID-19. Med 2022, 3, 204–215.e206. [Google Scholar] [CrossRef]
- Rahnavard, A.; Mann, B.; Giri, A.; Chatterjee, R.; Crandall, K.A. Metabolite, protein, and tissue dysfunction associated with COVID-19 disease severity. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.-B.; Lu, Q.-Q.; Yu, Y.; Zhang, S.-Q.; Ke, P.-F.; Zhang, F.; Li, J.-C. Metabolite profile of COVID-19 revealed by UPLC-MS/MS-based widely targeted metabolomics. Front. Immunol. 2022, 13, 894107. [Google Scholar] [CrossRef]
- Lee, J.W.; Su, Y.; Baloni, P.; Chen, D.; Pavlovitch-Bedzyk, A.J.; Yuan, D.; Duvvuri, V.R.; Ng, R.H.; Choi, J.; Xie, J.; et al. Integrated analysis of plasma and single immune cells uncovers metabolic changes in individuals with COVID-19. Nat. Biotechnol. 2022, 40, 110–120. [Google Scholar] [CrossRef]
- Dagla, I.; Iliou, A.; Benaki, D.; Gikas, E.; Mikros, E.; Bagratuni, T.; Kastritis, E.; Dimopoulos, M.A.; Terpos, E.; Tsarbopoulos, A. Plasma Metabolomic Alterations Induced by COVID-19 Vaccination Reveal Putative Biomarkers Reflecting the Immune Response. Cells 2022, 11, 1241. [Google Scholar] [CrossRef]
- Wu, D.; Shu, T.; Yang, X.; Song, J.-X.; Zhang, M.; Yao, C.; Liu, W.; Huang, M.; Yu, Y.; Yang, Q.J. Plasma metabolomic and lipidomic alterations associated with COVID-19. Natl. Sci. Rev. 2020, 7, 1157–1168. [Google Scholar] [CrossRef]
- Oliveira, L.B.; Mwangi, V.I.; Sartim, M.A.; Delafiori, J.; Sales, G.M.; de Oliveira, A.N.; Busanello, E.N.B.; e Val, F.F.d.A.; Xavier, M.S.; Costa, F.T.J. Metabolomic Profiling of Plasma Reveals Differential Disease Severity Markers in COVID-19 Patients. Front. Microbiol. 2022, 13, 844283. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef]
- Park, J.; Shrestha, R.; Qiu, C.; Kondo, A.; Huang, S.; Werth, M.; Li, M.; Barasch, J.; Suszták, K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 2018, 360, 758–763. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Limbutara, K.; Knepper, M.A.; Payne, D.M.; Pisitkun, T. From Molecules to Mechanisms: Functional Proteomics and Its Application to Renal Tubule Physiology. Physiol. Rev. 2018, 98, 2571–2606. [Google Scholar] [CrossRef] [PubMed]
- Kalim, S.; Rhee, E.P. Metabolomics and Kidney Precision Medicine. Clin. J. Am. Soc. Nephrol. 2017, 12, 1726–1727. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Schwedhelm, E.; Zeller, T. Integrated genomics and metabolomics in nephrology. Nephrol. Dial. Transplant. 2013, 29, 1467–1474. [Google Scholar] [CrossRef]
- Shah, V.O.; Townsend, R.R.; Feldman, H.I.; Pappan, K.L.; Kensicki, E.; Vander Jagt, D.L. Plasma metabolomic profiles in different stages of CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 363–370. [Google Scholar] [CrossRef]
- Schefold, J.C.; Zeden, J.P.; Fotopoulou, C.; von Haehling, S.; Pschowski, R.; Hasper, D.; Volk, H.D.; Schuett, C.; Reinke, P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transplant. 2009, 24, 1901–1908. [Google Scholar] [CrossRef]
- Hanna, M.H.; Brophy, P.D. Metabolomics in pediatric nephrology: Emerging concepts. Pediatr. Nephrol. 2015, 30, 881–887. [Google Scholar] [CrossRef]
- Suhre, K.; Shin, S.Y.; Petersen, A.K.; Mohney, R.P.; Meredith, D.; Wägele, B.; Altmaier, E.; Deloukas, P.; Erdmann, J.; Grundberg, E.; et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 2011, 477, 54–60. [Google Scholar] [CrossRef]
- Connaughton, D.M.; Kennedy, C.; Shril, S.; Mann, N.; Murray, S.L.; Williams, P.A.; Conlon, E.; Nakayama, M.; van der Ven, A.T.; Ityel, H.; et al. Monogenic causes of chronic kidney disease in adults. Kidney Int. 2019, 95, 914–928. [Google Scholar] [CrossRef]
- Cocchi, E.; Nestor, J.G.; Gharavi, A.G. Clinical Genetic Screening in Adult Patients with Kidney Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 1497–1510. [Google Scholar] [CrossRef]
- Lata, S.; Marasa, M.; Li, Y.; Fasel, D.A.; Groopman, E.; Jobanputra, V.; Rasouly, H.; Mitrotti, A.; Westland, R.; Verbitsky, M. Whole-exome sequencing in adults with chronic kidney disease: A pilot study. Ann. Intern. Med. 2018, 168, 100–109. [Google Scholar] [CrossRef]
- Provenzano, M.; Serra, R.; Garofalo, C.; Michael, A.; Crugliano, G.; Battaglia, Y.; Ielapi, N.; Bracale, U.M.; Faga, T.; Capitoli, G.; et al. OMICS in Chronic Kidney Disease: Focus on Prognosis and Prediction. Int. J. Mol. Sci. 2022, 23, 336. [Google Scholar] [CrossRef]
- Köttgen, A.; Glazer, N.L.; Dehghan, A.; Hwang, S.J.; Katz, R.; Li, M.; Yang, Q.; Gudnason, V.; Launer, L.J.; Harris, T.B.; et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 2009, 41, 712–717. [Google Scholar] [CrossRef]
- Köttgen, A.; Hwang, S.J.; Larson, M.G.; Van Eyk, J.E.; Fu, Q.; Benjamin, E.J.; Dehghan, A.; Glazer, N.L.; Kao, W.H.; Harris, T.B.; et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J. Am. Soc. Nephrol. 2010, 21, 337–344. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, K.; Saigusa, D.; Suzuki, T.; Takeuchi, Y.; Mishima, E.; Yamamoto, Y.; Ishida, A.; Sugawara, D.; Jinno, D.; et al. Indoxyl sulfate down-regulates SLCO4C1 transporter through up-regulation of GATA3. PLoS ONE 2013, 8, e66518. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Meisinger, C.; Döring, A.; Altmaier, E.; Belcredi, P.; Gieger, C.; Chang, D.; Milburn, M.V.; Gall, W.E.; Weinberger, K.M.; et al. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PLoS ONE 2010, 5, e13953. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, A.; Kawamura, Y.; Kanazawa, A.; Sekine, A.; Saito, S.; Tsunoda, T.; Koya, D.; Babazono, T.; Tanaka, Y.; Matsuda, M.; et al. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes 2005, 54, 1171–1178. [Google Scholar] [CrossRef]
- Salem, R.M.; Todd, J.N.; Sandholm, N.; Cole, J.B.; Chen, W.-M.; Andrews, D.; Pezzolesi, M.G.; McKeigue, P.M.; Hiraki, L.T.; Qiu, C.; et al. Genome-Wide Association Study of Diabetic Kidney Disease Highlights Biology Involved in Glomerular Basement Membrane Collagen. J. Am. Soc. Nephrol. 2019, 30, 2000. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, A.; Tanaka, Y.; Shinosaki, T.; Ikeda, M.; Watada, H.; Hirose, T.; Kawamori, R.; Maeda, S. ELMO1 increases expression of extracellular matrix proteins and inhibits cell adhesion to ECMs. Kidney Int. 2006, 70, 1769–1776. [Google Scholar] [CrossRef]
- Chiang, W.F.; Hsiao, P.J.; Chan, J.S. Vitamin D for Recovery of COVID-19 in Patients With Chronic Kidney Disease. Front. Nutr. 2022, 9, 930176. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Ioannou, G.N.; Kestenbaum, B.; Brunzell, J.D.; Weiss, N.S. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am. J. Kidney Dis. 2007, 50, 69–77. [Google Scholar] [CrossRef]
- Handelman, G.J. New insight on vitamin C in patients with chronic kidney disease. J. Ren. Nutr. 2011, 21, 110–112. [Google Scholar] [CrossRef]
- Biancatelli, R.; Berrill, M.; Marik, P. The antiviral properties of Vitamin C. Expert Rev. Anti-Infect. Ther. 2020, 18, 99–101. [Google Scholar] [CrossRef]
- Tavakol, S.; Seifalian, A.M. Vitamin E at a high dose as an anti-ferroptosis drug and not just a supplement for COVID-19 treatment. Biotechnol. Appl. Biochem. 2022, 69, 1058–1060. [Google Scholar] [CrossRef]
- Akhtar, S.; Das, J.K.; Ismail, T.; Wahid, M.; Saeed, W.; Bhutta, Z.A. Nutritional perspectives for the prevention and mitigation of COVID-19. Nutr. Rev. 2020, 79, 289–300. [Google Scholar] [CrossRef]
- Hulisz, D. Efficacy of zinc against common cold viruses: An overview. J. Am. Pharm. Assoc. 2004, 44, 594–603. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Chinni, V.; El-Khoury, J.; Perera, M.; Bellomo, R.; Jones, D.; Bolton, D.; Ischia, J.; Patel, O. Zinc supplementation as an adjunct therapy for COVID-19: Challenges and opportunities. Br. J. Clin. Pharmacol. 2021, 87, 3737–3746. [Google Scholar] [CrossRef]
- Mahajan, S.K. Zinc in kidney disease. J. Am. Coll. Nutr. 1989, 8, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Fakhrolmobasheri, M.; Mazaheri-Tehrani, S.; Kieliszek, M.; Zeinalian, M.; Abbasi, M.; Karimi, F.; Mozafari, A.M. COVID-19 and Selenium Deficiency: A Systematic Review. Biol. Trace Elem. Res. 2022, 200, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A. Chapter Five-Selenium and Selenium-Dependent Antioxidants in Chronic Kidney Disease. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 68, pp. 131–151. [Google Scholar]
- Pulido Perez, P.; Póndigo de Los Angeles, J.A.; Perez Peralta, A.; Ramirez Mojica, E.; Torres Rasgado, E.; Hernandez-Hernandez, M.E.; Romero, J.R. Reduction in Serum Magnesium Levels and Renal Function Are Associated with Increased Mortality in Obese COVID-19 Patients. Nutrients 2022, 14, 4054. [Google Scholar] [CrossRef]
- Tang, C.-F.; Ding, H.; Jiao, R.-Q.; Wu, X.-X.; Kong, L.-D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020, 886, 173546. [Google Scholar] [CrossRef]
- Barbosa, E.B.; Tomasi, C.D.; de Castro Damasio, D.; Vinhas, M.; Lichtenfels, B.; de Luca Francisco, V.; Fraga, C.M.; Ritter, C.; Dal-Pizzol, F. Effects of magnesium supplementation on the incidence of acute kidney injury in critically ill patients presenting with hypomagnesemia. Intensive Care Med. 2016, 42, 1084. [Google Scholar] [CrossRef]
- Hamroun, A.; Lenain, R.; Bigna, J.J.; Speyer, E.; Bui, L.; Chamley, P.; Pottier, N.; Cauffiez, C.; Dewaeles, E.; Dhalluin, X.; et al. Prevention of Cisplatin-Induced Acute Kidney Injury: A Systematic Review and Meta-Analysis. Drugs 2019, 79, 1567–1582. [Google Scholar] [CrossRef]
- Boga, J.A.; Coto-Montes, A.; Rosales-Corral, S.A.; Tan, D.X.; Reiter, R.J. Beneficial actions of melatonin in the management of viral infections: A new use for this “molecular handyman”? Rev. Med. Virol. 2012, 22, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Bahrampour Juybari, K.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res. 2020, 287, 198108. [Google Scholar] [CrossRef]
- Faridzadeh, A.; Tabashiri, A.; Miri, H.H.; Mahmoudi, M. The role of melatonin as an adjuvant in the treatment of COVID-19: A systematic review. Heliyon 2022, 8, e10906. [Google Scholar] [CrossRef] [PubMed]
- Shneider, A.; Kudriavtsev, A.; Vakhrusheva, A. Can melatonin reduce the severity of COVID-19 pandemic? Int. Rev. Immunol. 2020, 39, 153–162. [Google Scholar] [CrossRef]
- Hrenak, J.; Paulis, L.; Repova, K.; Aziriova, S.; J. Nagtegaal, E.; J. Reiter, R.; Simko, F.J. Melatonin and renal protection: Novel perspectives from animal experiments and human studies. Curr. Pharm. Des. 2015, 21, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Adikwu, E.; Brambaifa, N.; Obianime, W.A. Melatonin and alpha lipoic acid restore electrolytes and kidney morphology of lopinavir/ritonavir-treated rats. J. Nephropharmacol. 2020, 9, e06. [Google Scholar] [CrossRef]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 151–160. [Google Scholar] [CrossRef]
- Costa, R.L.D.; Sória, T.C.; Salles, E.F.; Gerecht, A.V.; Corvisier, M.F.; Menezes, M.A.M.; Ávila, C.D.S.; Silva, E.C.F.; Pereira, S.R.N.; Simvoulidis, L.F.N. Acute kidney injury in patients with Covid-19 in a Brazilian ICU: Incidence, predictors and in-hospital mortality. J. Bras. Nefrol. 2021, 43, 349–358. [Google Scholar] [CrossRef]
- Ronco, C.; Reis, T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 2020, 16, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, S.; Hajage, D.; Benichou, N.; Chaïbi, K.; Barbar, S.; Zarbock, A.; Lumlertgul, N.; Wald, R.; Bagshaw, S.M.; Srisawat, N.; et al. Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: A systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 2020, 395, 1506–1515. [Google Scholar] [CrossRef]
- Manns, B.; Doig, C.J.; Lee, H.; Dean, S.; Tonelli, M.; Johnson, D.; Donaldson, C. Cost of acute renal failure requiring dialysis in the intensive care unit: Clinical and resource implications of renal recovery*. Crit. Care Med. 2003, 31, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Joannidis, M.; Pani, A.; Floris, M.; De Rosa, S.; Kellum, J.A.; Ronco, C. Patient Selection and Timing of Continuous Renal Replacement Therapy. Blood Purif. 2016, 42, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sandoval, J.C.; Gaytan-Arocha, J.E.; Xolalpa-Chávez, P.; Mejia-Vilet, J.M.; Arvizu-Hernandez, M.; Rivero-Sigarroa, E.; Torruco-Sotelo, C.; Correa-Rotter, R.; Vega-Vega, O. Prolonged Intermittent Renal Replacement Therapy for Acute Kidney Injury in COVID-19 Patients with Acute Respiratory Distress Syndrome. Blood Purif. 2021, 50, 355–363. [Google Scholar] [CrossRef]
| Drug Class | Drug | Study Model | Kidney Injury | Mechanism | Reference |
|---|---|---|---|---|---|
| Antiviral | Remdesivir | Humans | AKI | ↑ proximal tubular epithelial cell necrosis | [162] |
| lopinavir/ritonavir | Humans | AKI | ↓ glomerular filtration rate, proteinuria, glycosuria | [163] | |
| Favipiravir | Humans | AKI | ↑ hyperuricemia | [164] | |
| Tenofovir | Humans | Subclinical tubular injury | ↑ proteinuria | [165] | |
| Antimalarial | Hydroxychloroquine and chloroquine | In-vivo: female C57BL/6JOlaHsd mice In-vitro: U2OS, HeLa cells, HeLa-RFP-GFP-LC3 cells, SNAP29- and GFP-STX17-expressing MEFs, MEFs | AKI | ↓ autophagy, ↑ lysosomal pH, mitochondrial damage | [166] |
| Antibiotic | Azithromycin | Humans | AKI | Induce interstitial nephritis | [167] |
| Amphotericin B | Humans | AKI | Arteriolar vasoconstriction; direct tubular injury | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murali, R.; Wanjari, U.R.; Mukherjee, A.G.; Gopalakrishnan, A.V.; Kannampuzha, S.; Namachivayam, A.; Madhyastha, H.; Renu, K.; Ganesan, R. Crosstalk between COVID-19 Infection and Kidney Diseases: A Review on the Metabolomic Approaches. Vaccines 2023, 11, 489. https://doi.org/10.3390/vaccines11020489
Murali R, Wanjari UR, Mukherjee AG, Gopalakrishnan AV, Kannampuzha S, Namachivayam A, Madhyastha H, Renu K, Ganesan R. Crosstalk between COVID-19 Infection and Kidney Diseases: A Review on the Metabolomic Approaches. Vaccines. 2023; 11(2):489. https://doi.org/10.3390/vaccines11020489
Chicago/Turabian StyleMurali, Reshma, Uddesh Ramesh Wanjari, Anirban Goutam Mukherjee, Abilash Valsala Gopalakrishnan, Sandra Kannampuzha, Arunraj Namachivayam, Harishkumar Madhyastha, Kaviyarasi Renu, and Raja Ganesan. 2023. "Crosstalk between COVID-19 Infection and Kidney Diseases: A Review on the Metabolomic Approaches" Vaccines 11, no. 2: 489. https://doi.org/10.3390/vaccines11020489
APA StyleMurali, R., Wanjari, U. R., Mukherjee, A. G., Gopalakrishnan, A. V., Kannampuzha, S., Namachivayam, A., Madhyastha, H., Renu, K., & Ganesan, R. (2023). Crosstalk between COVID-19 Infection and Kidney Diseases: A Review on the Metabolomic Approaches. Vaccines, 11(2), 489. https://doi.org/10.3390/vaccines11020489










