Adenovirus Vaccine Containing Truncated SARS-CoV-2 Spike Protein S1 Subunit Leads to a Specific Immune Response in Mice
Abstract
1. Introduction
2. Materials and Method
2.1. Cells and Animals
2.2. Vaccines
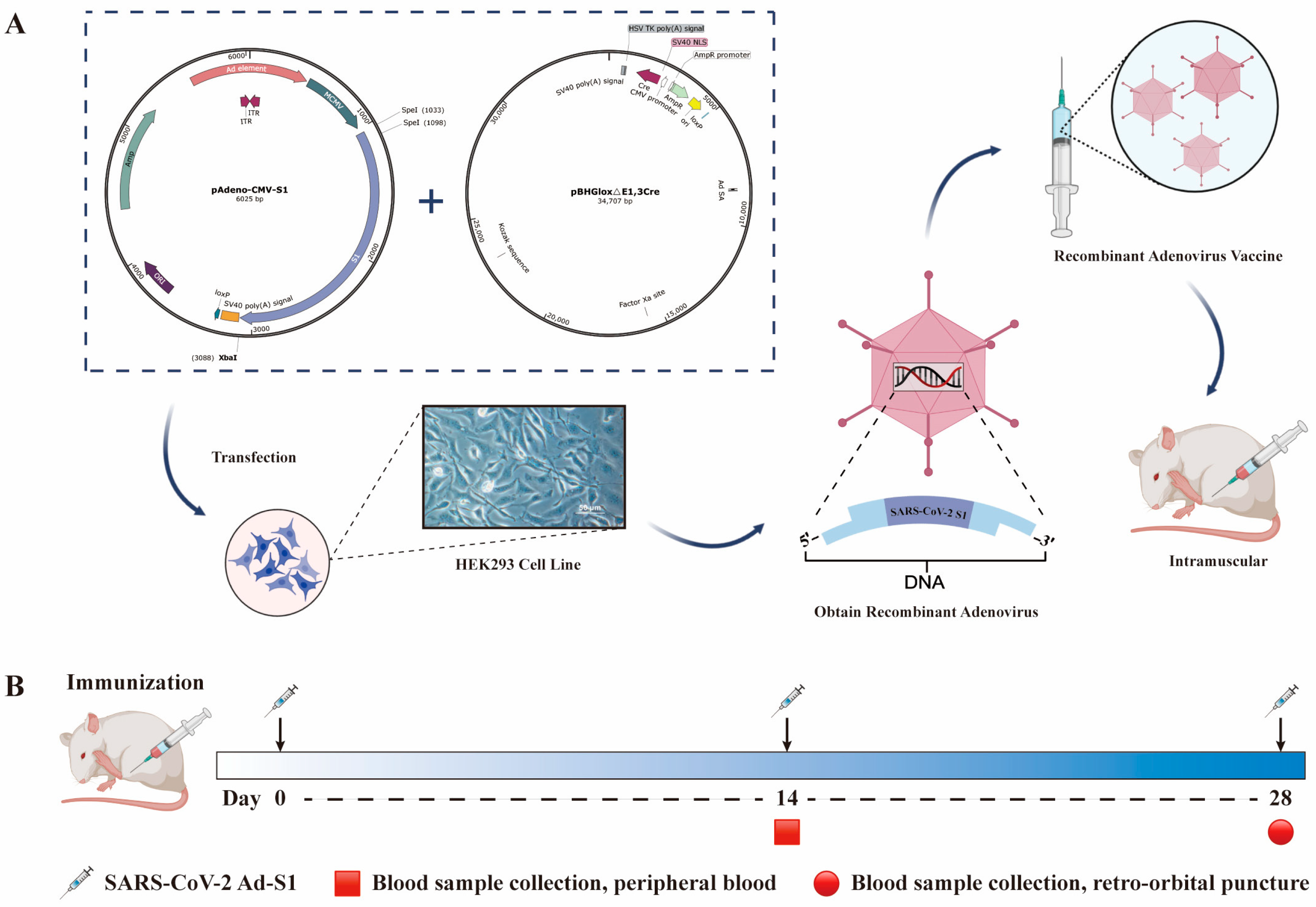
2.3. Recombinant Adenovirus Construction
2.4. Determination of Adenovirus Titer
2.5. Real-Time PCR
2.6. Western Blot
2.7. Immunofluorescence Assay
2.8. ELISA
2.9. Cytokine Determination
2.10. Neutralizing Antibody
2.10.1. Pseudovirus Neutralization Assay
2.10.2. Live Neutralization Assay
2.11. Statistical Analysis
3. Results
3.1. Recombinant Adenovirus Construction
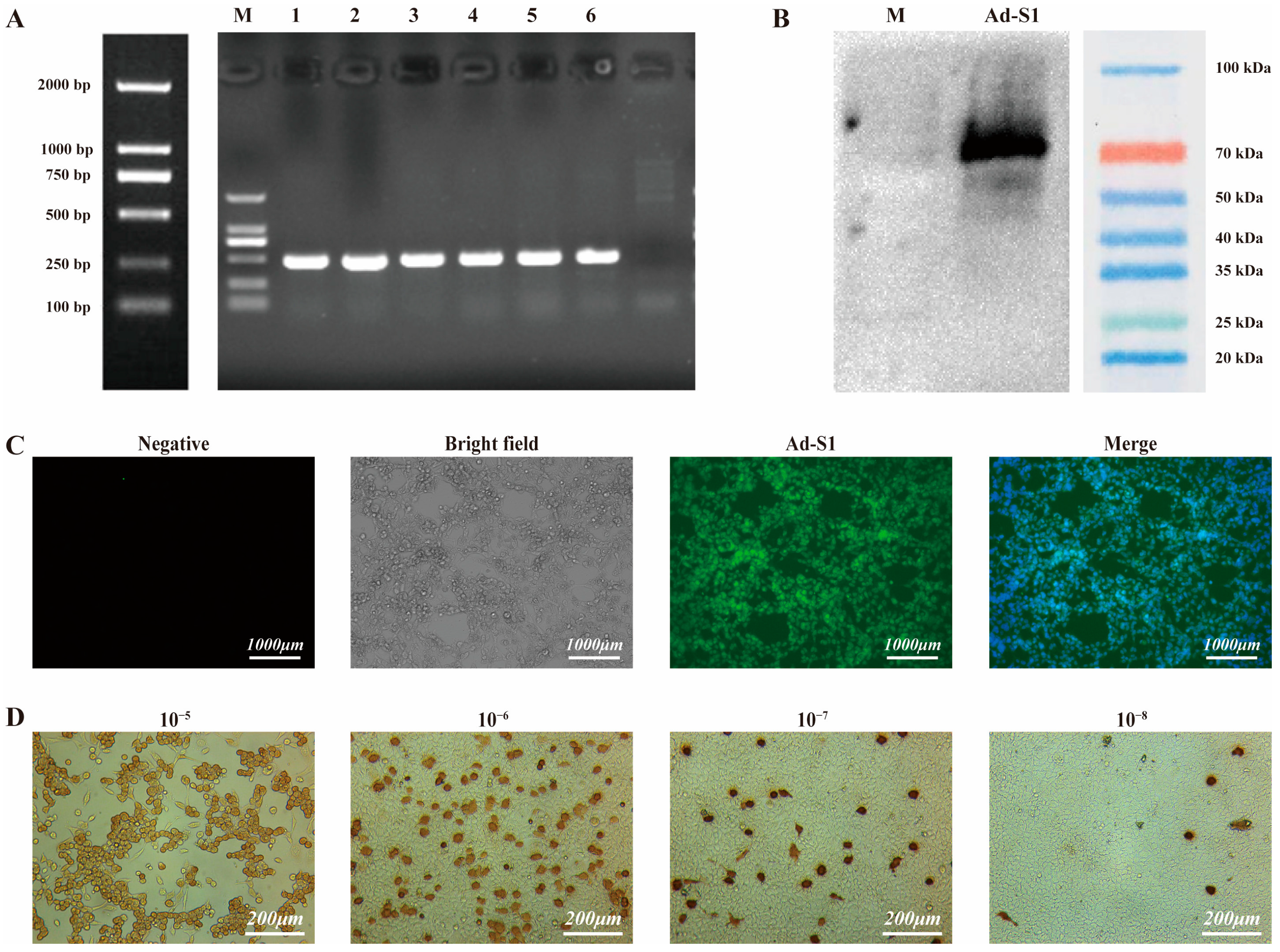
3.2. Characterization of Recombinant Adenovirus
3.3. Adenovirus Titer
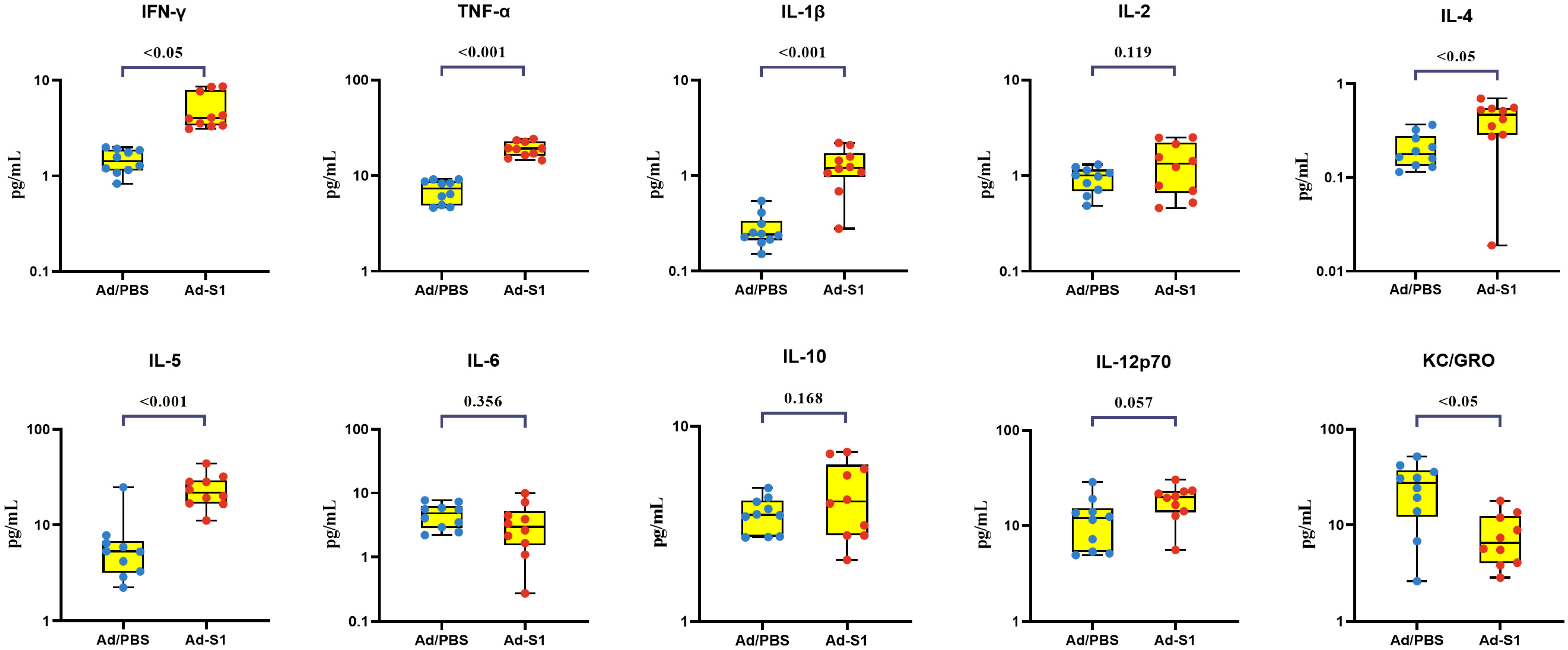
3.4. Post-Vaccination Cellular Immunity
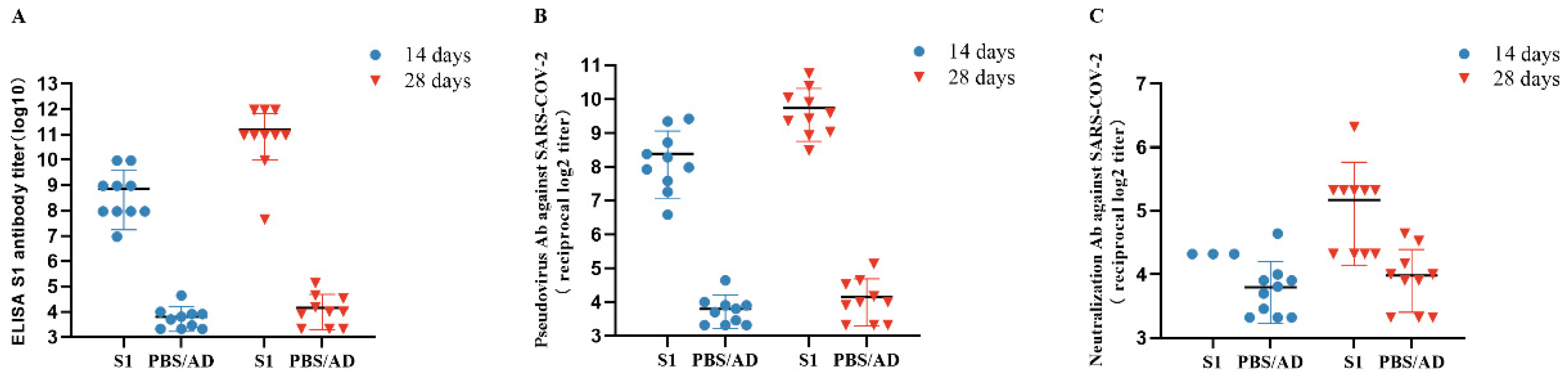
3.5. Detection of Anti-SARS-CoV-2 S1 Antibodies Post-Immunization
3.6. Neutralizing Antibody Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef]
- Pillay, T.S. Gene of the month: The 2019-nCoV/SARS-CoV-2 novel coronavirus spike protein. J. Clin. Pathol. 2020, 73, 366–369. [Google Scholar] [CrossRef]
- Sheinin, M.; Jeong, B.; Paidi, R.K.; Pahan, K. Regression of Lung Cancer in Mice by Intranasal Administration of SARS-CoV-2 Spike S1. Cancers 2022, 14, 5648. [Google Scholar] [CrossRef]
- Kadam, S.B.; Sukhramani, G.S.; Bishnoi, P.; Pable, A.A.; Barvkar, V.T. SARS-CoV-2, the pandemic coronavirus: Molecular and structural insights. J. Basic Microbiol. 2021, 61, 180–202. [Google Scholar] [CrossRef]
- Sternberg, A.; Naujokat, C. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci. 2020, 257, 118056. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Tortorici, M.A.; Frenz, B.; Snijder, J.; Li, W.; Rey, F.A.; DiMaio, F.; Bosch, B.J.; Veesler, D. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2016, 23, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Cao, H.; Liu, C. SARS-CoV-2 S1 is superior to the RBD as a COVID-19 subunit vaccine antigen. J. Med. Virol. 2021, 93, 892–898. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. Be aware of SARS-CoV-2 spike protein: There is more than meets the eye. J. Biol. Regul. Homeost. Agents 2021, 35, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Deng, Y.; Chen, H.; Lan, J.; Wang, W.; Zou, X.; Hung, T.; Lu, Z.; Tan, W. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology 2015, 145, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Mese, K.; Bunz, O.; Ehrhardt, A. State-of-the-art human adenovirus vectorology for therapeutic approaches. FEBS Lett. 2019, 593, 3609–3622. [Google Scholar] [CrossRef]
- Xing, K.; Tu, X.Y.; Liu, M.; Liang, Z.W.; Chen, J.N.; Li, J.J.; Jiang, L.G.; Xing, F.Q.; Jiang, Y. Efficacy and safety of COVID-19 vaccines: A systematic review. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 221–228. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, Y.; Fang, Z.; Zhang, H.; Zhang, Y.; Chen, K. Analysis of the Protective Efficacy of Approved COVID-19 Vaccines Against Various Mutants. Front. Immunol. 2022, 13, 804945. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Mallapaty, S. India’s DNA COVID vaccine is a world first-more are coming. Nature 2021, 597, 161–162. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C.; et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 2020, 182, 73–84.e16. [Google Scholar] [CrossRef]
- Amanat, F.; Krammer, F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Li, Y.; Bi, Y.; Xiao, H.; Yao, Y.; Liu, X.; Hu, Z.; Duan, J.; Yang, Y.; Li, Z.; Li, Y.; et al. A novel DNA and protein combination COVID-19 vaccine formulation provides full protection against SARS-CoV-2 in rhesus macaques. Emerg. Microbes Infect. 2021, 10, 342–355. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Cao, H.; Xie, T.; Long, R.; Li, H.; Yang, T.; Yan, M.; Xie, Z. N-terminally truncated nucleocapsid protein of SARS-CoV-2 as a better serological marker than whole nucleocapsid protein in evaluating the immunogenicity of inactivated SARS-CoV-2. J. Med. Virol. 2021, 93, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Loes, A.N.; Gentles, L.E.; Crawford, K.H.D.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. The SARS-CoV-2 mRNA-1273 vaccine elicits more RBD-focused neutralization, but with broader antibody binding within the RBD. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mantus, G.; Nyhoff, L.E.; Kauffman, R.C.; Edara, V.V.; Lai, L.; Floyd, K.; Shi, P.Y.; Menachery, V.D.; Edupuganti, S.; Scherer, E.M.; et al. Evaluation of Cellular and Serological Responses to Acute SARS-CoV-2 Infection Demonstrates the Functional Importance of the Receptor-Binding Domain. J. Immunol. 2021, 206, 2605–2613. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Y.; Liu, S.; Kou, Z.; Li, W.; Farzan, M.; Jiang, S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: Implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004, 324, 773–781. [Google Scholar] [CrossRef]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y.; et al. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms 2022, 10, 1450. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Li, M.; Guo, J.; Lu, S.; Zhou, R.; Shi, H.; Shi, X.; Cheng, L.; Liang, Q.; Liu, H.; Wang, P.; et al. Single-Dose Immunization With a Chimpanzee Adenovirus-Based Vaccine Induces Sustained and Protective Immunity Against SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 697074. [Google Scholar] [CrossRef]
- Guo, J.; Mondal, M.; Zhou, D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum. Vaccines Immunother. 2018, 14, 1679–1685. [Google Scholar] [CrossRef]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–157.e113. [Google Scholar] [CrossRef] [PubMed]
- Lexberg, M.H.; Taubner, A.; Albrecht, I.; Lepenies, I.; Richter, A.; Kamradt, T.; Radbruch, A.; Chang, H.D. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur. J. Immunol. 2010, 40, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, K.; Xing, M.; Zhuo, Y.; Guo, J.; Du, M.; Wang, Q.; An, Y.; Li, J.; Gao, P.; et al. Heterologous prime-boost immunizations with chimpanzee adenoviral vectors elicit potent and protective immunity against SARS-CoV-2 infection. Cell Discov. 2021, 7, 123. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Shi, D.; Li, C.; Fang, Z.; Guo, Y.; Jiang, W.; Li, J.; Li, H.; Yao, H. Adenovirus Vaccine Containing Truncated SARS-CoV-2 Spike Protein S1 Subunit Leads to a Specific Immune Response in Mice. Vaccines 2023, 11, 429. https://doi.org/10.3390/vaccines11020429
Chen K, Shi D, Li C, Fang Z, Guo Y, Jiang W, Li J, Li H, Yao H. Adenovirus Vaccine Containing Truncated SARS-CoV-2 Spike Protein S1 Subunit Leads to a Specific Immune Response in Mice. Vaccines. 2023; 11(2):429. https://doi.org/10.3390/vaccines11020429
Chicago/Turabian StyleChen, Keda, Danrong Shi, Chaonan Li, Zhongbiao Fang, Yikai Guo, Wenjie Jiang, Jiaxuan Li, Hongyu Li, and Hangping Yao. 2023. "Adenovirus Vaccine Containing Truncated SARS-CoV-2 Spike Protein S1 Subunit Leads to a Specific Immune Response in Mice" Vaccines 11, no. 2: 429. https://doi.org/10.3390/vaccines11020429
APA StyleChen, K., Shi, D., Li, C., Fang, Z., Guo, Y., Jiang, W., Li, J., Li, H., & Yao, H. (2023). Adenovirus Vaccine Containing Truncated SARS-CoV-2 Spike Protein S1 Subunit Leads to a Specific Immune Response in Mice. Vaccines, 11(2), 429. https://doi.org/10.3390/vaccines11020429






