Neutralization Effect of Sera against Delta and Omicron in Patients Recovering from COVID-19 and Inactivated Vaccine Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Pseudovirus Preparation
2.3. Pseudovirus Neutralization Assay (pVNT)
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
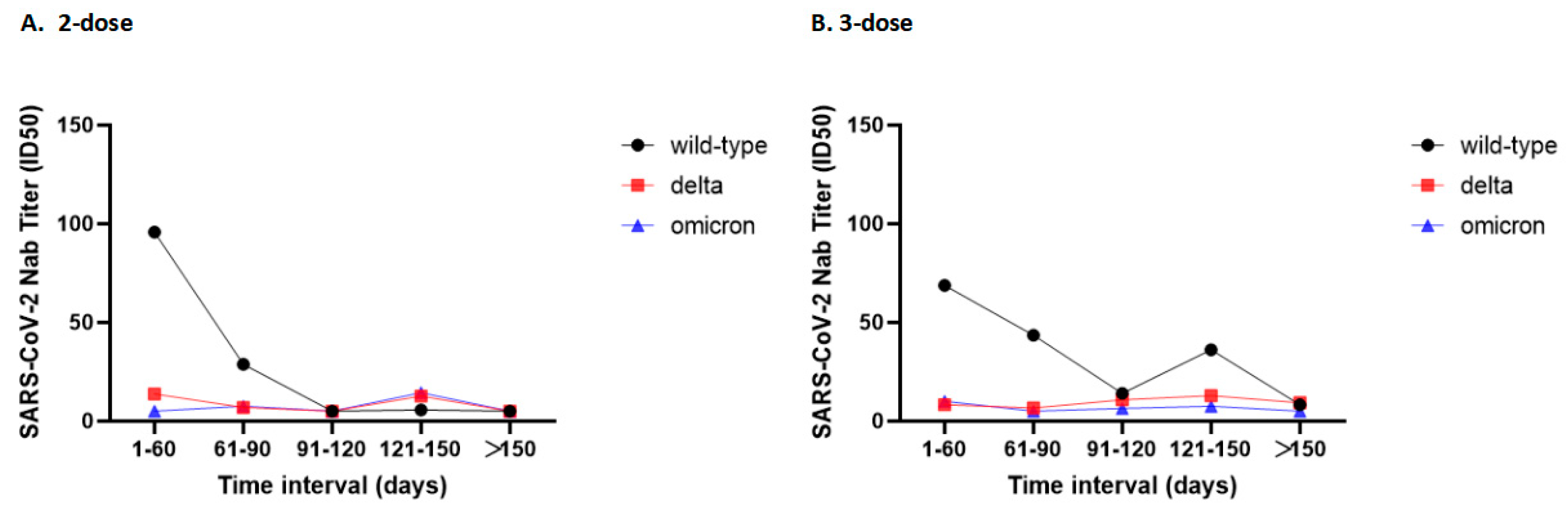
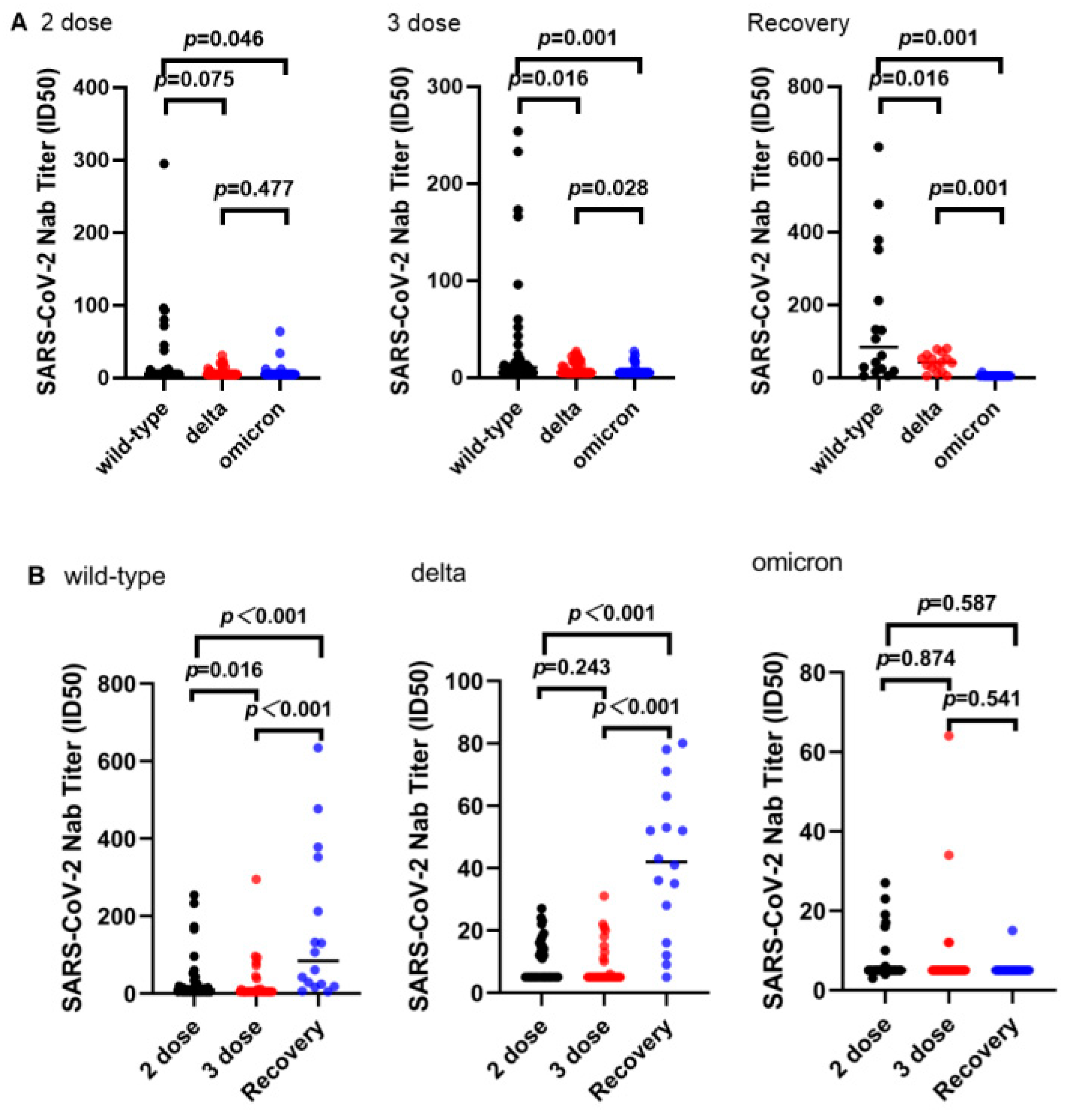
3.2. Neutralizing Ability
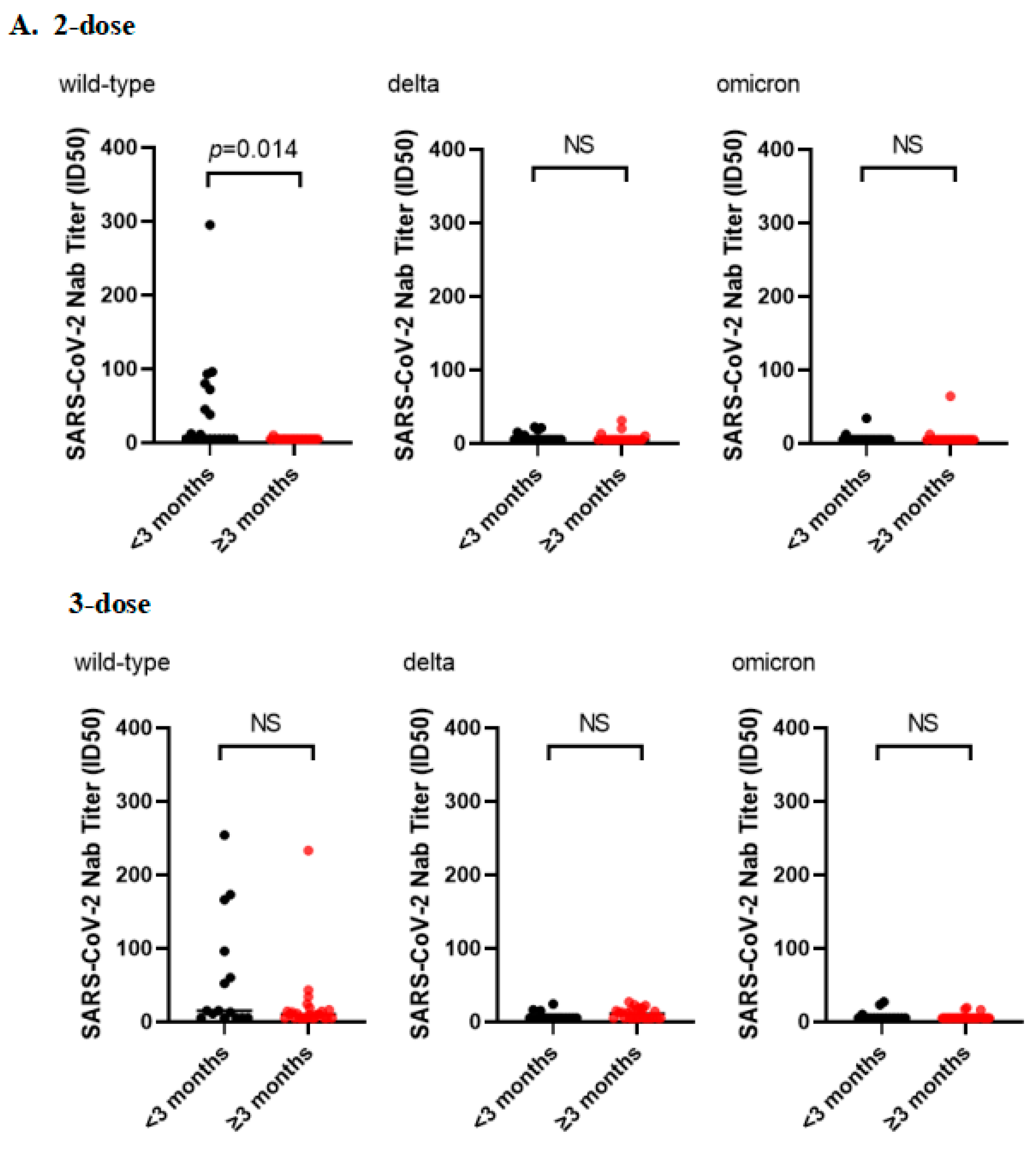
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard [EB/OL]. Available online: https://covid19.who.int/ (accessed on 9 October 2022).
- World Health Organization. Tracking SARS-CoV-2 Variants [EB/OL]. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 9 October 2022).
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Berkhout, B.; Herrera-Carrillo, E. SARS-CoV-2 Evolution: On the Sudden Appearance of the Omicron Variant. J. Virol. 2022, 96, e00090-22. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Muecksch, F.; Weisblum, Y.; Da Silva, J.; Bednarski, E.; Cho, A.; Wang, Z.; Gaebler, C.; Caskey, M.; Nussenzweig, M.C.; et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. N. Engl. J. Med. 2022, 386, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Pollett, S.D.; Neerukonda, S.N.; Wang, W.; Wang, R.; Vassell, R.; Epsi, N.J.; Fries, A.C.; Agan, B.K.; Lindholm, D.A.; et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster-elicited serum but evades most convalescent serum and therapeutic antibodies. Sci. Transl. Med. 2022, 14, eabn8543. [Google Scholar] [CrossRef]
- Ai, J.; Wang, X.; He, X.; Zhao, X.; Zhang, Y.; Jiang, Y.; Li, M.; Cui, Y.; Chen, Y.; Qiao, R.; et al. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe 2022, 30, 1077–1083. [Google Scholar] [CrossRef]
- Yu, X.; Qi, X.; Cao, Y.; Li, P.; Lu, L.; Wang, P.; Feng, Y.; Yang, J.; Wei, H.; Guo, L.; et al. Three doses of an inactivation-based COVID-19 vaccine induces cross-neutralizing immunity against the SARS-CoV-2 Omicron variant. Emerg. Microbes Infect. 2022, 11, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, J.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 2022, 376, eabn4947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg. Microbes Infect. 2022, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Marschalek, R.; et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. eBioMedicine 2022, 82, 104158. [Google Scholar] [CrossRef]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; St. Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, Y.; Ji, Y.; Cong, X.; Liu, Y.; Yang, R.; Kong, X.; Shi, Y.; Zhu, L.; Wang, Z.; et al. Inactivated Vaccines Against SARS-CoV-2: Neutralizing Antibody Titers in Vaccine Recipients. Front. Microbiol. 2022, 13, 816778. [Google Scholar] [CrossRef] [PubMed]

| Recovering Patients (n = 16) | Participants Who Had Received Two Doses of Inactivated Vaccine (n = 36) | Participants Who Had Received Three Doses of Inactivated Vaccine (n = 40) | Total (n = 92) | p | |
|---|---|---|---|---|---|
| Sex (n (% of total)) | 0.131 | ||||
| Male | 7 (43.8) | 19 (52.8) | 12 (30.0) | 38 (41.3) | |
| Female | 9 (56.2) | 17 (47.2) | 28 (70.0) | 54 (58.7) | |
| Age [Median (interquartile range)] | 35.5 (33.5–45.5) | 57 (35–71) | 40.5 (33–49.5) | 42.5 (34–56) | <0.001 |
| Sample collection window | June 2020–April 2021 | April 2021–February 2022 | January 2022–February 2022 | June 2020–February 2022 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhong, Q.; Ma, Z.; Liu, S.; Lan, Y.; Peng, B.; Zhang, X.; Shi, X.; Qu, J.; Wu, Z.; et al. Neutralization Effect of Sera against Delta and Omicron in Patients Recovering from COVID-19 and Inactivated Vaccine Recipients. Vaccines 2023, 11, 471. https://doi.org/10.3390/vaccines11020471
Zhu Y, Zhong Q, Ma Z, Liu S, Lan Y, Peng B, Zhang X, Shi X, Qu J, Wu Z, et al. Neutralization Effect of Sera against Delta and Omicron in Patients Recovering from COVID-19 and Inactivated Vaccine Recipients. Vaccines. 2023; 11(2):471. https://doi.org/10.3390/vaccines11020471
Chicago/Turabian StyleZhu, Yajuan, Qianhong Zhong, Zhanzhong Ma, Shuang Liu, Yunhua Lan, Bo Peng, Xiaomin Zhang, Xiaolu Shi, Jing Qu, Zhilong Wu, and et al. 2023. "Neutralization Effect of Sera against Delta and Omicron in Patients Recovering from COVID-19 and Inactivated Vaccine Recipients" Vaccines 11, no. 2: 471. https://doi.org/10.3390/vaccines11020471
APA StyleZhu, Y., Zhong, Q., Ma, Z., Liu, S., Lan, Y., Peng, B., Zhang, X., Shi, X., Qu, J., Wu, Z., Zhao, Z., Zhang, X., & Zhang, D. (2023). Neutralization Effect of Sera against Delta and Omicron in Patients Recovering from COVID-19 and Inactivated Vaccine Recipients. Vaccines, 11(2), 471. https://doi.org/10.3390/vaccines11020471









