Recent Developments in Oral Delivery of Vaccines Using Nanocarriers
Abstract
1. Introduction
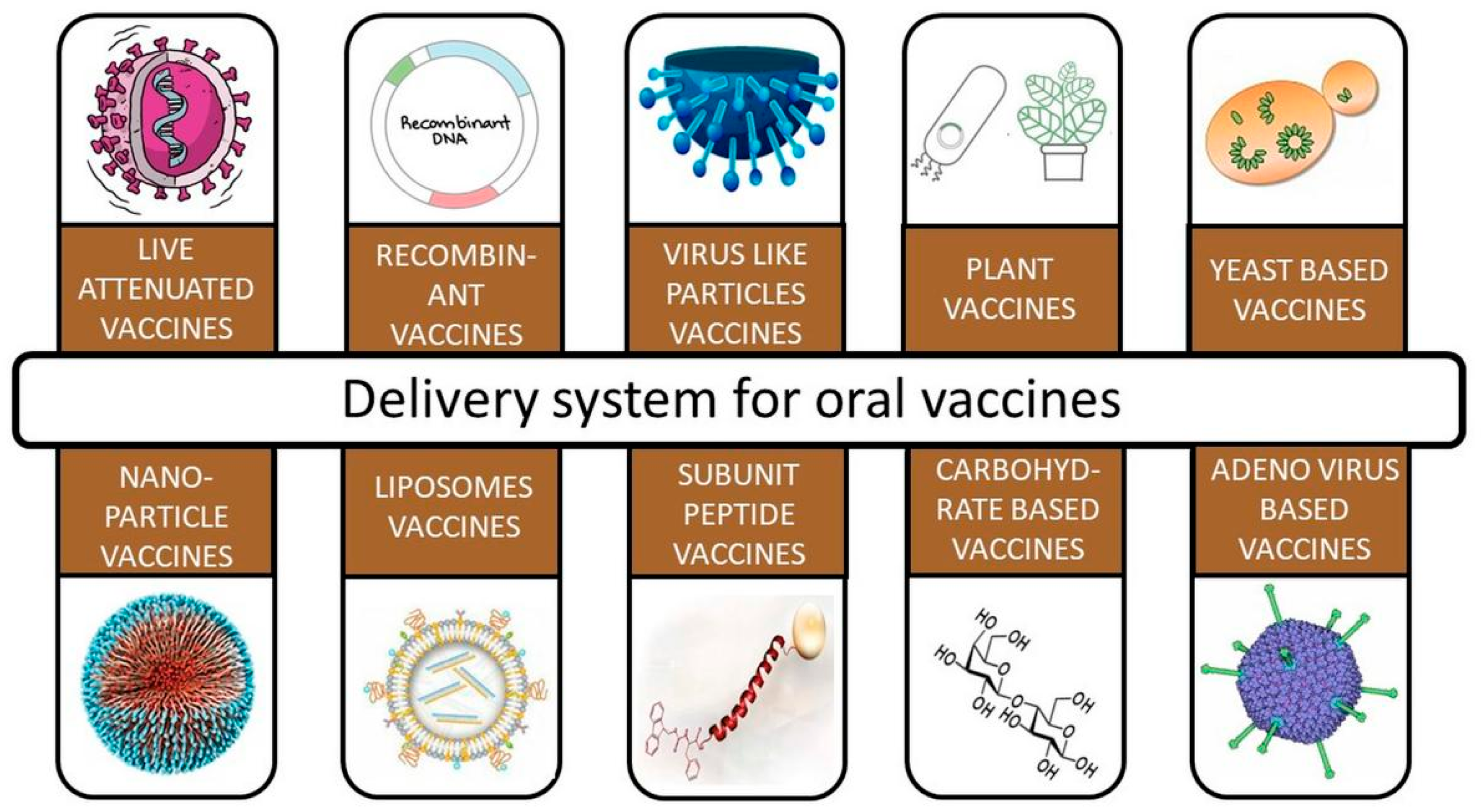
1.1. Oral Delivery of Vaccines
1.2. Yeast-Based Vaccines
1.3. Adenoviral-Based Vaccines
2. Pros and Cons of Oral Delivery of Vaccines
- Improved patient compliance:
- Capacity for mass immunization [20]
- Simplified production and storage [20]
- No needle-associated risks: Each year, 5% of healthcare professionals experience needle-related danger, putting them at risk for blood-borne infectious diseases, including HIV/AIDS and hepatitis [14].
- Both IgG- and IgA-specific response: Antigen-specific mucosal secretory IgA antibodies and antigen-specific systemic IgG antibodies can be produced by vaccinations given by mucosal routes at all mucosal locations, not only the site of delivery [21].
- An oral vaccination must first be exposed to a very acidic pH, proteolytic enzymes, and bile salts, which will cause it to degrade in the digestive system (GIT) [22].
- For successful absorption and penetration across the intestinal walls, vaccinations must pass a variety of biological obstacles (such as the existence of tight epithelial cellular junctions and a thick mucous layer) in the intestinal lumen [22].
- Additionally, the brief antigenic exposure period to mucosal tissues contributes to the decreased absorption of antigenic particles. As a result, compared to their systemic equivalents, oral vaccinations may need multiple and larger doses to have a powerful and long-lasting immunogenic impact [22].
- Another significant obstacle to the development of oral vaccinations is the scarcity of powerful immunostimulants or mucosal adjuvants with minimal toxicity [27].
- A significant barrier to oral vaccination is the difficulty in measuring the real intensity of the immune response, particularly IgA, at different mucosal sites following oral administration [21].
- A short vaccination half-life might result from enzymatic breakdown, among other issues. Therefore, the scientific challenge is to significantly increase oral vaccination absorption from the conventional 1% [28].
3. Approaches to Enhance Oral Delivery of Vaccines
3.1. Oral Adjuvants
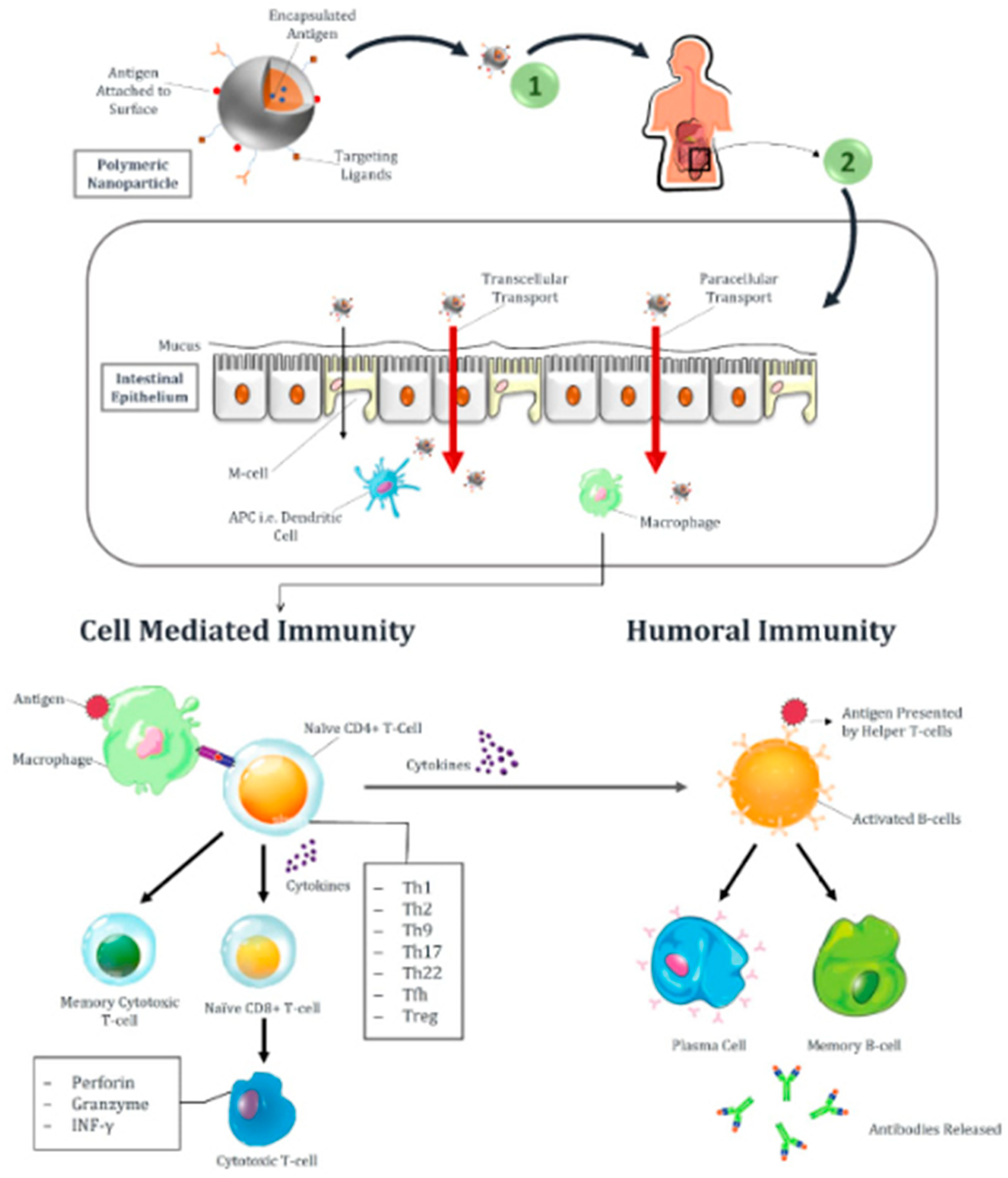
3.2. Targeting M Cells of Intestinal Epithelium
4. Nanoparticle-Based Oral Vaccination Strategies
- i.
- Polymeric NPs;
- ii.
- Lipid-based systems;
- iii.
- Inorganic NPs;
- iv.
- Niosomes;
- v.
- Advanced vesicular drug delivery systems.
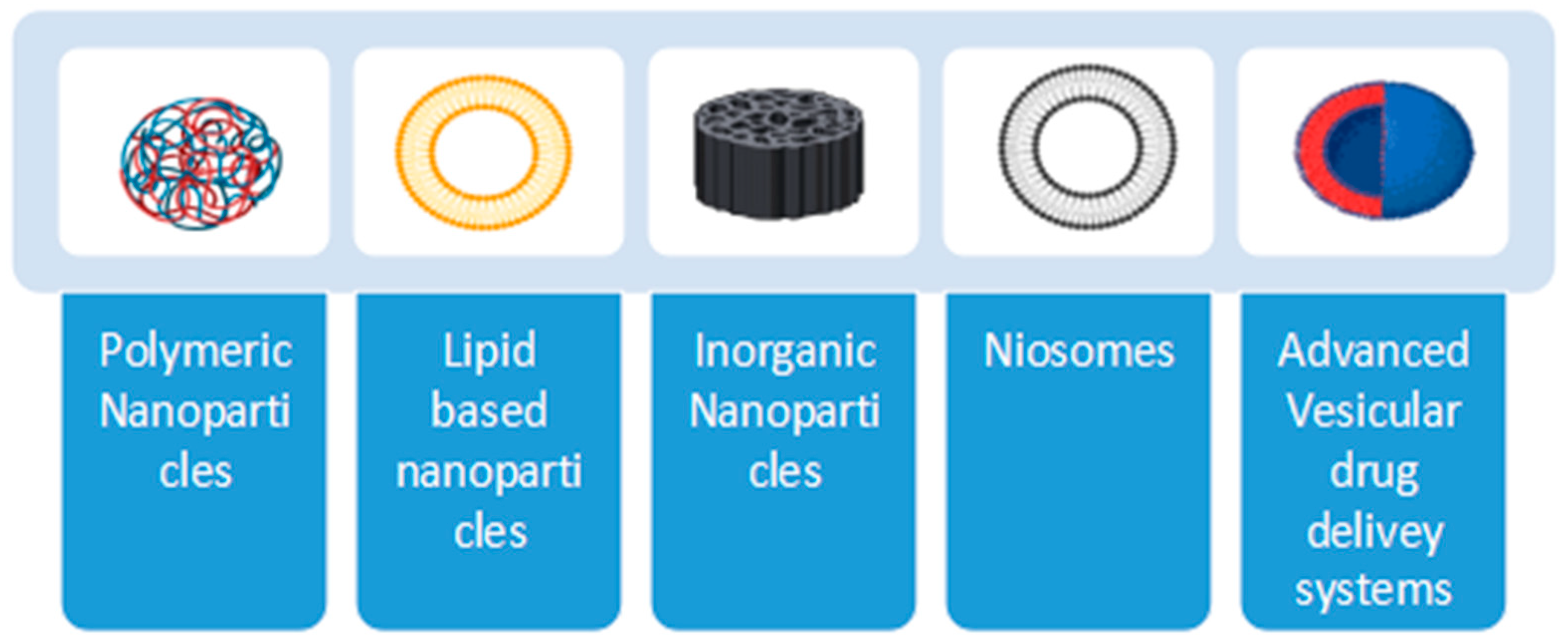
4.1. Polymeric NPs
- ➢
- Mucoadhesive NPs;
- ➢
- Stimuli-responsive NPs;
- ➢
- Specific ligand-bearing NPs.
4.2. Lipid-Based Nanoparticles
- ➢
- Liposomes
- ➢
- Nanoemulsion
- ➢
- Immunostimulating complexes (ISCOMs)
4.3. Inorganic NPs
4.4. Niosomes
4.5. Vesicular Drug Delivery Systems
- ➢
- Exosomes
- ➢
- Colloidosomes
- ➢
- Aquasomes
- ➢
- Polymersomes
- ➢
- Phytosomes
- ➢
- Emulsomes
- ➢
- Enzymosomes
- ➢
- Sphingosomes
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Declares the Coronavirus Outbreak a Pandemic. Available online: https://www.scientificamerican.com/article/who-declares-the-coronavirus-outbreak-a-pandemic/ (accessed on 11 March 2020).
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Bio. Med. Atenei Parm. 2020, 91, 157–160. [Google Scholar]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral delivery of nanoparticle-based vaccines. Expert Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef]
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 667–668. [Google Scholar] [CrossRef]
- Doherty, M.; Buchy, P.; Standaert, B.; Giaquinto, C.; Prado-Cohrs, D. Vaccine impact: Benefits for human health. Vaccine 2016, 34, 6707–6714. [Google Scholar] [CrossRef]
- Ellis, H. James Phipps, first to be vaccinated against smallpox by Edward Jenner. J. Perioper. Pract. 2021, 31, 51–52. [Google Scholar] [CrossRef]
- Li, R.Q.; Wang, W.; Yan, L.; Song, L.Y.; Guan, X.; Zhang, W.; Lian, J. Identification of tumor antigens and immune subtypes in breast cancer for mRNA vaccine development. Front. Oncol. 2022, 12, 973712. [Google Scholar] [CrossRef]
- Sauboin, C.; Van Bellinghen, L.-A.; Van De Velde, N.; Van Vlaenderen, I. Economic Impact of introducing the RTS, S malaria vaccine: Cost-effectiveness and budget impact analysis in 41 countries. MDM Policy Pract. 2019, 4, 2381468319873324. [Google Scholar] [CrossRef]
- Simerska, P.; Moyle, P.M.; Olive, C.; Toth, I. Oral vaccine delivery-new strategies and technologies. Curr. Drug Deliv. 2009, 6, 347–358. [Google Scholar] [CrossRef]
- Taddio, A.; Ipp, M.; Thivakaran, S.; Jamal, A.; Parikh, C.; Smart, S.; Sovran, J.; Stephens, D.; Katz, J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine 2012, 30, 4807–4812. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J.A. Vaccine hesitancy: An overview. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef]
- Kremsner, P.G.; Mann, P.; Kroidl, A.; Leroux-Roels, I.; Schindler, C.; Gabor, J.J.; Schunk, M.; Leroux-Roels, G.; Bosch, J.J.; Fendel, R. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2. Wien. Klin. Wochenschr. 2021, 133, 931–941. [Google Scholar] [CrossRef]
- Yeh, P.-Y.; Ellens, H.; Smith, P. Physiological considerations in the design of particulate dosage forms for oral vaccine delivery. Adv. Drug Deliv. Rev. 1998, 34, 123–133. [Google Scholar] [CrossRef]
- Ramirez, J.E.V.; Sharpe, L.; Peppas, N. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- New, R. Formulation technologies for oral vaccines. Clin. Exp. Immunol. 2019, 198, 153–169. [Google Scholar] [CrossRef]
- Johnson, S.; Martinez, C.I.; Jegede, C.B.; Gutierrez, S.; Cortese, M.; Martinez, C.J.; Garg, S.J.; Peinovich, N.; Dora, E.G.; Tucker, S.N. SARS-CoV-2 oral tablet vaccination induces neutralizing mucosal IgA in a phase 1 open label trial. MedRxiv 2022. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef]
- Majhen, D.; Calderon, H.; Chandra, N.; Fajardo, C.A.; Rajan, A.; Alemany, R.; Custers, J. Adenovirus-based vaccines for fighting infectious diseases and cancer: Progress in the field. Hum. Gene Ther. 2014, 25, 301–317. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- McNeela, E.A.; Mills, K. Manipulating the immune system: Humoral versus cell-mediated immunity. Adv. Drug Deliv. Rev. 2001, 51, 43–54. [Google Scholar] [CrossRef]
- Bergqvist, P.; Stensson, A.; Hazanov, L.; Holmberg, A.; Mattsson, J.; Mehr, R.; Bemark, M.; Lycke, N.Y. Re-utilization of germinal centers in multiple Peyer’s patches results in highly synchronized, oligoclonal, and affinity-matured gut IgA responses. Mucosal Immunol. 2013, 6, 122–135. [Google Scholar] [CrossRef]
- Aziz, M.A.; Midha, S.; Waheed, S.M.; Bhatnagar, R. Oral vaccines: New needs, new possibilities. Bioessays 2007, 29, 591–604. [Google Scholar] [CrossRef]
- Russell-Jones, G. Oral vaccine delivery. J. Control. Release 2000, 65, 49–54. [Google Scholar] [CrossRef]
- Mestecky, J.; Russell, M.; Elson, C. Perspectives on mucosal vaccines: Is mucosal tolerance a barrier? J. Immunol. 2007, 179, 5633–5638. [Google Scholar] [CrossRef]
- Pabst, O.; Mowat, A. Oral tolerance to food protein. Mucosal Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef]
- Premanand, B.; Prabakaran, M.; Kiener, T.K.; Kwang, J. Recombinant baculovirus associated with bilosomes as an oral vaccine candidate against HEV71 infection in mice. PLoS ONE 2013, 8, e55536. [Google Scholar] [CrossRef]
- Shaji, J.; Patole, V. Protein and peptide drug delivery: Oral approaches. Indian J. Pharm. Sci. 2008, 70, 269. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.; Chan, W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Kumar, S.; Anselmo, A.C.; Banerjee, A.; Zakrewsky, M.; Mitragotri, S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control. Release 2015, 220, 141–148. [Google Scholar] [CrossRef]
- Cruz, L.J.; Tacken, P.J.; Rueda, F.; Domingo, J.C.; Albericio, F.; Figdor, C.G. Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol. 2012, 509, 143–163. [Google Scholar]
- Devriendt, B.; De Geest, B.G.; Goddeeris, B.M.; Cox, E. Crossing the barrier: Targeting epithelial receptors for enhanced oral vaccine delivery. J. Control. Release 2012, 160, 431–439. [Google Scholar] [CrossRef]
- Courtney, A.N.; Nehete, P.N.; Nehete, B.P.; Thapa, P.; Zhou, D.; Sastry, K.J. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine 2009, 27, 3335–3341. [Google Scholar] [CrossRef]
- Davitt, C.J.; Longet, S.; Albutti, A.; Aversa, V.; Nordqvist, S.; Hackett, B.; McEntee, C.P.; Rosa, M.; Coulter, I.S.; Lebens, M. Alpha-galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines. Mucosal Immunol. 2019, 12, 1055–1064. [Google Scholar] [CrossRef]
- Kraehenbuhl, J.-P.; Neutra, M. Epithelial M cells: Differentiation and function. Annu. Rev. Cell Dev. Biol. 2000, 16, 301–332. [Google Scholar] [CrossRef]
- Gebert, A.; Rothkötter, H.-J.; Pabst, R. M cells in Peyer’s patches of the intestine. Int. Rev. Cytol. 1996, 167, 91–159. [Google Scholar]
- Jepson, M.A.; Clark, M. Studying M cells and their role in infection. Trends Microbiol. 1998, 6, 359–365. [Google Scholar] [CrossRef]
- Clark, M.A.; Hirst, B.; Jepson, M. Lectin-mediated mucosal delivery of drugs and microparticles. Adv. Drug Deliv. Rev. 2000, 43, 207–223. [Google Scholar] [CrossRef]
- Ermak, T.H.; Giannasca, P. Microparticle targeting to M cells. Adv. Drug Deliv. Rev. 1998, 34, 261–283. [Google Scholar] [CrossRef]
- Jepson, M.A.; Clark, M.A.; Foster, N.; Mason, C.M.; Bennett, M.K.; Simmons, N.L.; Hirst, B.H. Targeting to intestinal M cells. J. Anat. 1996, 189 Pt 3, 507. [Google Scholar]
- Clark, M.A.; Jepson, M.; Hirst, B. Exploiting M cells for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2001, 50, 81–106. [Google Scholar] [CrossRef]
- Lavelle, E.C.; O’Hagan, D. Delivery systems and adjuvants for oral vaccines. Expert Opin. Drug Deliv. 2006, 3, 747–762. [Google Scholar] [CrossRef]
- Seyfoori, A.; Shokrollahi Barough, M.; Mokarram, P.; Ahmadi, M.; Mehrbod, P.; Sheidary, A.; Madrakian, T.; Kiumarsi, M.; Walsh, T.; McAlinden, K.D. Emerging advances of nanotechnology in drug and vaccine delivery against viral associated respiratory infectious diseases (VARID). Int. J. Mol. Sci. 2021, 22, 6937. [Google Scholar] [CrossRef]
- Song, M.; Cui, M.; Fang, Z.; Liu, K. Advanced research on extracellular vesicles based oral drug delivery systems. J. Control. Release 2022, 351, 560–572. [Google Scholar] [CrossRef]
- Cao, P.; Xu, Z.; Li, L. Tailoring functional nanoparticles for oral vaccine delivery: Recent advances and future perspectives. Compos. Part B Eng. 2022, 236, 109826. [Google Scholar] [CrossRef]
- Su, C.J.; Murugan, A.; Linton, J.M.; Yeluri, A.; Bois, J.; Klumpe, H.; Langley, M.A.; Antebi, Y.E.; Elowitz, M.B. Ligand-receptor promiscuity enables cellular addressing. Cell Syst. 2022, 13, 408–425.e12. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Aminabhavi, T.M.; Torchilin, V.P. Nanovesicular systems in drug delivery. In Systems of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–15. [Google Scholar]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Perry, S.L.; McClements, D. Recent advances in encapsulation, protection, and oral delivery of bioactive proteins and peptides using colloidal systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef]
- Madani, F.; Hsein, H.; Busignies, V.; Tchoreloff, P. An overview on dosage forms and formulation strategies for vaccines and antibodies oral delivery. Pharm. Dev. Technol. 2020, 25, 133–148. [Google Scholar] [CrossRef]
- Jarai, B.M.; Kolewe, E.L.; Stillman, Z.S.; Raman, N.; Fromen, C.A. Polymeric nanoparticles. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–324. [Google Scholar]
- Khan, S.; Hossain, M. Classification and properties of nanoparticles. In Nanoparticle-Based Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 15–54. [Google Scholar]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021, 142, 110155. [Google Scholar] [CrossRef]
- Carreño, J.M.; Perez-Shibayama, C.; Gil-Cruz, C.; Printz, A.; Pastelin, R.; Isibasi, A.; Chariatte, D.; Tanoue, Y.; Lopez-Macias, C.; Gander, B. PLGA-microencapsulation protects Salmonella typhi outer membrane proteins from acidic degradation and increases their mucosal immunogenicity. Vaccine 2016, 34, 4263–4269. [Google Scholar] [CrossRef]
- Zhu, Q.; Talton, J.; Zhang, G.; Cunningham, T.; Wang, Z.; Waters, R.C.; Kirk, J.; Eppler, B.; Klinman, D.M.; Sui, Y. Large intestine–targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 2012, 18, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Tekale, S.U.; Rottenberg, Y.; Ingle, R.D.; Domb, A.J.; Pawar, R.P. Recent developments in biodegradable block copolymers. Polym. Adv. Technol. 2021, 32, 3877–3899. [Google Scholar] [CrossRef]
- Prokop, A.; Kozlov, E.; Newman, G.W.; Newman, M.J. Water-based nanoparticulate polymeric system for protein delivery: Permeability control and vaccine application. Biotechnol. Bioeng. 2002, 78, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Rhushikesh, S.; Suresh, S. A Review on Mucoadhesive Drug Delivery System. Int. J. Res. Anal. Rev. 2020, 7, 793–808. [Google Scholar]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dunn, E.; Grandmaison, E.; Goosen, M.F. Applications and properties of chitosan. In Applications of Chitin and Chitosan; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–29. [Google Scholar]
- Dong, W.; Ye, J.; Zhou, J.; Wang, W.; Wang, H.; Zheng, X.; Yang, Y.; Xia, X.; Liu, Y. Comparative study of mucoadhesive and mucus-penetrative nanoparticles based on phospholipid complex to overcome the mucus barrier for inhaled delivery of baicalein. Acta Pharm. Sin. B 2020, 10, 1576–1585. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195. [Google Scholar] [CrossRef]
- Yoo, M.-K.; Kang, S.-K.; Choi, J.-H.; Park, I.-K.; Na, H.-S.; Lee, H.-C.; Kim, E.-B.; Lee, N.-K.; Nah, J.-W.; Choi, Y.-J. Targeted delivery of chitosan nanoparticles to Peyer’s patch using M cell-homing peptide selected by phage display technique. Biomaterials 2010, 31, 7738–7747. [Google Scholar] [CrossRef]
- Gong, X.; Gao, Y.; Shu, J.; Zhang, C.; Zhao, K. Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines. Vaccines 2022, 10, 1906. [Google Scholar] [CrossRef]
- AbdelAllah, N.H.; Gaber, Y.; Rashed, M.E.; Azmy, A.F.; Abou-Taleb, H.A.; AbdelGhani, S. Alginate-coated chitosan nanoparticles act as effective adjuvant for hepatitis A vaccine in mice. Int. J. Biol. Macromol. 2020, 152, 904–912. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A. Applications of chitosan-alginate-based nanoparticles—An up-to-date review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Pedro, R.; Pereira, A.R.; Oliveira, O.N.; Miranda, P.B. Interaction of chitosan derivatives with cell membrane models in a biologically relevant medium. Colloids Surf. B Biointerfaces 2020, 192, 111048. [Google Scholar] [CrossRef] [PubMed]
- Farooque, F.; Wasi, M.; Mughees, M. Liposomes as Drug Delivery System: An Updated Review. J. Drug Deliv. Ther. 2021, 11, 149–158. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Liu, J.; Miao, L.; Sui, J.; Hao, Y.; Huang, G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian J. Pharm. Sci. 2020, 15, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Sonowal, K.; Laskar, R.E.; Deka, D.; Dey, B.K. Liposome: A carrier for effective drug delivery. J. Appl. Pharm. Res. 2020, 8, 22–28. [Google Scholar] [CrossRef]
- Marasini, N.; Giddam, A.K.; Ghaffar, K.A.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. Multilayer engineered nanoliposomes as a novel tool for oral delivery of lipopeptide-based vaccines against group A Streptococcus. Nanomedicine 2016, 11, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Mühlberg, E.; Burtscher, M.; Umstätter, F.; Fricker, G.; Mier, W.; Uhl, P. Trends in liposomal nanocarrier strategies for the oral delivery of biologics. Nanomedicine 2021, 16, 1813–1832. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C. Nanoemulsions for drug delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Eqbal, A.; Ansari, V.A.; Hafeez, A.; Ahsan, F.; Imran, M.; Tanweer, S. Recent applications of nanoemulsion based drug delivery system: A review. Res. J. Pharm. Technol. 2021, 14, 2852–2858. [Google Scholar] [CrossRef]
- Sheth, T.; Seshadri, S.; Prileszky, T.; Helgeson, M.E. Multiple nanoemulsions. Nat. Rev. Mater. 2020, 5, 214–228. [Google Scholar] [CrossRef]
- Soni, H.; Sharma, S. Current Update on Nanoemulsion: A Review. Sch. Int. J. Anat. Physiol. 2021, 4, 6–13. [Google Scholar]
- Shah, P.; Bhalodia, D.; Shelat, P. Nanoemulsion: A pharmaceutical review. Syst. Rev. Pharm. 2010, 1, 24–32. [Google Scholar] [CrossRef]
- Petkar, K.C.; Patil, S.M.; Chavhan, S.S.; Kaneko, K.; Sawant, K.K.; Kunda, N.K.; Saleem, I.Y. An overview of nanocarrier-based adjuvants for vaccine delivery. Pharmaceutics 2021, 13, 455. [Google Scholar] [CrossRef]
- Rhee, J.H. Current and new approaches for mucosal vaccine delivery. In Mucosal Vaccines; Elsevier: Amsterdam, The Netherlands, 2020; pp. 325–356. [Google Scholar]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.; Lee, Y.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020, 156, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Dewangan, H. Nanoparticles as adjuvants in vaccine delivery. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, S.; Aubert-Pouëssel, A.; Ouchait, L.; Mohamed, K.E.; Martineau, P.; Guglielmi, L.; Devoisselle, J.; Legrand, P.; Chopineau, J.; Morille, M. Nanotechnologies for Intracellular Protein Delivery: Recent Progress in Inorganic and Organic Nanocarriers. Adv. Ther. 2021, 4, 2100009. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C. Inorganic nanoparticles for targeted drug delivery. In Biointegration of Medical Implant Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–373. [Google Scholar]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Joy, C.; Nair, S.K.; Kumar, K.K.; Dineshkumar, B. Niosomes as nano-carrier based targeted drug delivery system. J. Drug Deliv. Ther. 2021, 11, 166–170. [Google Scholar] [CrossRef]
- Nasir, A.; Harikumar, S.; Amanpreet, K. Niosomes: An excellent tool for drug delivery. Int. J. Res. Pharm. Chem. 2012, 2, 479–487. [Google Scholar]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183. [Google Scholar] [CrossRef]
- Zani-Ruttenstock, E.; Antounians, L.; Khalaj, K.; Figueira, R.L.; Zani, A. The role of exosomes in the treatment, prevention, diagnosis, and pathogenesis of COVID-19. Eur. J. Pediatr. Surg. 2021, 31, 326–334. [Google Scholar] [CrossRef]
- Thomas, S.C.; Kim, J.; Pauletti, G.M.; Hassett, D.J.; Kotagiri, N. Exosomes: Biological Pharmaceutical Nanovectors for Enhanced Theranostics. Front. Bioeng. Biotechnol. 2022, 9, 1475. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Bagherifar, R.; Ansari Dezfouli, E.; Kiaie, S.H.; Jafari, R.; Ramezani, R. Exosomes as bio-inspired nanocarriers for RNA delivery: Preparation and applications. J. Transl. Med. 2022, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Ahmed, N. Nanovesicles for target specific drug delivery. In Applications of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 149–165. [Google Scholar]
- Aggarwal, N.; Nabi, B.; Aggarwal, S.; Baboota, S.; Ali, J. Nano-based drug delivery system: A smart alternative towards eradication of viral sanctuaries in management of NeuroAIDS. Drug Deliv. Transl. Res. 2022, 12, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Alenzi, A.M.; Albalawi, S.A.; Alghamdi, S.G.; Albalawi, R.F.; Albalawi, H.S.; Qushawy, M. Review on Different Vesicular Drug Delivery Systems (VDDSs) and Their Applications. Recent Pat. Nanotechnol. 2023, 17, 18–32. [Google Scholar] [PubMed]
- Attia, M.S.; Abdel-Mottaleb, M.; Mohamed, E. Smart nanovesicles for drug delivery. In Systems of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 367–385. [Google Scholar]
- Sallam, N.; Sanad, R.; KHAFAGY, E.; Ahmed, M.; Ghourab, M.; Gad, S. Colloidal delivery of drugs: Present strategies and conditions. Rec. Pharm. Biomed. Sci. 2021, 5, 40–51. [Google Scholar] [CrossRef]
- İlhan, M.; Gültekin, H.E.; Rençber, S.; Şenyiğit, Z.; Aydın, H.H. Aquasomes: A novel platform for drug delivery. In Systems of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 191–206. [Google Scholar]
- Banerjee, S.; Sen, K. Aquasomes: A novel nanoparticulate drug carrier. J. Drug Deliv. Sci. Technol. 2018, 43, 446–452. [Google Scholar] [CrossRef]
- Haranath, C.; Reddy, J.L.; Ahad, H.A.; Reshma, T.; Shubha, B.N. Aquasomes: A Novel Approach for the Delivery of Bioactive Molecules. J. Med. Pharm. Allied Sci. 2022, 11, 5325–5330. [Google Scholar] [CrossRef]
- Kulkarni, S.; Prabhakar, B.; Shende, P. Aquasomes: Advanced Vesicular-based Nanocarrier Systems. Curr. Pharm. Des. 2022, 28, 2404–2414. [Google Scholar] [PubMed]
- Jain, S.S.; Jagtap, P.S.; Dand, N.M.; Jadhav, K.R.; Kadam, V.J. Aquasomes: A novel drug carrier. J. Appl. Pharm. Sci. 2012, 2, 184–192. [Google Scholar]
- Hasannia, M.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Synthesis of block copolymers used in polymersome fabrication: Application in drug delivery. J. Control. Release 2022, 341, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.M.; Dua, K. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Park, Y. Application of Nanoparticles: Diagnosis, Therapeutics, and Delivery of Insulin/Anti-Diabetic Drugs to Enhance the Therapeutic Efficacy of Diabetes Mellitus. Life 2022, 12, 2078. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.; Ma’mon, M.H.; Alshaer, W.; Rahman, E.; Mohd-Zahid, M.H.; Alhaj-Qasem, D.M.; Yean, C.Y.; Alias, I.Z.; Jaafar, J.; Ferji, K. COVID-19 infection and nanomedicine applications for development of vaccines and therapeutics: An overview and future perspectives based on polymersomes. Eur. J. Pharmacol. 2021, 896, 173930. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M. Phytosomes as innovative delivery systems for phytochemicals: A comprehensive review of literature. Int. J. Nanomed. 2021, 16, 6983. [Google Scholar] [CrossRef]
- Rana, M.; Kumar, A.; Rana, A. Drug Delivery through Targeted Approach with Special References to Phytosomes. In Role of Novel Drug Delivery Vehicles in Nanobiomedicine; IntechOpen: London, UK, 2020; Volume 125. [Google Scholar]
- Ren, Y.; Nie, L.; Zhu, S.; Zhang, X. Nanovesicles-Mediated Drug Delivery for Oral Bioavailability Enhancement. Int. J. Nanomed. 2022, 17, 4861–4877. [Google Scholar] [CrossRef]
- Gill, V.; Nanda, A. EMULSOMES: A Lipid Bases Drug Delivery System. World J. Pharm. Res. 2021, 10, 113–129. [Google Scholar]
- Ucisik, M.H.; Sleytr, U.; Schuster, B. Emulsomes meet S-layer proteins: An emerging targeted drug delivery system. Curr. Pharm. Biotechnol. 2015, 16, 392–405. [Google Scholar] [CrossRef]
- Myneni, G.S.; Radha, G.; Soujanya, G. Novel Vesicular Drug Delivery Systems: A Review. J. Pharm. Res. 2021, 11, 1650–1664. [Google Scholar]
- Ghode, S.P.; Ghode, P. Applications Perspectives of Emulsomes Drug Delivery System. Int. J. Med. Phar. Sci. Vol. 2020, 10, 1. [Google Scholar] [CrossRef]
- Kadawla, M.; Rao, L.; Yadav, K. Emulsomes-based Formulations for Effective Delivery of Small Molecules: Recent Advances and Modern Applications Perspectives. Int. J. Ind. Biotechnol. Biomater. 2021, 7, 29–39. [Google Scholar]
- Sharadha, M.; Gowda, D.; Gupta, V.; Akhila, A. An overview on topical drug delivery system–updated review. Int. J. Res. Pharm. Sci. 2020, 11, 368–385. [Google Scholar] [CrossRef]
- Pattnaik, S.; Swain, K.; Singh, S.P.; Sirbaiya, A.K. Lipid vesicles: Potentials as drug delivery systems. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–180. [Google Scholar]
- Shefrin, S.; Sreelaxmi, C.; Vishnu, V.; Sreeja, C. Enzymosomes: A rising effectual tool for targeted drug delivery system. Int. J. Appl. Pharm. 2017, 9, 1–9. [Google Scholar]
- Chaudhari, S.P.; Gaikwad, S. Sphingosomes: A novel lipoidal vesicular drug delivery system. System 2020, 5, 261–267. [Google Scholar]
- Lakshmi Narayana Rao, B.; Krishnan, S.P.; Reddy, C.B. Vesicular and Stealth Vesicular Drug Delivery–A Review. J. Pharm. Res. Int. 2021, 33, 76–88. [Google Scholar]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef]
- Singh, N.; Joshi, A.; Toor, A.P.; Verma, G. Drug delivery: Advancements and challenges. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 865–886. [Google Scholar]
- Aguilar-Pérez, K.; Avilés-Castrillo, J.; Medina, D.; Parra-Saldivar, R.; Iqbal, H. Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front. Bioeng. Biotechnol. 2020, 8, 579536. [Google Scholar] [CrossRef]
- Ojha, B.; Jain, V.K.; Gupta, S.; Talegaonkar, S.; Jain, K. Nanoemulgel: A promising novel formulation for treatment of skin ailments. Polym. Bull. 2022, 79, 4441–4465. [Google Scholar] [CrossRef]
- Savardekar, P.; Bajaj, A. Nanoemulsions—A review. Int. J. Res. Pharm. Chem. 2016, 6, 312–322. [Google Scholar]
- Ghosn, Y.; Kamareddine, M.H.; Tawk, A.; Elia, C.; El Mahmoud, A.; Terro, K.; El Harake, N.; El-Baba, B.; Makdessi, J.; Makdessi, J. Inorganic nanoparticles as drug delivery systems and their potential role in the treatment of chronic myelogenous leukaemia. Technol. Cancer Res. Treat. 2019, 18, 1533033819853241. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef]
- Afreen, U.; Shailaja, A.K. Pharmacosomes and Emulsomes: An Emerging Novel Vesicular Drug Delivery System. Glob. J. Anes. Pain Med. 2020, 3, 287–297. [Google Scholar]
- Gupta, P.; Mazumder, R.; Padhi, S. Challenges of new generation liposomes—A review. Int. J. Pharm. Sci. Nanotechnol. 2020, 13, 4815–4825. [Google Scholar] [CrossRef]
- Saraf, S.; Paliwal, S.; Kaur, C.D.; Saraf, S. Sphingosomes a novel approach to vesicular drug delivery. Res. J. Pharm. Technol. 2011, 4, 661–666. [Google Scholar]
- Mohammad, Z.; Zeeshan, A.; Faisal, S.; Suhail, A.; Sahar, I.; Mohd, S.; Nazma, K. Vesicular drug delivery system used for liver diseases. World J. Pharm. Sci. 2017, 5, 28–35. [Google Scholar]
| Vaccines Available in Market [16] | ||
| Disease | Oral Vaccines | Examples |
| Polio | Polio vaccine | Inactivated poliovirus vaccine, OPV |
| Gastroenteritis | Rota vaccine | Rotarix, Rotateq |
| Typhoid fever | Typhoid vaccine | Vivotif, Ty21a |
| Acute respiratory disease | Adenovirus vaccine | Adenovirus type 4 and 7 |
| Cholera | Cholera vaccine | Vaxchora, Dukoral, Shanchol |
| Vaccines under Clinical Trial [17] | ||
| COVID-19 | COVID-19 vaccine | Under trial |
| Nanoparticle System | Advantages | Limitations | References |
|---|---|---|---|
| Polymeric NPs | Sustained and controlled drug release, more stable than lipid-based NPs | Use of organic solvents, limited toxicity assessment in literature, difficulty in scale-up | [121,122] |
| Liposomes | Biocompatible, biodegradable, nontoxic, can enclose both hydrophilic and hydrophobic agents, can enhance bioavailability | Instability, leakage of enclosed antigen, rapid clearance through RES | [123] |
| Nanoemulsion | High loading capacity, greater stability, drug protection from degradation, economical | Low permeation and bioavailability, use of a large concentration of surfactant for stabilizing nanoparticles | [124,125] |
| Immunostimulating complexes | Provide site-specific delivery by attaching antibodies or in diagnostic immunological techniques | Inability to incorporate most soluble proteins as do not have exposed hydrophobic regions | [82] |
| Inorganic NPs | Biocompatibility | Instability and low loading capacity | [126] |
| Niosomes | Higher stability as compared to liposomes, high loading capacity, biocompatible, ability to entrap both hydrophilic and lipophilic agents, nonimmunogenic, enhance therapeutic effect | Lengthy preparation process, fusion hydrolysis, aggregation, leakage of entrapped drugs on poor storage conditions | [127] |
| Exosomes | Provide site-oriented delivery, minimal toxicity, maximum bioavailability, extend circulation time of components in blood | Less stable upon storage impurity | [128] |
| Colloidosomes | Easy fabrication, can encapsulate sufficient amount of antigen, mechanically robust, provide regulated release | Poor yield, sometimes show coalescence | [119] |
| Aquasomes | Enhance stability of biological entities, bypass rapid clearance through reticuloendothelial system | Preparation method is time-consuming | [98] |
| Polymersomes | More stable than liposomes, can entrap both hydrophilic and lipophilic components, better retain enclosed agent | Polymer can cause toxicity, difficulty in large-scale production | [129] |
| Phytosomes | Good drug retention in carrier, highly stable, deliver drug at the intended site, high loading capacity | Tend to fuse, aggregate, and hydrolyze upon storage | [130] |
| Emulsomes | Enhance bioavailability of agents having less aqueous solubility, protect antigen from denaturation in acidic pH, provide targeted delivery | Less stable | [130] |
| Enzymosomes | Provide targeted delivery, biodegradable, biocompatible, nontoxic, improved therapeutic effectiveness | Cost-ineffectiveness, phospholipids can be oxidized and fused and are liable to leak entrapped components from carrier system | [131] |
| Sphingosomes | Enhance efficacy of therapeutic moiety, less toxic, more stable, improves pharmacokinetic parameters | Low entrapment efficiency, expensive | [132,133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, A.; Arshad, R.; Ur.Rehman, A.; Ahmed, N.; Akhtar, H. Recent Developments in Oral Delivery of Vaccines Using Nanocarriers. Vaccines 2023, 11, 490. https://doi.org/10.3390/vaccines11020490
Zafar A, Arshad R, Ur.Rehman A, Ahmed N, Akhtar H. Recent Developments in Oral Delivery of Vaccines Using Nanocarriers. Vaccines. 2023; 11(2):490. https://doi.org/10.3390/vaccines11020490
Chicago/Turabian StyleZafar, Amna, Raffia Arshad, Asim Ur.Rehman, Naveed Ahmed, and Hashaam Akhtar. 2023. "Recent Developments in Oral Delivery of Vaccines Using Nanocarriers" Vaccines 11, no. 2: 490. https://doi.org/10.3390/vaccines11020490
APA StyleZafar, A., Arshad, R., Ur.Rehman, A., Ahmed, N., & Akhtar, H. (2023). Recent Developments in Oral Delivery of Vaccines Using Nanocarriers. Vaccines, 11(2), 490. https://doi.org/10.3390/vaccines11020490







