Protective Effect of Inactivated COVID-19 Vaccines against Omicron BA.2 Infection in Guangzhou: A Test-Negative Case-Control Real-World Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources and Information Collection
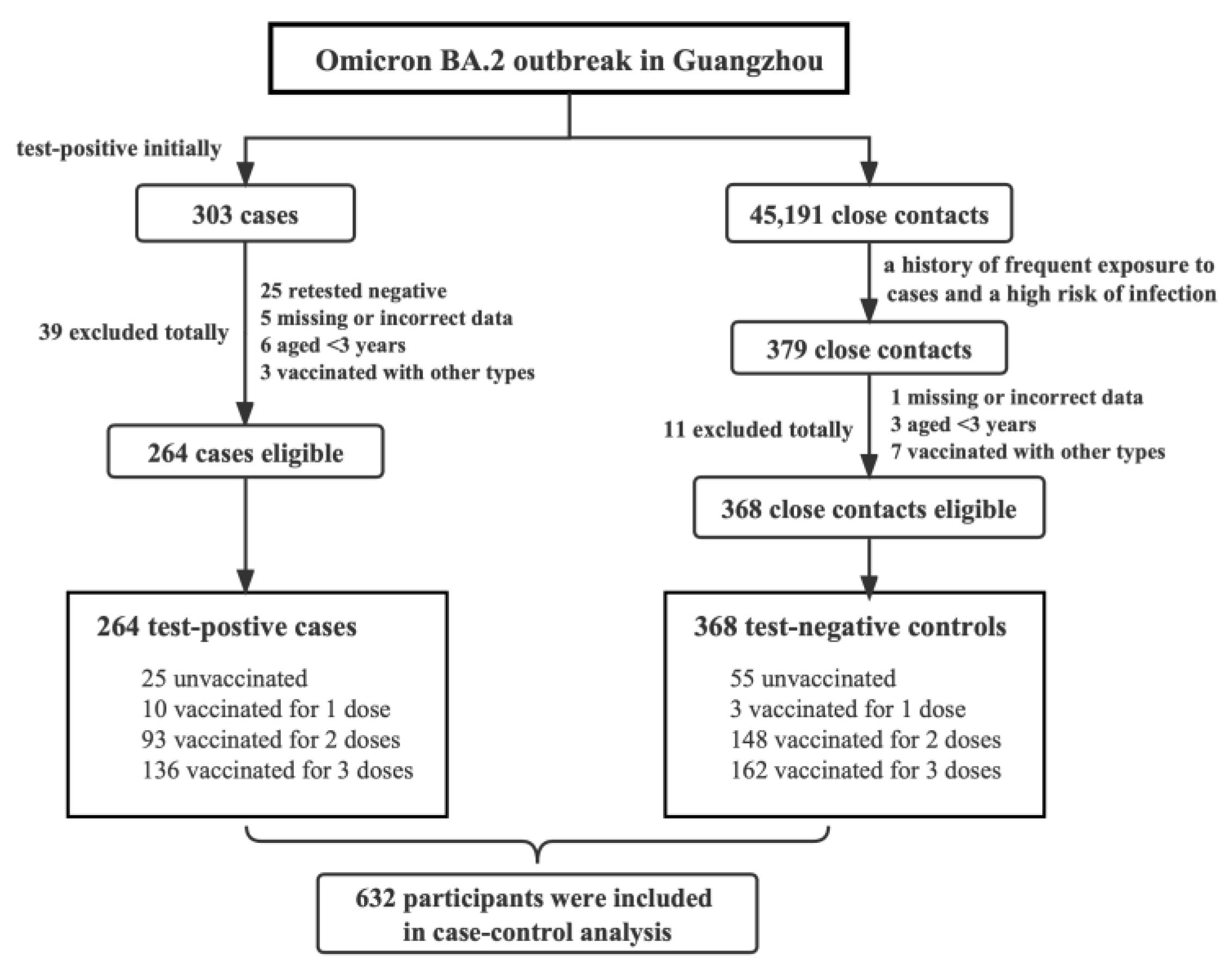
2.3. Selection of Case and Control Groups
2.4. Statistical Analysis
3. Results
3.1. Demographic and Epidemiological Characteristics of Included Cases and Controls
3.2. Protective Effect Induced by Inactivated COVID-19 Vaccines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Lf, A.-M.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar]
- Habas, K.; Nganwuchu, C.; Shahzad, F.; Gopalan, R.; Haque, M.; Rahman, S.; Majumder, A.A.; Nasim, T. Resolution of coronavirus disease 2019 (COVID-19). Expert Rev. Anti. Infect. Ther. 2020, 18, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.Y.; Wang, W.B.; Gao, R.D.; Zhou, A.M. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 2022, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron variant: Recent progress and future perspectives. Signal Transduct. Target Ther. 2022, 7, 141. [Google Scholar] [CrossRef]
- Thakur, V.; Ratho, R.K. OMICRON (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. J. Med. Virol. 2022, 94, 1821–1824. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes. Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Liu, G.; Luo, W.; Xia, N. COVID-19: Progress in diagnostics, therapy and vaccination. Theranostics 2020, 10, 7821–7835. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Moderna applies for US and EU approval as vaccine trial reports 94.1% efficacy. BMJ 2020, 371, m4709. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows. BMJ 2020, 371, m4826. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.C.; Tiruneh, G.M.M.; Behaile, T.M.A.; Dejenie, T.A.; Ayele, T.M.; Admasu, F.T.; Muche, Z.T.; Adela, G.A. Mutational Pattern, Impacts and Potential Preventive Strategies of Omicron SARS-CoV-2 Variant Infection. Infect. Drug Resist. 2022, 15, 1871–1887. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Q.; Wei, P.; Chen, Z.; Aviszus, K.; Yang, J.; Downing, W.; Jiang, C.; Liang, B.; Reynoso, L.; et al. The basis of a more contagious 501Y.V1 variant of SARS-COV-2. Cell Res. 2021, 31, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Bazargan, M.; Elahi, R.; Esmaeilzadeh, A. OMICRON: Virology, immunopathogenesis, and laboratory diagnosis. J. Gene Med. 2022, 24, e3435. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.; et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Jing, S.; Zhang, K.; Milne, R.; Wang, H. Infectivity versus fatality of SARS-CoV-2 mutations and influenza. Int. J. Infect. Dis. 2022, 121, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar]
- Suphanchaimat, R.; Nittayasoot, N.; Jiraphongsa, C.; Thammawijaya, P.; Bumrungwong, P.; Tulyathan, A.; Cheewaruangroj, N.; Pittayawonganon, C.; Tharmaphornpilas, P. Real-World Effectiveness of Mix-and-Match Vaccine Regimens against SARS-CoV-2 Delta Variant in Thailand: A Nationwide Test-Negative Matched Case-Control Study. Vaccines 2022, 10, 1080. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Peng, Y.; Shen, E.; Huang, Q.; Chen, Y.; Liu, P.; Guo, C.; Feng, Z.; Gao, L.; Zhang, X.; et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol. Ther. 2021, 29, 2794–2805. [Google Scholar] [CrossRef]
- Yang, B.; Wong, I.O.L.; Xiao, J.; Tsang, T.K.; Liao, Q.; Cowling, B.J. Effectiveness of CoronaVac and BNT162b2 Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 Omicron BA.2 Infections in Hong Kong. J. Infect. Dis. 2022, 226, 1382–1384. [Google Scholar] [CrossRef]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Toffa, S.; Sachdeva, R.; Gallagher, E.; Groves, N.; O’Connell, A.M.; Chand, M.; Ramsay, M.; et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect. Dis. 2022, 22, 931–933. [Google Scholar] [CrossRef]
- Wong, M.T.J.; Dhaliwal, S.S.; Balakrishnan, V.; Nordin, F.; Norazmi, M.N.; Tye, G.J. Effectiveness of Booster Vaccinations on the Control of COVID-19 during the Spread of Omicron Variant in Malaysia. Int. J. Environ. Res. Public Health 2023, 20, 1647. [Google Scholar] [CrossRef]
- Eggink, D.; Andeweg, S.P.; Vennema, H.; van Maarseveen, N.; Vermaas, K.; Vlaemynck, B.; Schepers, R.; van Gageldonk-Lafeber, A.B.; Hof, S.V.D.; Reusken, C.B.; et al. Increased risk of infection with SARS-CoV-2 Omicron BA.1 compared with Delta in vaccinated and previously infected individuals, the Netherlands, 22 November 2021 to 19 January 2022. Euro Surveil. 2022, 27, 2101196. [Google Scholar] [CrossRef]
- Andeweg, S.P.; Vennema, H.; Veldhuijzen, I.; Smorenburg, N.; Schmitz, D.; Zwagemaker, F.; van Gageldonk-Lafeber, A.B.; Hahné, S.J.M.; Reusken, C.; Knol, M.J.; et al. Elevated risk of infection with SARS-CoV-2 Beta, Gamma, and Delta variant compared to Alpha variant in vaccinated individuals. Sci. Transl. Med. 2023, 15, eabn4338. [Google Scholar] [CrossRef]
- Lyngse, F.P.; Kirkeby, C.T.; Denwood, M.; Christiansen, L.E.; Mølbak, K.; Møller, C.H.; Skov, R.L.; Krause, T.G.; Rasmussen, M.; Sieber, R.N.; et al. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat. Commun. 2022, 13, 5760. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, F. Evolving understanding of antibody-dependent enhancement (ADE) of SARS-CoV-2. Front. Immunol. 2022, 13, 1008285. [Google Scholar] [CrossRef] [PubMed]
| Test-Positive Cases (n = 264) | Test-Negative Controls (n = 368) | p-Value | |
|---|---|---|---|
| Sex | 0.564 | ||
| Male | 123 (46.6%) | 180 (48.9%) | |
| Female | 141 (53.4%) | 188 (51.1%) | |
| Age | |||
| Median [IQR] | 33.0 [24.0, 43.0] | 34.5 [19.0, 46.0] | 0.313 |
| Age group (years) | 0.026 | ||
| 3–17 | 25 (9.5%) | 62 (16.8%) | |
| 18–59 | 226 (85.6%) | 291 (79.1%) | |
| ≥60 | 13 (4.9%) | 15 (4.1%) | |
| Vaccination status | 0.004 | ||
| Unvaccinated | 25 (9.5%) | 55 (14.9%) | |
| One dose | 10 (3.8%) | 3 (0.9%) | |
| Two doses | 93 (35.2%) | 148 (40.2%) | |
| Three doses | 136 (51.5%) | 162 (44.0%) |
| Case Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Unvaccinated (n = 25) | Vaccinated (n = 239) | p-Value | Unvaccinated (n = 55) | Vaccinated (n = 313) | p-Value | |
| Sex | 0.158 | 0.231 | ||||
| Male | 15 (60.0%) | 108 (45.2%) | 31 (56.4%) | 149 (47.6%) | ||
| Female | 10 (40.0%) | 131 (54.8%) | 24 (43.6%) | 164 (52.4%) | ||
| Age | ||||||
| Median [IQR] | 32.0 [24.5, 42.5] | 33.0 [24.0, 43.0] | 0.733 | 34.5 [24.0, 49.0] | 34.5 [18.8, 45.0] | 0.129 |
| Age group (years) | 0.066 1 | 0.012 | ||||
| 3–17 | 4 (16.0%) | 21 (8.8%) | 6 (10.9%) | 56 (17.9%) | ||
| 18–59 | 18 (72.0%) | 208 (87.0%) | 43 (78.2%) | 248 (79.2%) | ||
| ≥60 | 3 (12.0%) | 10 (4.2%) | 6 (10.9%) | 9 (2.9%) | ||
| Vaccinated for 1 Dose | Vaccinated for 2 Doses | Vaccinated for 3 Doses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 10) | Controls (n = 3) | p-Value | Cases (n = 93) | Controls (n = 148) | p-Value | Cases (n = 136) | Controls (n = 162) | p-Value | |
| Vaccination interval 1 1 | |||||||||
| Median [IQR] | - | - | - | 273.0 [240.0, 286.0] | 246.0 [152.0, 277.8] | 0.001 | 317.5 [283.0, 333.0] | 317.0 [277.0, 337.8] | 0.563 |
| Vaccination interval 2 2 | |||||||||
| Median [IQR] | 234.5 [226.0, 258.5] | 155.0 [150.5, 232.0] | 0.785 3 | 236.0 [188.0, 261.0] | 215.0 [124.5, 255.0] | 0.001 | 93.0 [48.5, 113.0] | 83.5 [35.0, 110.5] | 0.562 |
| Cases (n = 264) | Controls (n = 368) | OR1 3 (95% CI) | OR2 3 (95% CI) | OR3 3 (95% CI) | |
|---|---|---|---|---|---|
| Overall participants 1 | |||||
| Unvaccinated | 25 (9.5%) | 55 (15.0%) | Reference | ||
| One dose | 10 (3.8%) | 3 (0.8%) | 7.715 (1.904, 31.254) ** | Reference | |
| Two doses | 93 (35.2%) | 148 (40.2%) | 1.474 (0.841, 2.585) | 0.191 (0.050, 0.727) * | Reference |
| Three doses | 136 (51.5%) | 162 (44.0%) | 2.055 (1.162, 3.635) * | 0.091 (0.011, 0.727) * | 1.069 (0.730, 1.568) |
| Male 2 | |||||
| Unvaccinated | 15 (5.7%) | 31 (8.4%) | Reference | ||
| One dose | 6 (2.3%) | 1 (0.4%) | 12.400 (1.367, 112.463) * | Reference | |
| Two doses | 42 (15.9%) | 78 (21.1%) | 1.113 (0.541, 2.290) | 0.090 (0.010, 0.770) * | Reference |
| Three doses | 60 (22.7%) | 70 (19.0%) | 1.771 (0.874, 3.590) | 0.143 (0.017, 1.220) | 1.592 (0.956, 2.650) |
| Female 2 | |||||
| Unvaccinated | 10 (3.8%) | 24 (6.5%) | Reference | ||
| One dose | 4 (1.5%) | 2 (0.6%) | 4.800 (0.754, 30.550) | Reference | |
| Two doses | 51 (19.3%) | 70 (19.0%) | 1.749 (0.769, 3.975) | 0.364 (0.064, 2.066) | Reference |
| Three doses | 76 (28.8%) | 92 (25.0%) | 1.983 (0.893, 4.403) | 0.413 (0.074, 2.317) | 1.134 (0.707, 1.817) |
| 3–17 years 2 | |||||
| Unvaccinated | 4 (1.5%) | 6 (1.6%) | Reference | ||
| One dose | 1 (0.4%) | 2 (0.6%) | 0.750 (0.050, 11.311) | Reference | |
| Two doses | 20 (7.6%) | 54 (14.6%) | 0.556 (0.142, 2.176) | 0.741 (0.064, 8.624) | Reference |
| Three doses | 0 (0.0%) | 0 (0.0%) | - 4 | - 4 | - 4 |
| 18–59 years 2 | |||||
| Unvaccinated | 18 (6.8%) | 43 (11.7%) | Reference | ||
| One dose | 9 (3.4%) | 1 (0.4%) | 21.500 (2.535, 182.373) ** | Reference | |
| Two doses | 72 (27.3%) | 91 (24.7%) | 1.890 (1.006, 3.553) * | 0.089 (0.011, 0.720) * | Reference |
| Three doses | 127 (48.1%) | 156 (42.4%) | 1.945 (1.069, 3.537) * | 0.090 (0.011, 0.723) * | 1.029 (0.698, 1.516) |
| ≥60 years 2 | |||||
| Unvaccinated | 3 (1.1%) | 6 (1.6%) | Reference | ||
| One dose | 0 (0.0%) | 0 (0.0%) | - 4 | Reference | |
| Two doses | 1 (0.4%) | 3 (0.8%) | 0.667 (0.047, 9.472) | - 4 | Reference |
| Three doses | 9 (3.4%) | 6 (1.6%) | 3.000 (0.533, 16.897) | - 4 | 4.500 (0.374, 54.155) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Zhong, J.; Xiong, H.; Li, Y.; Guo, T.; Peng, B.; Fang, C.; Kang, Y.; Tan, J.; Ma, Y. Protective Effect of Inactivated COVID-19 Vaccines against Omicron BA.2 Infection in Guangzhou: A Test-Negative Case-Control Real-World Study. Vaccines 2023, 11, 566. https://doi.org/10.3390/vaccines11030566
Zhang D, Zhong J, Xiong H, Li Y, Guo T, Peng B, Fang C, Kang Y, Tan J, Ma Y. Protective Effect of Inactivated COVID-19 Vaccines against Omicron BA.2 Infection in Guangzhou: A Test-Negative Case-Control Real-World Study. Vaccines. 2023; 11(3):566. https://doi.org/10.3390/vaccines11030566
Chicago/Turabian StyleZhang, Dingmei, Jiayi Zhong, Husheng Xiong, Yufen Li, Tong Guo, Bo Peng, Chuanjun Fang, Yan Kang, Jinlin Tan, and Yu Ma. 2023. "Protective Effect of Inactivated COVID-19 Vaccines against Omicron BA.2 Infection in Guangzhou: A Test-Negative Case-Control Real-World Study" Vaccines 11, no. 3: 566. https://doi.org/10.3390/vaccines11030566
APA StyleZhang, D., Zhong, J., Xiong, H., Li, Y., Guo, T., Peng, B., Fang, C., Kang, Y., Tan, J., & Ma, Y. (2023). Protective Effect of Inactivated COVID-19 Vaccines against Omicron BA.2 Infection in Guangzhou: A Test-Negative Case-Control Real-World Study. Vaccines, 11(3), 566. https://doi.org/10.3390/vaccines11030566







