Emerging Trends in Nano-Driven Immunotherapy for Treatment of Cancer
Abstract
1. Introduction
2. The Immune Suppressive Tumour Microenvironment—A Formidable Fortress
3. Nanoparticle-Mediated Immunotherapy in the Treatment of Cancer
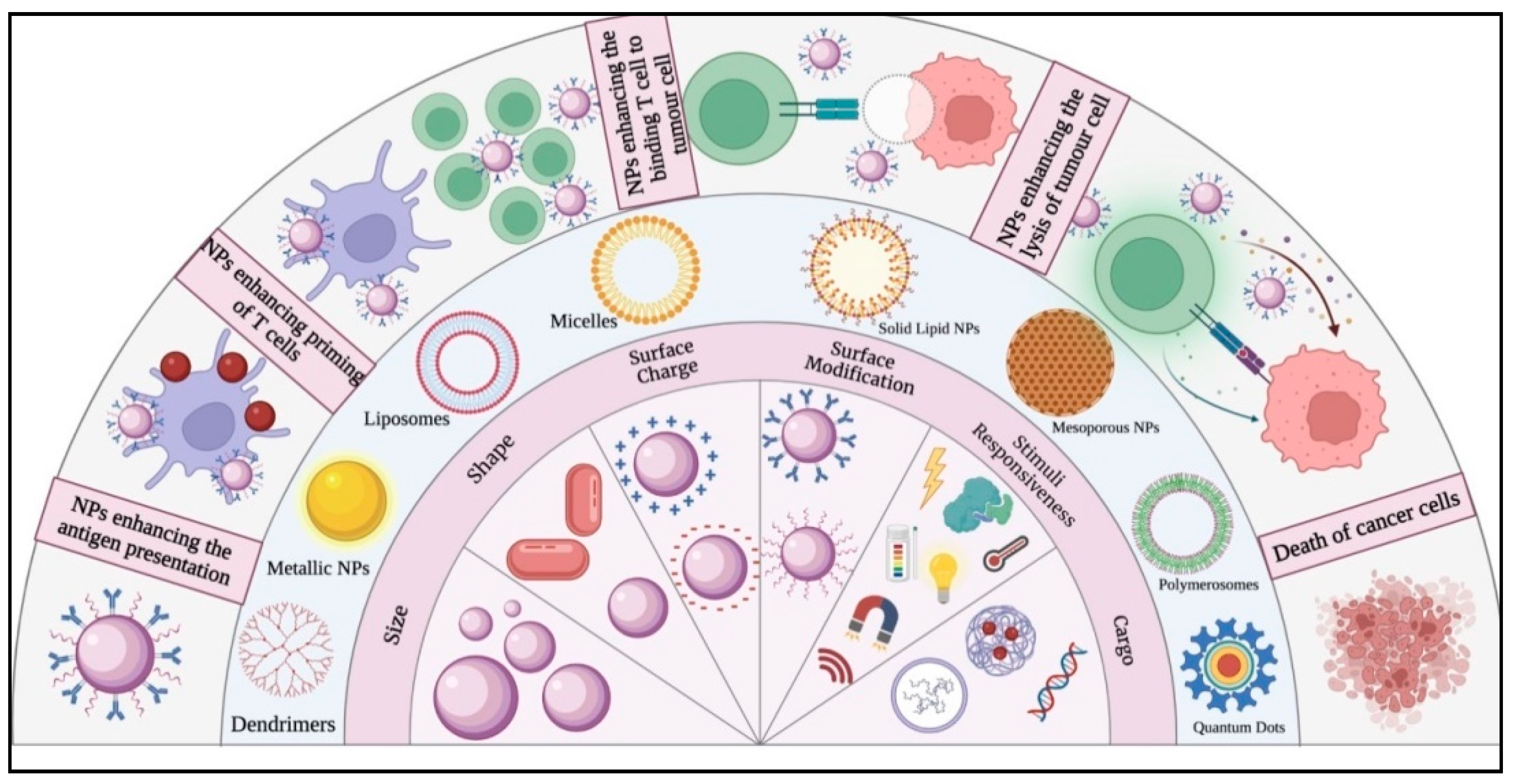
4. Modulation of Tumour Immunity Using Nanoparticles
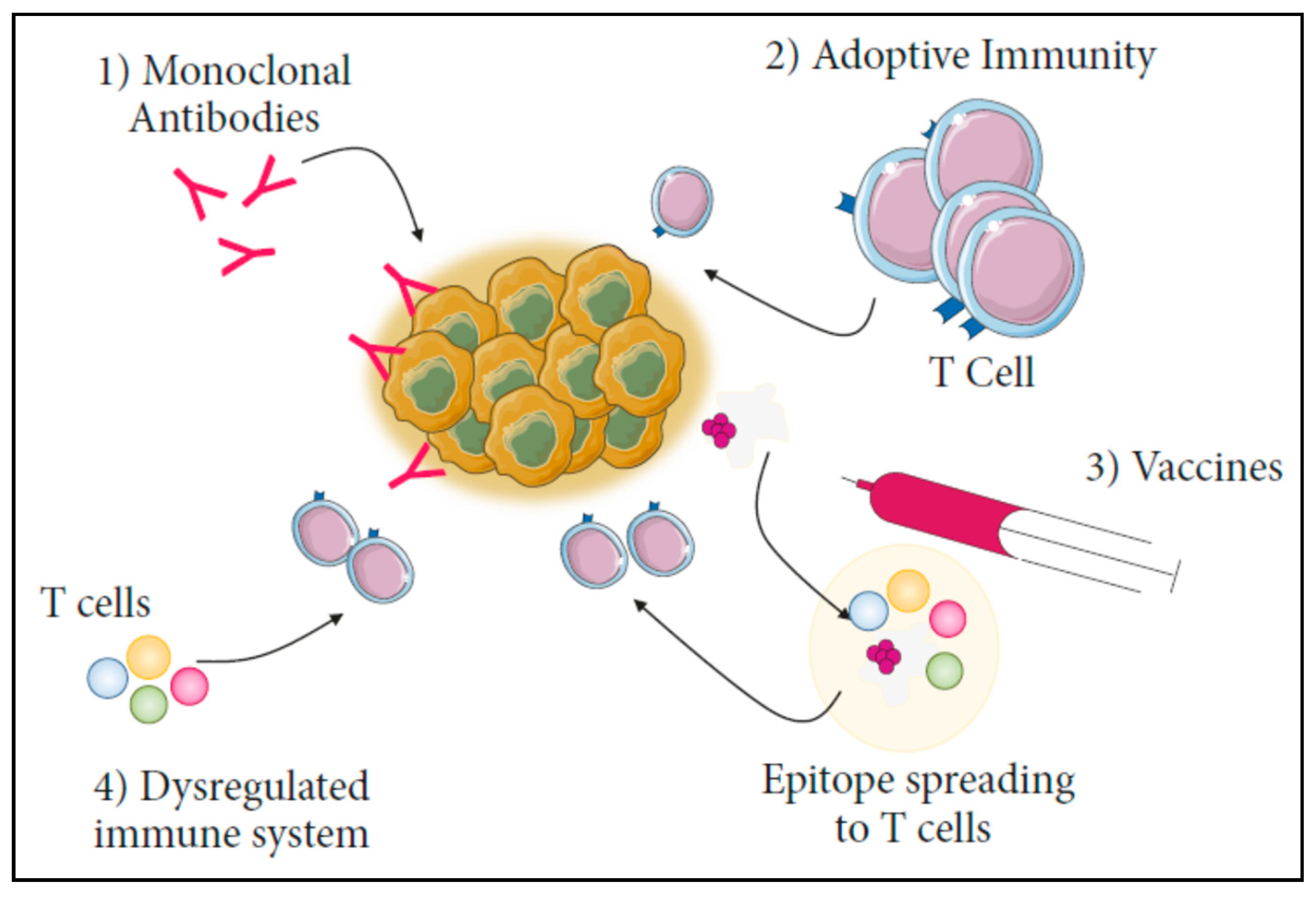

5. Limitations in Using Nanoparticles for Conventional CANCER Immunotherapy
6. Recent Developments in Cancer Immunotherapy
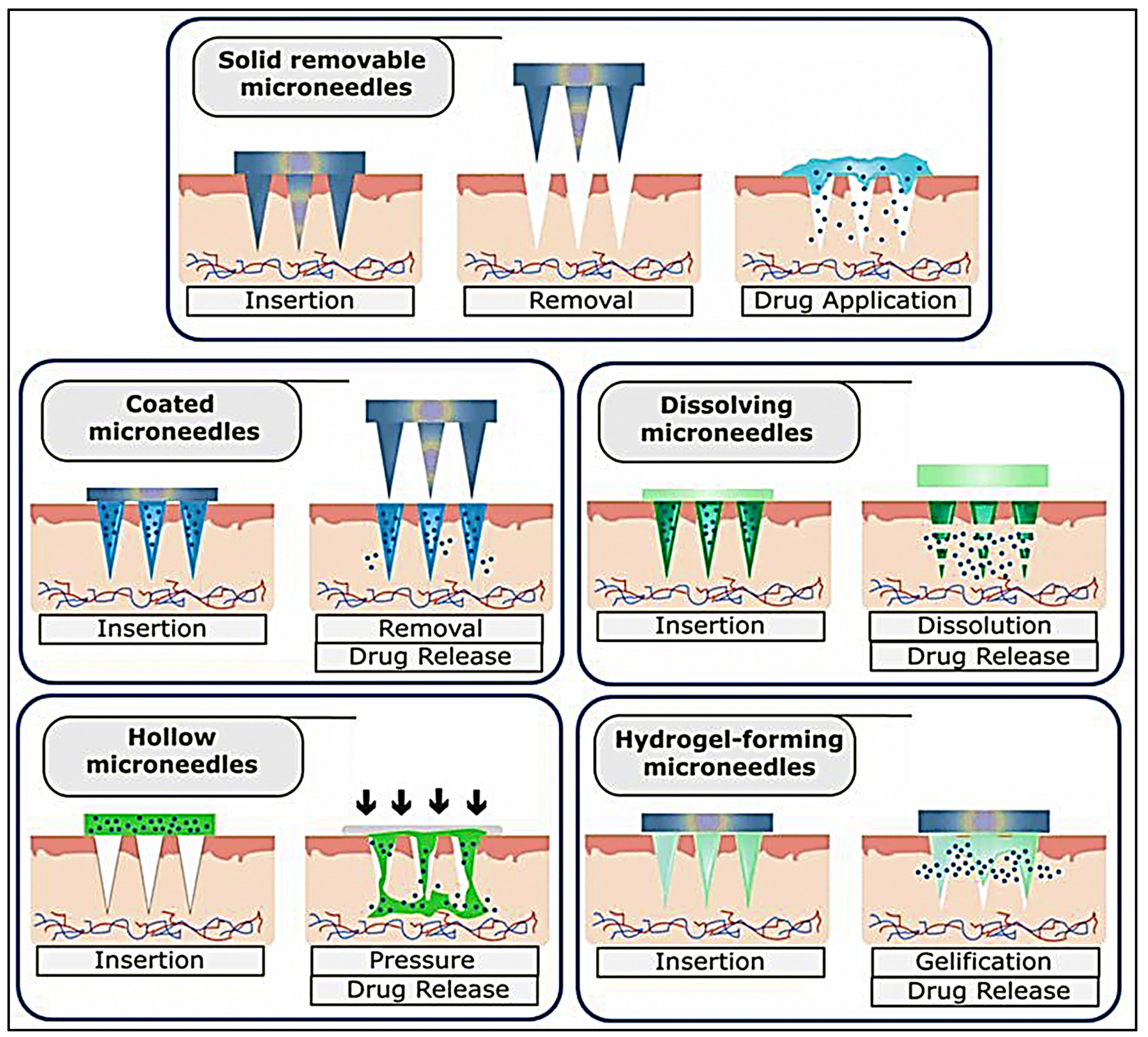
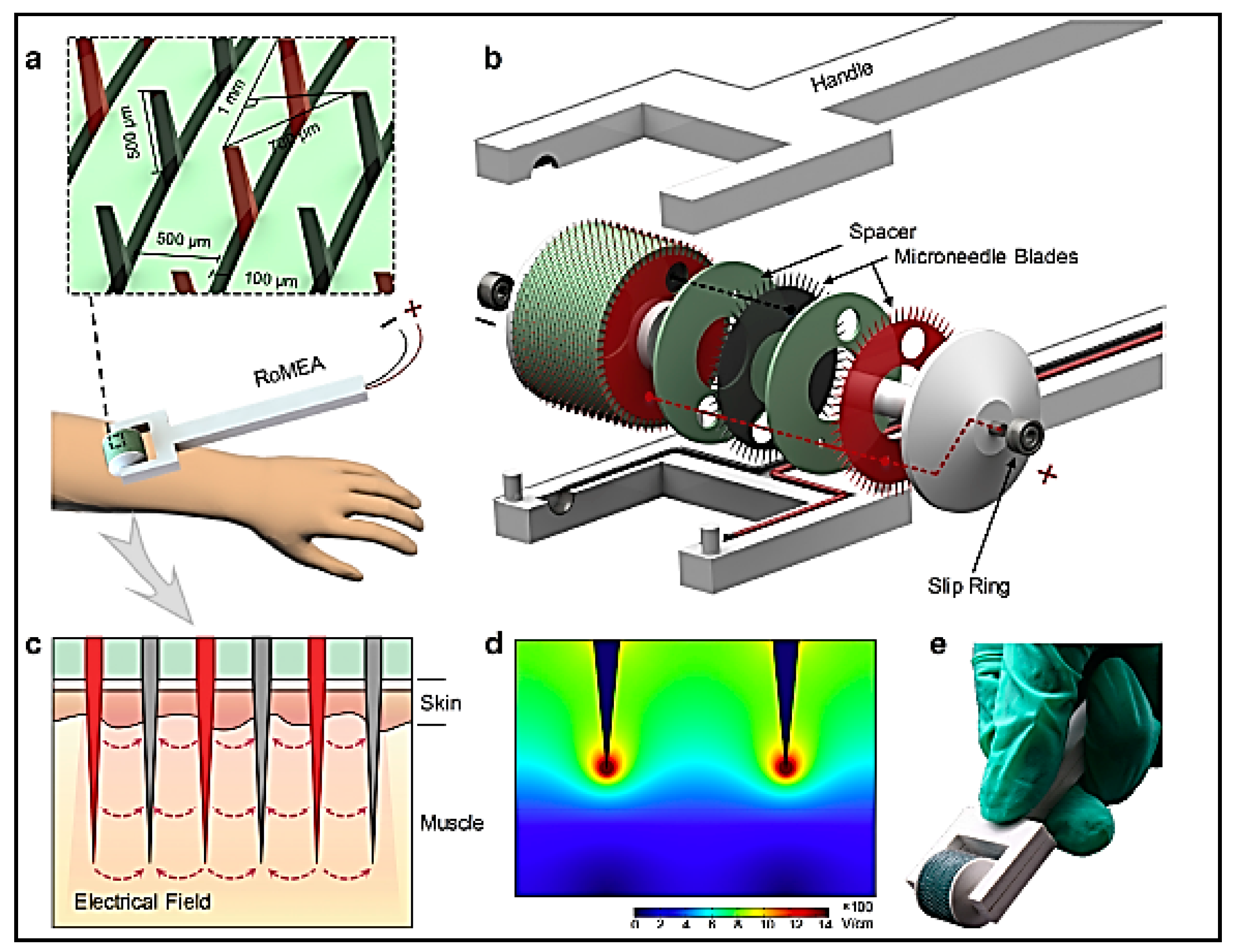
6.1. Microneedle-Based Immunotherapy
6.2. Nucleic Acid-Mediated Immunotherapy
6.3. Gene Editing Strategies in Immunotherapy
6.4. Exosome-Based Immunotherapy
6.5. Engineered Cells for Immunotherapy
6.6. CAR-T Therapy
6.7. Nano-Optogenetics for Immunotherapy
6.8. Virus and Viral Components for Immunotherapy
6.9. Oncolytic Virotherapy
6.10. Bacterial Immunotherapeutics
6.11. Fungal Derivatives as Immunotherapeutics
6.12. Herbal Interventions for Immunotherapy
6.13. Regulating 3D Matrix Architecture for Immunotherapy
7. Nanoparticles with Immunotherapy in Clinical Trials
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICG | Indocyanine green (ICG) |
| JQ1 | Thienotriazolodiazepine |
| BMS (BMS986205) | Linrodostat |
| ICD | Immunogenic cell death |
| PTT | Photothermal therapy |
| CpG | cytosine-guanine dinucleotides |
| PD-1/PD-L1 | Programmed cell death-1/Programmed cell death ligand-1 |
| IDO-1 | Indoleamine 2,3-dioxygenase 1 |
| MIRDs | Polyphenols coating ICG loaded magnetic nanoparticles |
| DPA-PEG | Dopamine-Poly(ethylene glycol) |
| R837 | Near-infrared heptamethine cyanine dye |
| NC | Nanocomplex |
| Ce6 | Chlorin e6 (Photosensitizer) |
| PyroR | Pyropheophorbide-A and Resiquimod |
| ROS | Reactive oxygen species |
| CTTPA-G | (Cancer cell membranes (CC-Ms) and TTPA and Glutamine) |
| Type I AIE photosensitizer (TTPA): AIE | Aggregation Induced Emission |
| TTPA | Terephthalic acid |
| Pep-PAPM | (peptide PEP-associated protein micelles) |
| PEP | Phosphoenolpyruvate |
| TNBC | Triple-negative breast cancer |
| 231MARS | 231 membrane artesunate |
| PLGA | Poly(lactic-co-glycolic acid) |
| SK | Shikonin |
| TGF-β | Tumour Growth Factor-β |
| 5-ASA | (5-aminosalicylic acid) |
| DOX | Doxorubicin |
| IR783 | Near-infrared heptamethine cyanine dye |
| mTOR | mammalian Target of Rapamycin |
| CTL | Cytotoxic T lymphocyte |
| BMS/RA@CC Liposome | (BMS-202/RA-V@CT26 cancer cells biomimetic) |
| BMS202 | PD-1/PD-L1 inhibitor 2 |
| RA-V | Deoxybouvardin (Chemotherapeutic drug) |
| MGTe | Fusing TM and BM on the surface of glutathione (GSH) decorated Te nanoparticles (GTe) |
| TM | Tumour cell membrane |
| BM | Bacterial cell membrane |
| APC | Antigen-presenting cells |
| PGA | Poly (glycolic acid) |
| IFN | Interferon |
| mAb | monoclonal antibody |
| HSP 70/90 | Heat shock protein 70/90 |
| MHC-I/II | Major Histocompatibility Complex-I/II |
| TGF β | Tumour growth factor |
| EZH2 | Enhancer of zeste homolog 2 |
| LPS | Lipopolysaccharide |
| TLR | Toll-like receptor |
| MUC1 | Mucin 1 |
| Dex | Dexamethasone |
| ZNPs/I@CML | ZSTK carrier-free nanoparticle/Indomethacin@cancer-derived membrane/liposome |
| PI3K | Phosphoinositide 3-kinase |
| PLG | Poly(DL-Lactide-co-Glycolide) |
| ZSTK | ZSTK474 (PI3K inhibitor) |
| MCP 1 | Monocyte chemoattractant protein-1 |
| TNF 2 | Tumour necrosis factor |
| UPP | Unripe plantain peel |
| OVA | Ovalbumin |
| YP and Er NaY/GdF4 | Yttrium phosphide and Erbium Sodium;Yttrium/Gadolinium fluoride |
| UCNPs | Up conversion nanoparticles |
| iRGD peptide | 9-amino acid cyclic peptide (sequence: CRGDKGPDC) |
| NSCLC | Non-small cell lung cancer |
| PCL | Polycaprolactone |
| CaCO3 | Calcium carbonate |
| EPC | Egg yolk phosphatidylcholine |
| Span85 | Sorbitan trioleate |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| ODN | oligodeoxynucleotides |
| CS/γ-PGA | (Chitosan/Poly(gamma glutamic acid)) |
| IgG | Immunoglobulin G |
| CTX | cyclophosphamide |
| PLEL | poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide) |
| HPAA | Hydroxyphosphono-Acetic Acid |
| PpASE | PEG-poly (AGE-Suc) with etiphosphate |
| ANLs | Synthetic long peptides |
| ANSs | MHC-I epitope restricted peptides |
| Mn-NP | Manganese nanoparticle |
| cGAS | cyclic GMP-AMP Synthase |
| STING | Stimulation of interferon genes |
| s.c. | Subcutaneous |
| i.v. | Intravenous |
| DGBA | Guanidinobenzoic acid |
| NLRP3 | NLR (Nod-like receptor) family pyrin domain containing 3 |
| iNOS | Inducible nitric oxide synthase |
References
- Gowd, V.; Ahmad, A.; Tarique, M.; Suhail, M.; Zughaibi, T.A.; Tabrez, S.; Khan, R. Advancement of Cancer Immunotherapy Using Nanoparticles-Based Nanomedicine. Semin. Cancer Biol. 2022, 86, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Buabeid, M.A.; Arafa, E.S.A.; Murtaza, G. Emerging Prospects for Nanoparticle-Enabled Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 9624532. [Google Scholar] [CrossRef] [PubMed]
- Aikins, M.E.; Xu, C.; Moon, J.J. Engineered Nanoparticles for Cancer Vaccination and Immunotherapy. Accounts Chem. Res. 2020, 53, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Muluh, T.A.; Chen, Z.; Li, Y.; Xiong, K.; Jin, J.; Fu, S.; Wu, J. Enhancing Cancer Immunotherapy Treatment Goals by Using Nanoparticle Delivery System. Int. J. Nanomed. 2021, 16, 2389–2404. [Google Scholar] [CrossRef]
- Lim, S.; Park, J.; Shim, M.K.; Um, W.; Yoon, H.Y.; Ryu, J.H.; Lim, D.K.; Kim, K. Recent Advances and Challenges of Repurposing Nanoparticle-Based Drug Delivery Systems to Enhance Cancer Immunotherapy. Theranostics 2019, 9, 7906–7923. [Google Scholar] [CrossRef]
- Yu, Y.R.; Ho, P.C. Sculpting Tumor Microenvironment with Immune System: From Immunometabolism to Immunoediting. Clin. Exp. Immunol. 2019, 197, 153–160. [Google Scholar] [CrossRef]
- Munn, D.H.; Bronte, V. Immune Suppressive Mechanisms in the Tumor Microenvironment. Curr. Opin. Immunol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Kapitanova, K.S.; Naumenko, V.A.; Garanina, A.S.; Melnikov, P.A.; Abakumov, M.A.; Alieva, I.B. Advances and Challenges of Nanoparticle-Based Macrophage Reprogramming for Cancer Immunotherapy. Biochemistry 2019, 84, 729–745. [Google Scholar] [CrossRef]
- Larionova, I.; Kazakova, E.; Patysheva, M.; Kzhyshkowska, J. Transcriptional, Epigenetic and Metabolic Programming of Tumor-Associated Macrophages. Cancers 2020, 12, 1411. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer Immunoediting and Resistance to T Cell-Based Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising Targets for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shi, T.; Zhang, H.; Hu, J.; Song, Y.; Wei, J.; Ren, S.; Zhou, C. Tumor Neoantigens: From Basic Research to Clinical Applications. J. Hematol. Oncol. 2019, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Sheppard, N.C.; Billingsley, M.M.; June, C.H.; Mitchell, M.J. Nanomaterials for T-Cell Cancer Immunotherapy. Nat. Nanotechnol. 2021, 16, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Hasan, M.; Tariq, T.; Dai, Z. Enhancing Cancer Immunotherapeutic Efficacy with Sonotheranostic Strategies. Bioconjugate Chem. 2022, 33, 1011–1034. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current Trends and Challenges in Cancer Management and Therapy Using Designer Nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Holay, M.; Park, J.H.; Fang, R.H.; Zhang, J.; Zhang, L. Nanoparticle Delivery of Immunostimulatory Agents for Cancer Immunotherapy. Theranostics 2019, 9, 7826–7848. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Z.; Jaklenec, A.; Hu, Q. Vaccine delivery systems toward lymph nodes. Adv. Drug Deliv. Rev. 2021, 179, 113914. [Google Scholar] [CrossRef]
- Ou, B.S.; Saouaf, O.M.; Baillet, J.; Appel, E.A. Sustained Delivery Approaches to Improving Adaptive Immune Responses. Adv. Drug Deliv. Rev. 2022, 187, 114401. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, J.; Pan, J.; Liu, C.; Guo, X.; Zhou, S. Personalized Nanovaccine Coated with Calcinetin-Expressed Cancer Cell Membrane Antigen for Cancer Immunotherapy. Nano Lett. 2021, 21, 8418–8425. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Hong, S.; Dong, X.; Zheng, D.W.; Liang, J.L.; Bai, X.F.; Wang, X.N.; Han, Z.Y.; Zhang, X.Z. Bioinspired Nano-Vaccine Construction by Antigen Pre-Degradation for Boosting Cancer Personalized Immunotherapy. Biomaterials 2022, 287, 121628. [Google Scholar] [CrossRef]
- Sambi, M.; Bagheri, L.; Szewczuk, M.R. Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy and Patient Response Rates. J. Oncol. 2019, 2019, 4508794. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; He, R.; Liu, Y.; Su, R.; Zhao, Y.; Zheng, X.; Liu, Y.; Chong, G.; Ruan, S.; Wang, H.; et al. A Size/Charge/Targeting Changeable Nano-Booster to Realize Synergistic Photodynamic-Immunotherapy with High Safety. Chem. Eng. J. 2022, 434, 134585. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, R.; Zhao, L.; Kong, R.; Yang, N.; Liu, Y.; Chen, A.; Wang, C.; Cheng, H.; Li, S. Photodynamic Therapy Initiated Immunotherapy of Self-Delivery Re-Educator by Inducing Immunogenic Cell Death and Macrophage Polarization. Chem. Eng. J. 2022, 435, 134783. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, J.; Li, C.; Lu, Y.; Cheng, L.; Liu, J. A Targeting Black Phosphorus Nanoparticle Based Immune Cells Nano-Regulator for Photodynamic/Photothermal and Photo-Immunotherapy. Bioact. Mater. 2021, 6, 472–489. [Google Scholar] [CrossRef]
- Ou, W.; Nam, K.S.; Park, D.H.; Hwang, J.; Ku, S.K.; Yong, C.S.; Kim, J.O.; Byeon, J.H. Artificial Nanoscale Erythrocytes from Clinically Relevant Compounds for Enhancing Cancer Immunotherapy. Nano-Micro Lett. 2020, 12, 90. [Google Scholar] [CrossRef]
- Yang, J.; Pan, X.; Zhang, J.; Ma, S.; Zhou, J.; Jia, Z.; Wei, Y.; Liu, Z.; Yang, N.; Shen, Q. Reprogramming Dysfunctional Dendritic Cells by a Versatile Metabolism Nano-Intervenor for Enhancing Cancer Combinatorial Immunotherapy. Nano Today 2022, 46, 101618. [Google Scholar] [CrossRef]
- Park, K.S.; Nam, J.; Son, S.; Moon, J.J. Personalized Combination Nano-Immunotherapy for Robust Induction and Tumor Infiltration of CD8+ T Cells. Biomaterials 2021, 274, 120844. [Google Scholar] [CrossRef]
- Yang, A.; Dong, X.; Bai, Y.; Sheng, S.; Zhang, Y.; Liu, T.; Zhu, D.; Lv, F. Doxorubicin/CpG Self-Assembled Nanoparticles Prodrug and Dendritic Cells Co-Laden Hydrogel for Cancer Chemo-Assisted Immunotherapy. Chem. Eng. J. 2021, 416, 129192. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef]
- Huo, W.; Yang, X.; Wang, B.; Cao, L.; Fang, Z.; Li, Z.; Liu, H.; Liang, X.-J.; Zhang, J.; Jin, Y. Biomineralized Hydrogel DC Vaccine for Cancer Immunotherapy: A Boosting Strategy via Improving Immunogenicity and Reversing Immune-Inhibitory Microenvironment. Biomaterials 2022, 288, 121722. [Google Scholar] [CrossRef]
- Tahara, Y.; Mizuno, R.; Nishimura, T.; Mukai, S.-A.; Wakabayashi, R.; Kamiya, N.; Akiyoshi, K.; Goto, M. A Solid-in-Oil-in-Water Emulsion: An Adjuvant-Based Immune-Carrier Enhances Vaccine Effect. Biomaterials 2022, 282, 121385. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, K.; Jia, Y.; Hao, Y.; Peng, J.; Yuan, L.; Chen, Y.; Pan, M.; Qian, Z. A Biodegradable Thermosensitive Hydrogel Vaccine for Cancer Immunotherapy. Appl. Mater. Today 2020, 19, 100608. [Google Scholar] [CrossRef]
- Chen, P.-G.; Huang, Z.-H.; Sun, Z.-Y.; Gao, Y.; Liu, Y.-F.; Shi, L.; Chen, Y.-X.; Zhao, Y.-F.; Li, Y.-M. Chitosan Nanoparticles Based Nanovaccines for Cancer Immunotherapy. Pure Appl. Chem 2016, 89, 931–939. [Google Scholar] [CrossRef]
- Yang, F.; Shi, K.; Hao, Y.; Jia, Y.; Liu, Q.; Chen, Y.; Pan, M.; Yuan, L.; Yu, Y.; Qian, Z. Cyclophosphamide Loaded Thermo-Responsive Hydrogel System Synergize with a Hydrogel Cancer Vaccine to Amplify Cancer Immunotherapy in a Prime-Boost Manner. Bioact. Mater. 2021, 6, 3036–3048. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Iqbal, M.Z.; Liu, C.; Xing, J.; Akakuru, O.U.; Fang, Q.; Li, Z.; Dai, Y.; Li, A.; Guan, Y.; et al. Engineered Nano-Immunopotentiators Efficiently Promote Cancer Immunotherapy for Inhibiting and Preventing Lung Metastasis of Melanoma. Biomaterials 2019, 223, 119464. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhan, X.; Lin, Z.; Liu, Z.; Yang, J.; Zhang, Y. Bacteria-like Tumor Vaccines Progressively Initiate Cascade Reaction for Precise Antigen Delivery and Induction of Anti-Tumor Cellular Immune Response. Chem. Eng. J. 2022, 450, 138136. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, H.; Song, Y.; Zhu, Y.; Qin, M.; He, C.; Du, G.; Sun, X. Aluminum Nanoparticles Deliver a Dual-Epitope Peptide for Enhanced Anti-Tumor Immunotherapy. J. Control. Release 2022, 344, 134–146. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Y.; Meng, M.; Li, Z.; Li, H.; Lin, L.; Xu, C.; Chen, J.; Hao, K.; Tang, Z.; et al. A Generally Minimalist Strategy of Constructing Biomineralized High-Efficiency Personalized Nanovaccine Combined with Immune Checkpoint Blockade for Cancer Immunotherapy. Biomaterials 2022, 289, 121794. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Xu, L.; Chao, Y.; Wang, C.; Han, X.; Dong, Z.; Chang, H.; Peng, R.; Cheng, Y.; et al. Nanovaccine Based on a Protein-Delivering Dendrimer for Effective Antigen Cross-Presentation and Cancer Immunotherapy. Biomaterials 2019, 207, 1–9. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, B.; Hao, J.; Zheng, Y.; Chai, J.; Zhang, Z.; Liu, Y.; Shi, L. Bi-Specific Macrophage Nano-Engager for Cancer Immunotherapy. Nano Today 2021, 41, 101313. [Google Scholar] [CrossRef]
- Gong, N.; Zhang, Y.; Teng, X.; Wang, Y.; Huo, S.; Qing, G.; Ni, Q.; Li, X.; Wang, J.; Ye, X.; et al. Proton-Driven Transformable Nanovaccine for Cancer Immunotherapy. Nat. Nanotechnol. 2020, 15, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Song, R.; Sun, F.; Saeed, M.; Guo, X.; Ye, J.; Chen, F.; Hou, B.; Zhu, Q.; Wang, Y.; et al. Bioinspired Magnetic Nanocomplexes Amplifying STING Activation of Tumor-Associated Macrophages to Potentiate Cancer Immunotherapy. Nano Today 2022, 43, 101400. [Google Scholar] [CrossRef]
- Adibfar, S.; Masjedi, A.; Nazer, A.; Rashidi, B.; Karpisheh, V.; Izadi, S.; Hassannia, H.; Gholizadeh Navashenaq, J.; Mohammadi, H.; Hojjat-Farsangi, M.; et al. Combined Inhibition of EZH2 and CD73 Molecules by Folic Acid-Conjugated SPION-TMC Nanocarriers Loaded with SiRNA Molecules Prevents TNBC Progression and Restores Anti-Tumor Responses. Life Sci. 2022, 309, 121008. [Google Scholar] [CrossRef] [PubMed]
- Shetab Boushehri, M.A.; Abdel-Mottaleb, M.M.A.; Béduneau, A.; Pellequer, Y.; Lamprecht, A. A Nanoparticle-Based Approach to Improve the Outcome of Cancer Active Immunotherapy with Lipopolysaccharides. Drug Deliv. 2018, 25, 1414–1425. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, K.; Wang, Z.; Wang, D.; Yin, X.; Liu, Y.; Yu, F.; Zhao, W. An Efficient and Safe MUC1-Dendritic Cell-Derived Exosome Conjugate Vaccine Elicits Potent Cellular and Humoral Immunity and Tumor Inhibition in Vivo. Acta Biomater. 2022, 138, 491–504. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, L.; Yu, Z.; Xu, Z.; Li, S.; Wang, Y.; Liu, H.; Zhao, B.; Liu, R.; Wang, W.; et al. Biomimetic Small-Molecule Self-Assembly of PI3K Inhibitor Integrated with Immunomodulator to Amplify Anticancer Efficacy. Chem. Eng. J. 2022, 433, 133747. [Google Scholar] [CrossRef]
- Zhang, Y.; Hughes, K.R.; Raghani, R.M.; Ma, J.; Orbach, S.; Jeruss, J.S.; Shea, L.D. Cargo-Free Immunomodulatory Nanoparticles Combined with Anti-PD-1 Antibody for Treating Metastatic Breast Cancer. Biomaterials 2021, 269, 120666. [Google Scholar] [CrossRef]
- Wilson, D.R.; Sen, R.; Sunshine, J.C.; Pardoll, D.M.; Green, J.J.; Kim, Y.J. Biodegradable STING Agonist Nanoparticles for Enhanced Cancer Immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 237–246. [Google Scholar] [CrossRef]
- Xiang, J.; Xu, L.; Gong, H.; Zhu, W.; Wang, C.; Xu, J.; Feng, L.; Cheng, L.; Peng, R.; Liu, Z. Antigen-Loaded Upconversion Nanoparticles for Dendritic Cell Stimulation, Tracking, and Vaccination in Dendritic Cell-Based Immunotherapy. ACS Nano 2015, 9, 6401–6411. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Lyu, J.; Xiang, Y.; Chen, L.; Zhou, Z.; Huang, Y. Split Bullets Loaded Nanoparticles for Amplified Immunotherapy. J. Control. Release 2022, 347, 199–210. [Google Scholar] [CrossRef]
- Yuba, E.; Kono, Y.; Harada, A.; Yokoyama, S.; Arai, M.; Kubo, K.; Kono, K. The Application of PH-Sensitive Polymer-Lipids to Antigen Delivery Forcancer Immunotherapy. Biomaterials 2013, 34, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Choi, H.; Kim, H.; Choi, A.; Kwon, S.W.; Mouli, S.K.; Lewandowski, R.J.; Kim, D.H. Z-Domain Protein Nano-Bio Interfaced MRI Visible Anti-Program Death Ligand-1 Nanoconjugates for Enhanced Local Immune Checkpoint Inhibitor Immunotherapy. Nano Today 2022, 45, 101552. [Google Scholar] [CrossRef]
- Ma, Z.; Wong, S.W.; Forgham, H.; Esser, L.; Lai, M.; Leiske, M.N.; Kempe, K.; Sharbeen, G.; Youkhana, J.; Mansfeld, F.; et al. Aerosol Delivery of Star Polymer-SiRNA Nanoparticles as a Therapeutic Strategy to Inhibit Lung Tumor Growth. Biomaterials 2022, 285, 121539. [Google Scholar] [CrossRef] [PubMed]
- Mennati, A.; Rostamizadeh, K.; Manjili, H.K.; Fathi, M.; Danafar, H. Co-Delivery of SiRNA and Lycopene Encapsulated Hybrid Lipid Nanoparticles for Dual Silencing of Insulin-like Growth Factor 1 Receptor in MCF-7 Breast Cancer Cell Line. Int. J. Biol. Macromol. 2022, 200, 335–349. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, W.; Xiang, J.; Li, D.; Chen, Y.; Yuan, P.; Shao, S.; Zhou, Z.; Shen, Y.; Tang, J. A ROS-Responsive Synergistic Delivery System for Combined Immunotherapy and Chemotherapy. Mater. Today Bio 2022, 14, 100284. [Google Scholar] [CrossRef]
- Zheng, D.; Zhou, J.; Qian, L.; Liu, X.J.; Chang, C.; Tang, S.; Zhang, H.; Zhou, S. Biomimetic Nanoparticles Drive the Mechanism Understanding of Shear-Wave Elasticity Stiffness in Triple Negative Breast Cancers to Predict Clinical Treatment. Bioact. Mater. 2023, 22, 567–587. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.; Liang, W.; Wu, S.; Wang, Z.; Wang, D. Codelivery of Shikonin and SiTGF-$β$ for Enhanced Triple Negative Breast Cancer Chemo-Immunotherapy. J. Control. Release 2022, 342, 308–320. [Google Scholar] [CrossRef]
- Huang, H.; Huang, Y.; Chen, Y.; Luo, Z.; Zhang, Z.; Sun, R.; Wan, Z.; Sun, J.; Lu, B.; Zhang, L.; et al. A Novel Immunochemotherapy Based on Targeting of Cyclooxygenase and Induction of Immunogenic Cell Death. Biomaterials 2021, 270, 120708. [Google Scholar] [CrossRef]
- Jeon, I.S.; Yoo, J.D.; Gurung, S.; Kim, M.; Lee, C.; Park, E.J.; Park, R.-W.; Lee, B.; Kim, S. Anticancer Nanocage Platforms for Combined Immunotherapy Designed to Harness Immune Checkpoints and Deliver Anticancer Drugs. Biomaterials 2021, 270, 120685. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, Y.; Liu, Q.; Xu, X.; Peng, J. Carrier-Free Small-Molecule Drug Nanoassembly Elicits Chemoimmunotherapy via Co-Inhibition of PD-L1/MTOR. ACS Appl. Bio Mater. 2020, 3, 4543–4555. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Z.; Sun, D.; Zou, Y.; Liu, Y.; Huang, L. Two Nanoformulations Induce Reactive Oxygen Species and Immunogenetic Cell Death for Synergistic Chemo-Immunotherapy Eradicating Colorectal Cancer and Hepatocellular Carcinoma. Mol. Cancer 2021, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, M.; Liu, M.; An, S.; Guan, K.; Wang, M.; Li, L.; Zhang, J.; Li, J.; Huang, L. Nano-Puerarin Regulates Tumor Microenvironment and Facilitates Chemo- and Immunotherapy in Murine Triple Negative Breast Cancer Model. Biomaterials 2020, 235, 119769. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, H.; Tan, N. Cancer-Cell-Biomimetic Nanoparticles Systemically Eliminate Hypoxia Tumors by Synergistic Chemotherapy and Checkpoint Blockade Immunotherapy. Acta Pharm. Sin. B 2022, 12, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, C.; Xiang, Y.; Lyu, J.; Zhou, Z.; Gong, T.; Gao, H.; Li, L.; Huang, Y. Exocytosis Blockade of Endoplasmic Reticulum-Targeted Nanoparticle Enhances Immunotherapy. Nano Today 2022, 42, 101356. [Google Scholar] [CrossRef]
- Pan, P.; Dong, X.; Chen, Y.; Ye, J.J.; Sun, Y.X.; Zhang, X.Z. A Heterogenic Membrane-Based Biomimetic Hybrid Nanoplatform for Combining Radiotherapy and Immunotherapy against Breast Cancer. Biomaterials 2022, 289, 121810. [Google Scholar] [CrossRef]
- Castro, F.; Pinto, M.L.; Pereira, C.L.; Serre, K.; Barbosa, M.A.; Vermaelen, K.; Gärtner, F.; Gonçalves, R.M.; De Wever, O.; Oliveira, M.J. Chitosan/$γ$-PGA Nanoparticles-Based Immunotherapy as Adjuvant to Radiotherapy in Breast Cancer. Biomaterials 2020, 257, 120218. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, B.; Wang, H.; Wang, D.; Li, Y. A Bispecific Nanomodulator to Potentiate Photothermal Cancer Immunotherapy. Nano Today 2022, 44, 101466. [Google Scholar] [CrossRef]
- Shukla, A.; Cano-Mejia, J.; Andricovich, J.; Burga, R.A.; Sweeney, E.E.; Fernandes, R. An Engineered Prussian Blue Nanoparticles-Based Nanoimmunotherapy Elicits Robust and Persistent Immunological Memory in a TH-MYCN Neuroblastoma Model. Adv. NanoBiomed Res. 2021, 1, 2100021. [Google Scholar] [CrossRef]
- Seth, A.; Gholami Derami, H.; Gupta, P.; Wang, Z.; Rathi, P.; Gupta, R.; Cao, T.; Morrissey, J.J.; Singamaneni, S. Polydopamine-Mesoporous Silica Core-Shell Nanoparticles for Combined Photothermal Immunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 42499–42510. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, G.; Wen, X.; Li, F.; Ji, X.; Li, Q.; Wu, M.; Cheng, Q.; Yu, Y.; Tang, J.; et al. Magnetic Nanoparticles Coated with Polyphenols for Spatio-Temporally Controlled Cancer Photothermal/Immunotherapy. J. Control. Release 2020, 326, 131–139. [Google Scholar] [CrossRef]
- Xie, W.; Chen, B.; Wen, H.; Xiao, P.; Wang, L.; Liu, W.; Wang, D.; Tang, B.Z. Biomimetic Nanoplatform Loading Type I Aggregation-Induced Emission Photosensitizer and Glutamine Blockade to Regulate Nutrient Partitioning for Enhancing Antitumor Immunotherapy. ACS Nano 2022, 16, 10742–10753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, Y.; Cao, K.; Du, Y.; Han, M.; Shi, Z.; Yan, F.; Feng, S. Hepcidin-Based Nanocomposites for Enhanced Cancer Immunotherapy by Modulating Iron Export-Mediated N6-Methyladenosine RNA Transcript. Adv. Funct. Mater. 2022, 32, 2107195. [Google Scholar] [CrossRef]
- Pakravan, A.; Azizi, M.; Rahimi, F.; Bani, F.; Mahmoudzadeh, F.; Salehi, R.; Mahkam, M. Comparative Effect of Thermo/PH-Responsive Polymer-Coated Gold Nanocages and Hollow Nanostars on Chemo-Photothermal Therapy of Breast Cancer Cells. Cancer Nanotechnol. 2021, 12, 19. [Google Scholar] [CrossRef]
- Sun, Y.; Han, R.; Wang, J.; Qin, Y.; Ren, Z.; Feng, X.; Liu, Q.; Wang, X. A Single-Beam of Light Priming the Immune Responses and Boosting Cancer Photoimmunotherapy. J. Control. Release 2022, 350, 734–747. [Google Scholar] [CrossRef]
- Gupta, J.; Safdari, H.A.; Hoque, M. Nanoparticle Mediated Cancer Immunotherapy. Semin. Cancer Biol. 2021, 69, 307–324. [Google Scholar] [CrossRef]
- Zang, X.; Zhao, X.; Hu, H.; Qiao, M.; Deng, Y.; Chen, D. Nanoparticles for Tumor Immunotherapy. Eur. J. Pharm. Biopharm. 2017, 115, 243–256. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Xu, Z.P. Nanoparticle-Based Nanomedicines to Promote Cancer Immunotherapy: Recent Advances and Future Directions. Small 2019, 15, 1900262. [Google Scholar] [CrossRef]
- Gupta, B.; Kim, J.O. Recent Progress in Cancer Immunotherapy Approaches Based on Nanoparticle Delivery Devices. J. Pharm. Investig. 2021, 51, 399–412. [Google Scholar] [CrossRef]
- Becicka, W.M.; Bielecki, P.A.; Lorkowski, M.E.; Moon, T.J.; Zhang, Y.; Atukorale, P.U.; Covarrubias, G.; Karathanasis, E. The Effect of PEGylation on the Efficacy and Uptake of an Immunostimulatory Nanoparticle in the Tumor Immune Microenvironment. Nanoscale Adv. 2021, 3, 4961–4972. [Google Scholar] [CrossRef]
- An, J.; Forchheimer, D.; Sävmarker, J.; Brülls, M.; Frenning, G. Nanoscale Characterization of PEGylated Phospholipid Coatings Formed by Spray Drying on Silica Microparticles. J. Colloid Interface Sci. 2020, 577, 92–100. [Google Scholar] [CrossRef]
- Lôbo, G.C.N.B.; Paiva, K.L.R.; Silva, A.L.G.; Simões, M.M.; Báo, S.N.; Radicchi, M.A. Nanocarriers Used in Drug Delivery to Enhance Immune System in Cancer Therapy. Pharmaceutics 2021, 13, 1167. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Shahbazi, M.A.; D’Amico, C.; Fontana, F.; Abbaszadeh, S.; Santos, H.A. Microneedles for Painless Transdermal Immunotherapeutic Applications. J. Control. Release 2021, 330, 185–217. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Seong, K.Y.; Han, H.H.; Yang, S.Y.; Hahn, S.K. Dissolving Microneedles Delivering Cancer Cell Membrane Coated Nanoparticles for Cancer Immunotherapy. RSC Adv. 2021, 11, 10393–10399. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seong, K.Y.; Lee, J.H.; Park, W.; Yang, S.Y.; Hahn, S.K. Biodegradable Microneedle Patch Delivering Antigenic Peptide-Hyaluronate Conjugate for Cancer Immunotherapy. ACS Biomater. Sci. Eng. 2019, 5, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Nguyen, T.L.; Giang Phan, V.H.; Lee, M.S.; Lee, J.E.; Kim, J.; Jeong, J.H.; Lee, D.S. Smart Vaccine Delivery Based on Microneedle Arrays Decorated with Ultra-PH-Responsive Copolymers for Cancer Immunotherapy. Biomaterials 2018, 185, 13–24. [Google Scholar] [CrossRef]
- Lan, X.; Zhu, W.; Huang, X.; Yu, Y.; Xiao, H.; Jin, L.; Pu, J.J.; Xie, X.; She, J.; Lui, V.W.Y.; et al. Microneedles Loaded with Anti-PD-1-Cisplatin Nanoparticles for Synergistic Cancer Immuno-Chemotherapy. Nanoscale 2020, 12, 18885–18898. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch -Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, C.; Zhang, Q.; Cheng, K.; Shan, W.; Wang, X.; Yang, J.; Wang, Y.; Ren, L. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of HBc VLPs Based Cancer Vaccine. Appl. Mater. Today 2021, 24, 101110. [Google Scholar] [CrossRef]
- Chen, S.X.; Ma, M.; Xue, F.; Shen, S.; Chen, Q.; Kuang, Y.; Liang, K.; Wang, X.; Chen, H. Construction of Microneedle-Assisted Co-Delivery Platform and Its Combining Photodynamic/Immunotherapy. J. Control. Release 2020, 324, 218–227. [Google Scholar] [CrossRef]
- Yang, T.; Huang, D.; Li, C.; Zhao, D.; Li, J.; Zhang, M.; Chen, Y.; Wang, Q.; Liang, Z.; Liang, X.J.; et al. Rolling Microneedle Electrode Array (RoMEA) Empowered Nucleic Acid Delivery and Cancer Immunotherapy. Nano Today 2020, 36, 101017. [Google Scholar] [CrossRef]
- Panigaj, M.; Dobrovolskaia, M.A.; Afonin, K.A. 2021: An Immunotherapy Odyssey and the Rise of Nucleic Acid Nanotechnology. Nanomedicine 2021, 16, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Yata, T.; Takahashi, Y.; Tan, M.; Nakatsuji, H.; Ohtsuki, S.; Murakami, T.; Imahori, H.; Umeki, Y.; Shiomi, T.; Takakura, Y.; et al. DNA Nanotechnology-Based Composite-Type Gold Nanoparticle-Immunostimulatory DNA Hydrogel for Tumor Photothermal Immunotherapy. Biomaterials 2017, 146, 136–145. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Liu, Y.; Chen, J.; Sun, Y.; Pan, X.; Xu, J.; Xu, S.; Liu, Z.; Tan, W. DNA-Based MXFs to Enhance Radiotherapy and Stimulate Robust Antitumor Immune Responses. Nano Lett. 2022, 22, 2826–2834. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Y.; Chao, Y.; Xiao, Z.; Xu, J.; Wang, C.; Dong, Z.; Hou, L.; Li, Q.; Liu, Z. Equipping Cancer Cell Membrane Vesicles with Functional DNA as a Targeted Vaccine for Cancer Immunotherapy. Nano Lett. 2021, 21, 9410–9418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, Y.; Wang, D.; Liu, J.; An, J.; Li, Y.; Ma, C.; Pei, Y.; Zhang, Z.; Liu, J.; et al. In Vivo Activation of T-Cell Proliferation by Regulating Cell Surface Receptor Clustering Using a PH-Driven Interlocked DNA Nano-Spring. Nano Lett. 2022, 22, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
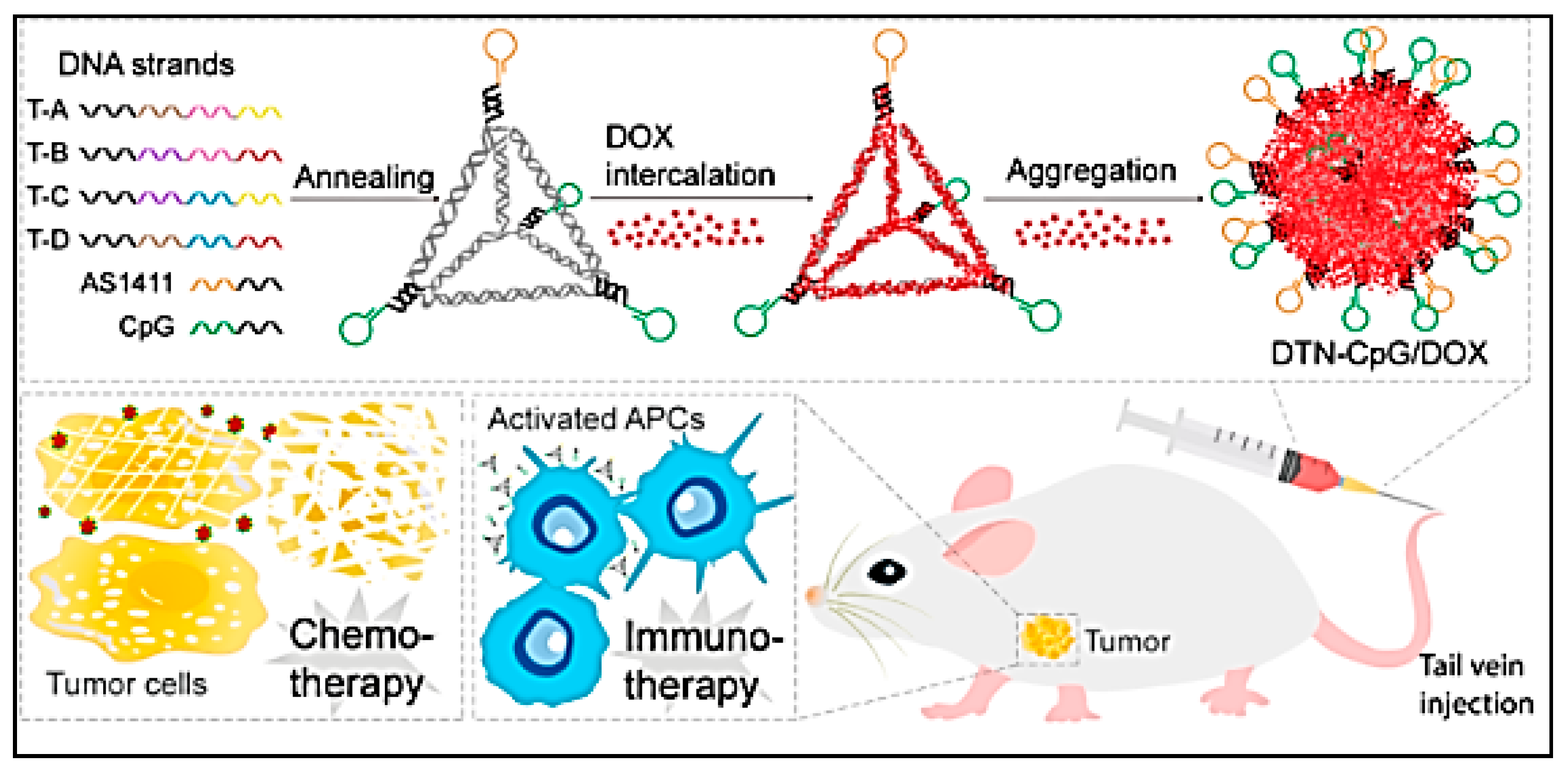
- Wang, Q.; Ma, Y.; Lu, Z.; Yu, H.; Li, Z. Co-Delivery of Chemotherapeutic Drugs and Immune Adjuvants by Nanoscale DNA Tetrahedrons for Synergistic Cancer Therapy. ACS Appl. Nano Mater. 2022, 5, 101–106. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, K.; Gao, M.; Wang, Z.; Gu, Y.; Ma, Y. Transformable DNA Octahedron for Remodeling Tumor Immune Microenvironment with Alleviated Toxicity. Chem. Eng. J. 2022, 440, 135813. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Xiong, Z.; Yang, H.; Dong, Z.; Xiang, J.; Gu, Q.; Ji, Q.; Fan, C.; Liu, Z. DNA Engineered Lymphocyte-Based Homologous Targeting Artificial Antigen-Presenting Cells for Personalized Cancer Immunotherapy. J. Am. Chem. Soc. 2022, 144, 7634–7645. [Google Scholar] [CrossRef]
- Farheen, J.; Hosmane, N.S.; Zhao, R.; Zhao, Q.; Iqbal, M.Z.; Kong, X. Nanomaterial-Assisted CRISPR Gene-Engineering–A Hallmark for Triple-Negative Breast Cancer Therapeutics Advancement. Mater. Today Bio 2022, 16, 100450. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, C.; An, C.; Zheng, X.; Wen, S.; Chen, W.; Liu, X.; Lv, Z.; Yang, P.; Xu, W.; et al. Application of the CRISPR/Cas9-Based Gene Editing Technique in Basic Research, Diagnosis, and Therapy of Cancer. Mol. Cancer 2021, 20, 126. [Google Scholar] [CrossRef]
- Deng, H.; Tan, S.; Gao, X.; Zou, C.; Xu, C.; Tu, K.; Song, Q.; Fan, F.; Huang, W.; Zhang, Z. Cdk5 Knocking out Mediated by CRISPR-Cas9 Genome Editing for PD-L1 Attenuation and Enhanced Antitumor Immunity. Acta Pharm. Sin. B 2020, 10, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, Y.K.; Oh, E.; Jeong, J.; Im, S.H.; Kim, D.K.; Lee, H.; Kim, S.G.; Jung, K.; Chung, H.J. Nano-Assembly of a Chemically Tailored Cas9 Ribonucleoprotein for in Vivo Gene Editing and Cancer Immunotherapy. Chem. Mater. 2022, 34, 547–561. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Zheng, Y.; Zhao, Y.; Wang, Y.; Hao, J.; Zhao, X.; Yi, K.; Shi, L.; Kang, C.; et al. Virus-like Nanoparticle as a Co-Delivery System to Enhance Efficacy of CRISPR/Cas9-Based Cancer Immunotherapy. Biomaterials 2020, 258, 120275. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.; Shen, M.; Wang, Y.; Wang, L.; Li, J.; Yang, W.; Li, J.; Li, H.; Wang, X.; et al. Programmable Unlocking Nano-Matryoshka-CRISPR Precisely Reverses Immunosuppression to Unleash Cascade Amplified Adaptive Immune Response. Adv. Sci. 2021, 8, 2100292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, D.; Zhang, Y.; Huang, Q.; Zhang, Z.; Chen, C.; Xu, C.F.; Chu, X.; Zhang, Y.; Yang, X. HSP70-Promoter-Driven CRISPR/Cas9 System Activated by Reactive Oxygen Species for Multifaceted Anticancer Immune Response and Potentiated Immunotherapy. ACS Nano 2022, 16, 13821–13833. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fu, Y.; Huang, C.; Hu, D.; Zhou, K.; Hao, Y.; Chu, B.; Yang, Y.; Qian, Z. Chlorin E6 and CRISPR-Cas9 Dual-Loading System with Deep Penetration for a Synergistic Tumoral Photodynamic-Immunotherapy. Biomaterials 2020, 255, 120194. [Google Scholar] [CrossRef]
- Lin, M.; Yang, Z.; Yang, Y.; Peng, Y.; Li, J.; Du, Y.; Sun, Q.; Gao, D.; Yuan, Q.; Zhou, Y.; et al. CRISPR-Based In Situ Engineering Tumor Cells to Reprogram Macrophages for Effective Cancer Immunotherapy. Nano Today 2022, 42, 101359. [Google Scholar] [CrossRef]
- Ju, H.; Kim, D.; Oh, Y.-K. Lipid Nanoparticle-Mediated CRISPR/Cas9 Gene Editing and Metabolic Engineering for Anticancer Immunotherapy. Asian J. Pharm. Sci. 2022, 17, 641–652. [Google Scholar] [CrossRef]
- Tran, T.H.; Mattheolabakis, G.; Aldawsari, H.; Amiji, M. Exosomes as Nanocarriers for Immunotherapy of Cancer and Inflammatory Diseases. Clin. Immunol. 2015, 160, 46–58. [Google Scholar] [CrossRef]
- Pi, Y.N.; Xia, B.R.; Jin, M.Z.; Jin, W.L.; Lou, G. Exosomes: Powerful Weapon for Cancer Nano Immunoengineering. Biochem Pharm. 2021, 186, 114487. [Google Scholar] [CrossRef]
- Taghikhani, A.; Farzaneh, F.; Sharifzad, F.; Mardpour, S.; Ebrahimi, M.; Hassan, Z.M. Engineered Tumor-Derived Extracellular Vesicles: Potentials in Cancer Immunotherapy. Front. Immunol. 2020, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; McGill, J.; Gamero-Kubota, P.; He, M. Microfluidic On-Demand Engineering of Exosomes towards Cancer Immunotherapy. Lab Chip 2019, 19, 1877–1886. [Google Scholar] [CrossRef]
- Khosravi, N.; Pishavar, E.; Baradaran, B.; Oroojalian, F.; Mokhtarzadeh, A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: A promising biomimetic nanoplatforms for cancer theranostics. J. Control. Release 2022, 348, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, H.; Zhang, W.; Li, Z.; Gao, J. Engineered tumor cell-derived vaccines against cancer: The art of combating poison with poison. Bioact. Mater. 2023, 22, 491–517. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Zhou, Y.; Kramer, A.; Yang, L. Engineering Stem Cells for Cancer Immunotherapy. Trends Cancer 2021, 7, 1059–1073. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, J.Y.; Han, J.; Hwang, H.S.; Lee, J.; Na, K. Local Immune-Triggered Surface-Modified Stem Cells for Solid Tumor Immunotherapy. Adv. Funct. Mater. 2019, 29, 1900773. [Google Scholar] [CrossRef]
- Zou, W.; Zheng, H.; He, T.C.; Chang, J.; Fu, Y.X.; Fan, W. LIGHT Delivery to Tumors by Mesenchymal Stem Cells Mobilizes an Effective Antitumor Immune Response. Cancer Res. 2012, 72, 2980–2989. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Sweeney, E.E. Nanoparticles for Enhanced Adoptive T Cell Therapies and Future Perspectives for CNS Tumors. Front. Immunol. 2021, 12, 600659. [Google Scholar] [CrossRef]
- Mi, Y.; Hagan, C.T.; Vincent, B.G.; Wang, A.Z. Emerging Nano-/Microapproaches for Cancer Immunotherapy. Adv. Sci. 2019, 6, 1801847. [Google Scholar] [CrossRef]
- Billingsley, M.M.; Singh, N.; Ravikumar, P.; Zhang, R.; June, C.H.; Mitchell, M.J. Ionizable Lipid Nanoparticle-Mediated MRNA Delivery for Human CAR T Cell Engineering. Nano Lett. 2020, 20, 1578–1589. [Google Scholar] [CrossRef]
- Lee, J.M. When CAR Meets Stem Cells. Int. J. Mol. Sci. 2019, 20, 1825. [Google Scholar] [CrossRef] [PubMed]
- Kiru, L.; Zlitni, A.; Tousley, A.M.; Dalton, G.N.; Wu, W.; Lafortune, F.; Liu, A.; Cunanan, K.M.; Nejadnik, H.; Sulchek, T.; et al. In Vivo Imaging of Nanoparticle-Labeled CAR T Cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2102363119. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Rubart, M.; Zhu, W. Optogenetics: Background, Methodological Advances and Potential Applications for Cardiovascular Research and Medicine. Front. Bioeng. Biotechnol. 2020, 7, 466. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Tan, X.; Zheng, X.; Wang, F.; Ke, K.; Zhang, C.; Liao, N.; Dang, Y.; Shi, Y.; et al. An Optogenetic Controllable T Cell System for Hepatocellular Carcinoma Immunotherapy. Theranostics 2019, 9, 1837–1850. [Google Scholar] [CrossRef]
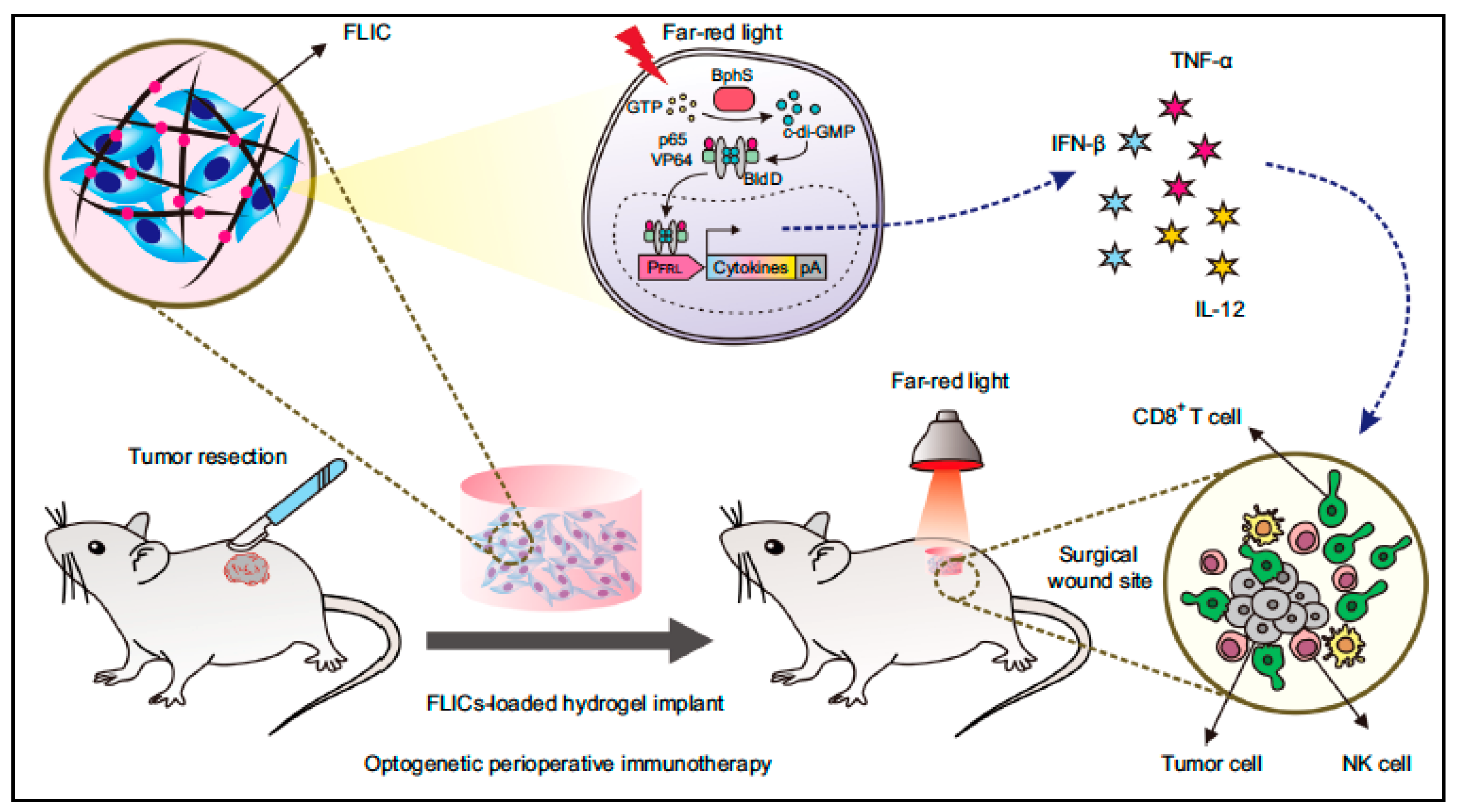
- Yu, Y.; Wu, X.; Wang, M.; Liu, W.; Zhang, L.; Zhang, Y.; Hu, Z.; Zhou, X.; Jiang, W.; Zou, Q.; et al. Optogenetic-controlled immunotherapeutic designer cells for post-surgical cancer immunotherapy. Nat. Commun. 2022, 13, 6357. [Google Scholar] [CrossRef]
- Huang, K.; Liu, X.; Han, G.; Zhou, Y. Nano-optogenetic immunotherapy. Clin. Transl. Med. 2022, 12, e1020. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Huang, K.; Zeng, H.; Jing, J.; Wang, R.; Fang, S.; Chen, J.; Liu, X.; Huang, Z.; You, M.J.; et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nat. Nanotechnol. 2021, 16, 1424–1434. [Google Scholar] [CrossRef]
- Shoeb, E.; Hefferon, K. Future of cancer immunotherapy using plant virus-based nanoparticles. Futur. Sci. OA 2019, 5, FSO401. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, B.; Huang, M.; Li, X.; Wang, Z.; Chang, J.; Wang, T. Sendai Virus Acts as a Nano-Booster to Excite Dendritic Cells for Enhancing the Efficacy of CD47-Directed Immune Checkpoint Inhibitors against Breast Carcinoma. Mater. Chem. Front. 2021, 5, 223–237. [Google Scholar] [CrossRef]
- Lebel, M.È.; Chartrand, K.; Tarrab, E.; Savard, P.; Leclerc, D.; Lamarre, A. Potentiating Cancer Immunotherapy Using Papaya Mosaic Virus-Derived Nanoparticles. Nano Lett. 2016, 16, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Wang, C.; Beiss, V.; Steinmetz, N.F. Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer. ACS Nano 2020, 14, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Beiss, V.; Wang, C.; Wang, L.; Steinmetz, N.F. Plant Viral Nanoparticle Conjugated with Anti-PD-1 Peptide for Ovarian Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 17233. [Google Scholar] [CrossRef]
- Boone, C.E.; Wang, C.; Lopez-Ramirez, M.A.; Beiss, V.; Shukla, S.; Chariou, P.L.; Kupor, D.; Rueda, R.; Wang, J.; Steinmetz, N.F. Active Microneedle Administration of Plant Virus Nanoparticles for Cancer in Situ Vaccination Improves Immunotherapeutic Efficacy. ACS Appl. Nano Mater. 2020, 3, 8037–8051. [Google Scholar] [CrossRef] [PubMed]
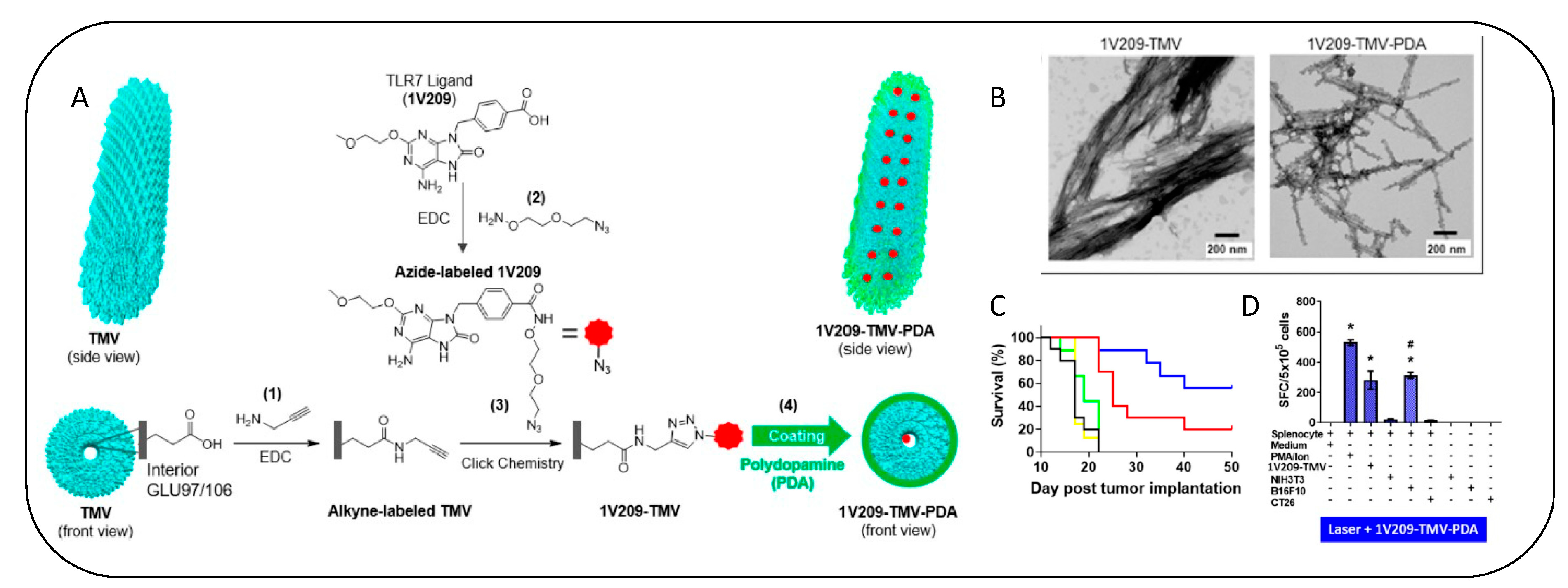
- Nkanga, C.I.; Ortega-Rivera, O.A.; Steinmetz, N.F. Photothermal Immunotherapy of Melanoma Using TLR-7 Agonist Laden Tobacco Mosaic Virus with Polydopamine Coat. Nanomed. Nanotechnol. Biol. Med. 2022, 44, 102573. [Google Scholar] [CrossRef]
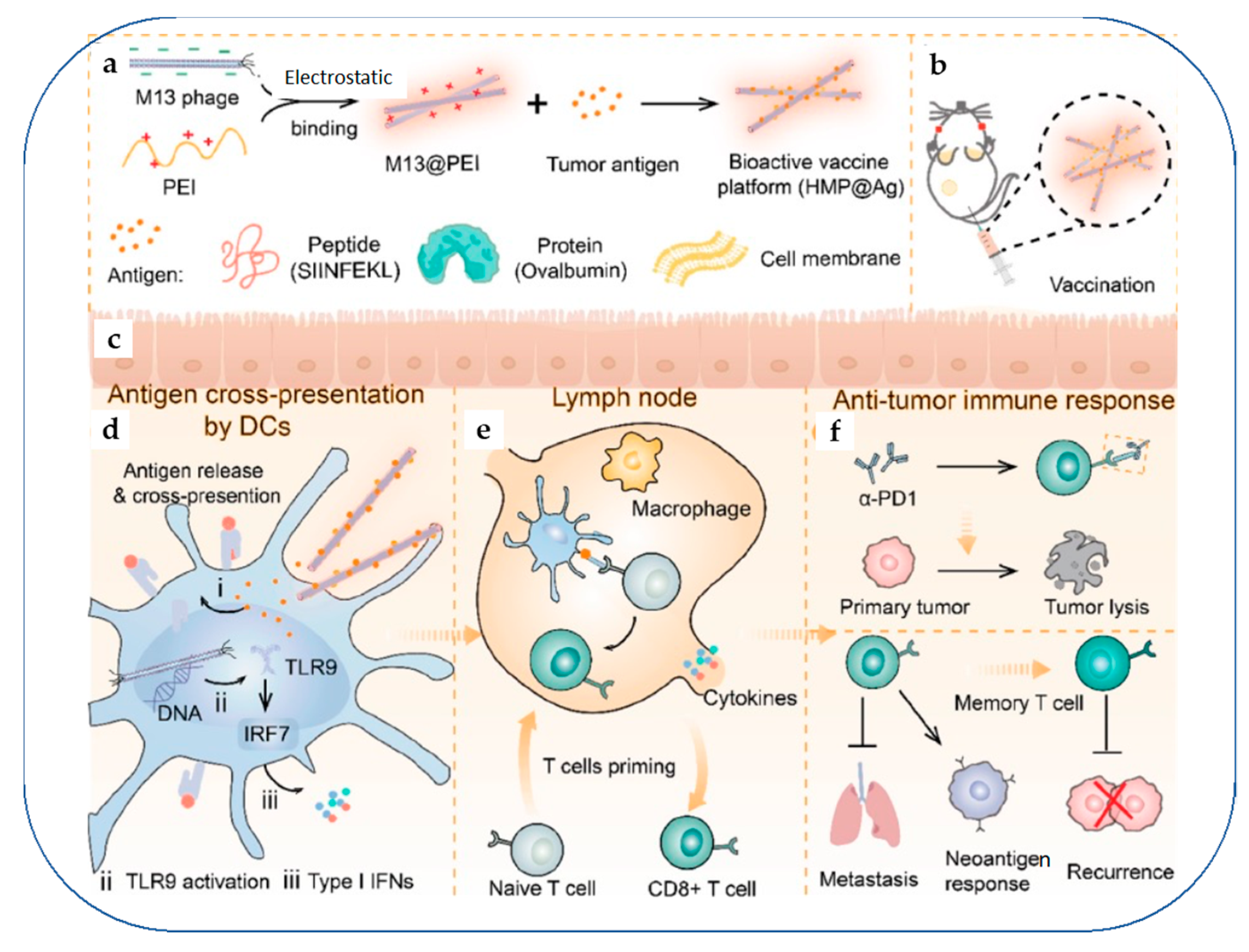
- Dong, X.; Pan, P.; Ye, J.J.; Zhang, Q.L.; Zhang, X.Z. Hybrid M13 Bacteriophage-Based Vaccine Platform for Personalized Cancer Immunotherapy. Biomaterials 2022, 289, 121763. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic Virus Therapy: A New Era of Cancer Treatment at Dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Ban, W.; Guan, J.; Huang, H.; He, Z.; Sun, M.; Liu, F.; Sun, J. Emerging Systemic Delivery Strategies of Oncolytic Viruses: A Key Step toward Cancer Immunotherapy. Nano Res. 2022, 15, 4137–4153. [Google Scholar] [CrossRef]
- Fusciello, M.; Fontana, F.; Tähtinen, S.; Capasso, C.; Feola, S.; Martins, B.; Chiaro, J.; Peltonen, K.; Ylösmäki, L.; Ylösmäki, E.; et al. Artificially Cloaked Viral Nanovaccine for Cancer Immunotherapy. Nat. Commun. 2019, 10, 5747. [Google Scholar] [CrossRef]
- Huang, X.; Pan, J.; Xu, F.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Bacteria-Based Cancer Immunotherapy. Adv. Sci. 2021, 8, 2003572. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, M.; Fang, C.; Cheng, C.; Zhao, M.; Fang, W.; Chu, P.K.; Ping, Y.; Tang, G. Engineering Nanoparticle-Coated Bacteria as Oral DNA Vaccines for Cancer Immunotherapy. Nano Lett. 2015, 15, 2732–2739. [Google Scholar] [CrossRef]
- Pan, H.; Li, L.; Pang, G.; Han, C.; Liu, B.; Zhang, Y.; Shen, Y.; Sun, T.; Liu, J.; Chang, J.; et al. Engineered NIR Light-Responsive Bacteria as Anti-Tumor Agent for Targeted and Precise Cancer Therapy. Chem. Eng. J. 2021, 426, 130842. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, X.; Fang, M.; Pang, G.; Xing, Y.; Zhang, X.; Li, L.; Chen, Q.; Wang, Y.; Chang, J.; et al. Upconversion Optogenetic Engineered Bacteria System for Time-Resolved Imaging Diagnosis and Light-Controlled Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 46351–46361. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; D’Souza, A.; Huang, D.; Bhavsar, A.; Amiji, M. Bacterial Extracellular Vesicle Applications in Cancer Immunotherapy. Bioact. Mater. 2023, 22, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Bai, H.; Wu, W.; Huang, G.; Li, Y.; Wu, M.; Tang, G.; Ping, Y. Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention. Nano Lett. 2020, 20, 11–21. [Google Scholar] [CrossRef]
- Chen, M.H.; Liu, T.Y.; Chen, Y.C.; Chen, M.H. Combining Augmented Radiotherapy and Immunotherapy through a Nano-Gold and Bacterial Outer-Membrane Vesicle Complex for the Treatment of Glioblastoma. Nanomaterials 2021, 11, 1661. [Google Scholar] [CrossRef]
- Qin, J.; Yang, T.; Li, J.; Zhan, G.; Li, X.; Wei, Z.; Chen, Z.; Zheng, W.; Chen, H.; Yang, X.; et al. Bacterial Outer Membrane Vesicle-Templated Biomimetic Nanoparticles for Synergistic Photothermo-Immunotherapy. Nano Today 2022, 46, 101591. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.D.; Jiang, Z. The Application of Fungal Beta-Glucans for the Treatment of Colon Cancer. Anticancer. Agents Med. Chem. 2013, 13, 725–730. [Google Scholar] [CrossRef]
- Wold, C.W.; Christopoulos, P.F.; Arias, M.A.; Dzovor, D.E.; Øynebråten, I.; Corthay, A.; Inngjerdingen, K.T. Polysaccharides from the fungus Inonotus obliquus activate macrophages into a tumoricidal phenotype via interaction with TLR2, TLR4 and Dectin-1a. bioRxiv 2020. [Google Scholar] [CrossRef]
- Netea, M.G.; Mulder, W.J.M. TH17 cells boosted by nanoparticle-bound fungal motifs. Nat. Biomed. Eng. 2022, 7, 10–11. [Google Scholar] [CrossRef]
- Wang, M.Z.; He, X.; Yu, Z.; Wu, H.; Yang, T.H. A Nano Drug Delivery System Based on Angelica Sinensis Polysaccharide for Combination of Chemotherapy and Immunotherapy. Molecules 2020, 25, 3096. [Google Scholar] [CrossRef]
- Sun, K.; Wu, L.; Wang, S.; Deng, W. Antitumor effects of Chinese herbal medicine compounds and their nano-formulations on regulating the immune system microenvironment. Front Oncol. 2022, 12, 949332. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, H.; Chen, X.; Gao, J.; Duan, Y.; Wei, D.; Zhang, J.; Ge, K.; Liang, X.J.; Huang, Y.; et al. Nano-Herb Medicine and PDT Induced Synergistic Immunotherapy for Colon Cancer Treatment. Biomaterials 2021, 269, 120654. [Google Scholar] [CrossRef]
- Yu, L.; Jin, Y.; Song, M.; Zhao, Y.; Zhang, H. When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy. Pharmaceutics 2022, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, Z.; Sun, L.; Lin, Y.; Yang, Y.; Cui, X.; Wang, C. Herb Polysaccharide-Based Drug Delivery System: Fabrication, Properties, and Applications for Immunotherapy. Pharmaceutics 2022, 14, 1703. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wu, J. Three-Dimensional (3D) Scaffolds as Powerful Weapons for Tumor Immunotherapy. Bioact. Mater. 2022, 17, 300–319. [Google Scholar] [CrossRef]
- Hyun, J.; Kim, H.W. Leveraging Cellular Mechano-Responsiveness for Cancer Therapy. Trends Mol. Med. 2022, 28, 155–169. [Google Scholar] [CrossRef]
- Panagi, M.; Mpekris, F.; Chen, P.; Voutouri, C.; Nakagawa, Y.; Martin, J.D.; Hiroi, T.; Hashimoto, H.; Demetriou, P.; Pierides, C.; et al. Polymeric micelles effectively reprogram the tumor microenvironment to potentiate nano-immunotherapy in mouse breast cancer models. Nat. Commun. 2022, 13, 7165. [Google Scholar] [CrossRef]
- Mpekris, F.; Panagi, M.; Voutouri, C.; Martin, J.D.; Samuel, R.; Takahashi, S.; Gotohda, N.; Suzuki, T.; Papageorgis, P.; Demetriou, P.; et al. Normalizing the Microenvironment Overcomes Vessel Compression and Resistance to Nano-Immunotherapy in Breast Cancer Lung Metastasis. Adv. Sci. 2021, 8, 2001917. [Google Scholar] [CrossRef]
- Gao, S.; Yang, X.; Xu, J.; Qiu, N.; Zhai, G. Nanotechnology for Boosting Cancer Immunotherapy and Remodeling Tumor Microenvironment: The Horizons in Cancer Treatment. ACS Nano 2021, 15, 12567–12603. [Google Scholar] [CrossRef]
- Erfanian, N.; Derakhshani, A.; Nasseri, S.; Fereidouni, M.; Baradaran, B.; Jalili Tabrizi, N.; Brunetti, O.; Bernardini, R.; Silvestris, N.; Safarpour, H. Immunotherapy of Cancer in Single-Cell RNA Sequencing Era: A Precision Medicine Perspective. Biomed. Pharmacother. 2022, 146, 112558. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.; Chen, X.; You, Z.; Gao, F.; Liu, T.; Ren, J.; Liu, J.; Xiong, Z.; Liu, Y.; et al. Graphdiyne Oxide Nanosheets Reprogram Immunosuppressive Macrophage for Cancer Immunotherapy. Nano Today 2022, 45, 101543. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawakami, K.; Nomura, M.; Sato, Y.; Hyodo, M.; Hatakeyama, H.; Hayakawa, Y.; Harashima, H. Combined Nano Cancer Immunotherapy Based on Immune Status in a Tumor Microenvironment. J. Control. Release 2022, 345, 200–213. [Google Scholar] [CrossRef]
- Pérez del Río, E.; Santos, F.; Rodriguez Rodriguez, X.; Martínez-Miguel, M.; Roca-Pinilla, R.; Arís, A.; Garcia-Fruitós, E.; Veciana, J.; Spatz, J.P.; Ratera, I.; et al. CCL21-Loaded 3D Hydrogels for T Cell Expansion and Differentiation. Biomaterials 2020, 259, 120313. [Google Scholar] [CrossRef] [PubMed]
- Dunn, Z.S.; Mac, J.; Wang, P. T Cell Immunotherapy Enhanced by Designer Biomaterials. Biomaterials 2019, 217, 119265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Fei, Z.; Dai, H.; Fan, Q.; Yang, Q.; Chen, Y.; Wang, B.; Wang, C. 3D Printing Scaffold Vaccine for Antitumor Immunity. Adv. Mater. 2021, 33, 2106768. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; De, S. Nanoparticle Design Strategies for Effective Cancer Immunotherapy. J Biomed 2017, 43, 909–932. [Google Scholar] [CrossRef]
- Choi, R.C.Y.; Gao, Q.T.; Cheung, A.W.H.; Zhu, J.T.T.; Lau, F.T.C.; Li, J.; Li, W.Z.M.; Chu, G.K.Y.; Duan, R.; Cheung, J.K.H.; et al. A Chinese Herbal Decoction, Danggui Buxue Tang, Stimulates Proliferation, Differentiation and Gene Expression of Cultured Osteosarcoma Cells: Genomic Approach to Reveal Specific Gene Activation. Evid.-Based Complement. Altern. Med. 2011, 2011, 307548. [Google Scholar] [CrossRef]
- Liu, S.; Deng, B.; Yin, Z.; Lin, Y.; An, L.; Liu, D.; Pan, J.; Yu, X.; Chen, B.; Wu, T.; et al. Combination of CD19 and CD22 CAR-T Cell Therapy in Relapsed B-Cell Acute Lymphoblastic Leukemia after Allogeneic Transplantation. Am. J. Hematol. 2021, 96, 671–679. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Calugaru, V.; Takacsi-Nagy, Z.; Liem, X.; Papai, Z.; Fijuth, J.; Moreno, V.; Giralt, J.; Salas, S.; Poissonnet, G.; et al. Phase I study of functionalized hafnium oxide nanoparticles (NBTXR3) activated by radiotherapy in cisplatin-ineligible locally advanced HNSCC patients. J. Clin. Oncol. 2021, 39, 6051. [Google Scholar] [CrossRef]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I Study of MRX34, a Liposomal MiR-34a Mimic, Administered Twice Weekly in Patients with Advanced Solid Tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef]
- Burris, H.A., III; Patel, M.R.; Cho, D.C.; Clarke, J.M.; Gutierrez, M.; Zaks, T.Z.; Frederick, J.; Hopson, K.; Mody, K.; Binanti-Berube, A.; et al. A phase 1, open-label, multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in subjects with resected solid tumors and in combination with pembrolizumab in subjects with unresectable solid tumors (Keynote-603). J. Glob. Oncol. 2019, 5, 93. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]


| Type of Therapy | Nanoparticle | Drug/Reactive Component | Cancer Type | Effect | References |
|---|---|---|---|---|---|
| Cancer Vaccine | Hydrogel | CaCO3 | TNBC | DC maturation and T-cell activation | [30] |
| OVA-EPC-Span85 complex | OVA | Mouse lymphoma | Activates both cellular and humoral immunity | [31] | |
| Hydrogel-encapsulated GM-CSF, CpG-ODN | GM-CSF, CpG-ODN, a TLR 9 agonist, and tumour cell lysates | Mouse colon carcinoma and Melanoma | Dendritic cell maturation and Immune system activation | [32] | |
| CaP-peptide vaccine | Calcium phosphate (CaP) and Peptides | Colon cancer and Breast cancer | Dendritic cell maturation | [20] | |
| CS/γ-PGA nanoparticle | MUC1 glycopeptide antigens | Breast cancer | Produce significantly high titers of IgG antibody | [33] | |
| A novel polyethyleneimine (PEI)-based personalized vaccine—NP vaccination combined with STING agonist therapy | Neoantigen peptides and CpG adjuvants in a compact nanoparticle | Colon carcinoma and melanoma | Tumour infiltration of CD8+ T cells | [27] | |
| CTX-loaded hydrogel and PLEL hydrogel | CpG and tumour lysates | Colon carcinoma | Produces the cytotoxic T lymphocyte and Immunogenic cell death | [34] | |
| Fe3O4 nanocomposite | OVA | Melanoma | Efficiently stimulate dendritic cell-based immunotherapy and potentially-activate macrophages | [35] | |
| CaCO3 Nanoparticle | CaCO3@(OVA/HPAA-CpG)3 vaccines | Lymphoma | Dendritic cell maturation and CD8+ T-cell proliferation | [36] | |
| A PEG derivative (PpASE) stabilized aluminium nanoparticle for delivering the synthetic long peptides (ANLs) | ANLs ANSs | Melanoma | Activation and proliferation of CD8+ T cells | [37] | |
| Mn-NP (Carrier and adjuvant) | OVA (Model Antigen), CpG (Adjuvant), Anti-PDL1 | Melanoma | Activation of the cGAS-STING pathway. Nanovaccine (NV) or Personalized NV (s.c.) Anti-PD-L1 (i.v.) | [38] | |
| DGBA-OVA-CpG nanovaccine | unmethylated cytosine-guanine dinucleotides (CpG) (adjuvant) | Melanoma | Controlled tumour growth along with anti-PD1 checkpoint inhibition | [39] | |
| Bi-specific macrophage nano-engager (BiME) | Serum albumin and targeted moiety | Melanoma | Robust T-cell activation | [40] | |
| Nanotransformer- based vaccine with anti-PD-L1 antibodies | A polymer–peptide conjugate-based nanotransformer and loaded antigenic pep | Melanoma | Activates the NLRP3- inflammasome pathway and thus boosts antitumour immunity and stimulation of CD8+ T cells | [41] | |
| Immunotherapy | Tumour exosomes (TEX) | HSP70, HSP90, MHC I, MHC II, TGF-β, and PD-L1 | TNBC | Dendritic cell activation, Cytotoxic T-cell-mediated immune response | [24] |
| Magnetic nanocomplexes (Iron oxide) | - | TNBC | STING activation and Macrophage polarization | [42] | |
| Folic acid conjugated superparamagnetic iron oxide, Trimethyl chitosan (TMC) nanoparticles | EZH2/CD73 siRNA | TNBC | Gene silencing | [43] | |
| LPS-decorated PLGA nanoparticles | LPS | Murine colon adeno-carcinoma and glioma | Activation of TLR4 Macrophage and DCs Proliferation | [44] | |
| MUC1-Dex | - | Melanoma | Activation of CD8+ T cells | [45] | |
| ZNPs/I@CML | Indomethacin | Prostate cancer | ZSTK is an effective pan-PI3K inhibitor, Macrophage polarization | [46] | |
| Cargo-free PLG nanoparticles | Anti-PD-L1 antibody | TNBC | Decrease the expression of MCP-1 by 5-fold and increase the expression of TNF-α by more than 2-fold upon uptake by innate immune cells | [47] | |
| Poly (beta-amino ester) (PBAE) nanoparticle | Cyclic dinucleotides (CDNs) | Melanoma | Stimulator of interferon receptor (STING) enhanced cancer immunotherapy | [48] | |
| UPP@OVA complex | Yb and Er-doped NaY/GdF4 UCNPs | Melanoma | Enhanced T-cell proliferation, interferon gamma production and cytotoxic T lymphocyte (CTL) mediated responses | [49] | |
| Split bullet nanoparticle | Doxorubicin and iRGD peptide | Melanoma | Suppress primary melanoma and initiate immune memory against tumour recurrence | [50] | |
| pH sensitive liposomes | Pyranine and antigenic protein Ovalbumin (OVA) | Lymphoma | Increased specific immunity and tumour regression occurred | [51] | |
| Immune checkpoint inhibitor (ICI) therapy | Z-domain conjugated ferumoxytol nanocarrier | Nanointerface (aPD-L1-Z-Fer) | Hepato-cellular carcinoma | Block the PD-1/PD-L1 (Programmed death ligand) | [52] |
| Immunogene therapy | Miktoarm star polymer (PDMAEMA-POEGMA) nanoparticles | βIII-tubulin, Polo-Like Kinase 1 (PLK1)—siRNA | NSCLC | Gene silencing | [53] |
| Methoxypoly (ethylene glycol)—Poly(caprolactone) was hybridized with Dimethyldioctadecyl-ammonium bromide (DDAB) cationic lipid (mPEG-PCL-DDAB) nanoparticles“mPEG-PCL-DDAB nanoparticle” | Anti-insulin-like growth factor 1 receptor-siRNA and lycopene | Breast cancer | Apoptosis and arrested cell cycle | [54] | |
| Chemoimmunotherapy | Pep-PAPM | Anti-PD-L1 peptide and Paclitaxel | TNBC | PD-L1 blockade and ROS-induced damage | [55] |
| 231MARS@PLGA | PD-L1 inhibitor and Paclitaxel | TNBC | Affect the tumour stiffness | [56] | |
| SK/siTGF-β NPs | Shikonin and siTGF-β | TNBC | Dendritic cell activation, Cytotoxic cell-mediated immune response | [57] | |
| PEG-b-PNHS polymer-conjugated 5-ASA (PASA) Folate-PEG-NH2-conjugated PASA (FASA) | 5-ASA and DOX | Mouse breast and colon cancer models | Anti-PD-L1 Activation. Macrophage activation and proliferation | [58] | |
| Ferritin nanocages | PD-L1pep1 and Doxorubicin | Human breast tumour and mouse colon tumour | Inhibited PD-1/PD-L1 interaction and restored T-cell activity | [59] | |
| Nano assembly | JQ1/Rapa-IR783 | TNBC | Co-inhibition of PD-L1/mTOR | [60] | |
| Doxorubicin/CpG self-assembled nanoparticles | Doxorubicin/CpG self-assembled nanoparticles, prodrug and dendritic cells (DC) co-encapsulated hydrogel system | Melanoma | Enhanced antigen presentation in DCs and CTL mediated tumour killing | [28] | |
| Nano-Folox (Nanoprecipitate of Folinic acid and Oxaliplatin) | Folinic acid (FnA), 5-fluorouracil (5-Fu), and oxaliplatin (OxP) | Colorectal cancer and hepatocellular carcinoma | Induce apoptosis and immunogenic cell death | [61] | |
| Nano-emulsion | Puerarin (nanoPue) and paclitaxel | TNBC | Deactivated tumour-associated fibroblast (TAFs) and 2-fold times increased the intra-tumoural infiltration of cytotoxic T cells | [62] | |
| Chemotherapy and immune checkpoint blockade therapy | BMS/RA@CC-Liposome | Chemotherapeutic drug (RA-V) and PD-1/PD-L1 blockade inhibitor (BMS-202) | Colorectal carcinoma | Dendritic cell maturation, Cytotoxic T-cell-mediated immune response | [63] |
| A metabolism nano-intervenor of DCs (Man-OVA(RSV) NPs) was loaded in a versatile hydrogel system | Metformin hydrochloride (MET), Rosuvastatin (RSV) | Melanoma | DC-mediated immunotherapy | [26] | |
| Exocytosis blockade of ER along with anti-PD-L1 therapy | Homologous cancer cell membrane coated nanoparticle (HCC@NP) | Brefeldin A (BFA) | Melanoma | Antitumour immunity and reversing immune suppression | [64] |
| Radioimmunotherapy | Hybrid nanoplatform (MGTe) composed of gTe (glutathione (GSH) decorated Te nanoparticles) | gTe was designed for radiotherapy sensitization, concurrently the fusion of TM and BM was expected for amplifying antitumour immune response | Breastcancer | X-Ray irradiation: ROS production and Immunogenic death (ICD) APC maturation and T-cell stimulation. | [65] |
| Chitosan/γ-PGA nanoparticles | - | TNBC | Decrease in the percentage of immunosuppressive myeloid cells and an increase in the antitumoural CD4+IFN-γ+ population | [66] | |
| Photothermal immunotherapy | Nano modulator IQS (ICG/JQ1/BMS nanoparticles) | ICG/JQ1/BMS | Mouse colon carcinoma | Immunogenic cell death (ICD) upon laser irradiation (PTT) and dual-block PD-L1 and IDO-1 pathways | [67] |
| Prussian blue nanoparticles (PBNP) | CpG-PBNP-PTT | Neuro-blastoma | T-cell activation and robust memory generation | [68] | |
| Polydopamine–Mesoporous Silica Core–Shell Nanoparticles | Polydopamine nanoparticle—Photothermal agent Gardiquimod—Immunomodulatory drug | Murine melanoma | Photothermal ablation of the cancer cells | [69] | |
| ICG-loaded magnetic nanoparticles (MIRDs) | Polyethylene glycol polyphenols (DPA-PEG)-R837 loaded | Breast cancer | Inhibited tumour growth and metastasis and recurrence | [70] | |
| Photodynamic Immuno therapy | Nano-booster (NC@Ce6) | Anti-programmed death-ligand 1 (aPDL1) and photosensitizer (Ce6) into the acid-responsive nanocomplex (NC) | Melanoma | ROS generation and Immunogenic cell death. Increases the intra-tumoural infiltration of CD8+ T cells | [22] |
| PyroR | Photosensitizer pyropheophorbide-a (Pyro) and TLR agonist resiquimod (R848) | Breast cancer | ROS generation. Dendritic cells (DCs) maturation and activate cytotoxic T lymphocytes. R848 induces macrophage repolarization. | [23] | |
| Hybrid CTTPA-G using cancer cell membranes (CC-Ms) and mesoporous silica nanoparticles (MSNs) | Type I AIE photosensitizer (TTPA) and glutamine antagonist | Melanoma | Regulate nutrition partitioning and remodelling the immune suppressive microenvironment | [71] | |
| Ferrotherapy and immunotherapy | Nanoparticle—fusion of hepcidin and leukemia cell membrane vesicles on gold nanoparticles (AuNPs) | Hollow mesoporous Prussian blue (AuPB@LMHep) | Leukemia | Immune response amplification via Ferrotherapy against tumour | [72] |
| Chemophotothermal therapy | Hollow gold nanostars (HGNSs) and gold nanocages (GNCs) | Doxorubicin | Breast cancer | Apoptosis | [73] |
| Photoimmunotherapy (Photodynamic/photo-thermal and immune-modulatory effects) | Nanoporphyrin platform | Mouse mAb anti-PD-L1 | TNBC | Sensitizing the “cold” tumour microenvironment via laser therapy followed by Immune checkpoint Blockade (PD-L1 blockade) | [74] |
| Black phosphorus and PEGylated Hyaluronic acid (HA-BP nanoparticle) | HA-BP | TNBC | Macrophage polarization. Immunogenic cell death and maturation of DCs | [24] |
| S. No. | Therapy | Nanoparticle/Material | Salient Characteristics | Cancer Model | Reference |
|---|---|---|---|---|---|
| 1 | Microneedle based Immuno-therapy | Rolling stainless steel microneedle electrode array (RoMEA) | Merit: Efficient siRNA delivery and gene silencing. Demerit: Currently, RoMEA is limited to nucleic acid delivery only | B16F10/CT26 xenograft mouse models | [90] |
| Hyaluronic acid integrated with pH-sensitive dextran nanoparticles (NPs) encapsulating anti-PD1 and glucose oxidase (GOx) | Merit: Triggered release of anti-PD1 antibody and immunomodulators (anti-CTLA4). Demerit: Focused only on skin cancer | B16F10 mouse model | [87] | ||
| pH-responsive tumour-targeted lipid nanoparticles (NPs) | Merit: Local delivery of aPD-1 and cisplatin Demerit: Shelf-life and stability issues | SCC VII mouse model | [86] | ||
| F127 nanoparticles loaded with R837 and coated with cancer cell membranes | Merit: Suppression of tumour growth by inhibiting angiogenesis | HCT116 mouse model | [83] | ||
| DNA vaccine delivery system with a layer-by-layer coating of ultra-pH-responsive OSM-(PEG-PAEU) and immunostimulatory adjuvant | Merit: Increase the immunogenecity Demerit: Risk of affecting host genome | B16/OVA melanoma tumours in mouse model | [85] | ||
| 2 | Nucleic acid-mediated therapy | Nucleic acid nanoassembly (NAN)-based technology for functionalization of hydrogels using isothermal toehold-mediated reassociation of RNA/DNA heteroduplexes. | Merit: Efficient capture of human T-lymphocytes and tunable activation of TCR Demerit: No in vivo studies for validation | - | [166] |
| Immunostimulatory DNA hydrogel consisting of a hexapod-like structured DNA (hexapodna) with CpG sequence and gold nanoparticles | Merit: Interferon- gamma production from splenocytes. Demerit: Irradiation causes adverse effects | EG7-OVA tumour-bearing mouse model | [92] | ||
| Targeted nano vaccine equipping cell membrane vesicles (CMVs) from tumour cells with functional DNA, including CpG oligonucleotide | Merit: Long-term immune memory to prevent tumour recurrence Demerit: Isolation of CMVs is dificult due to tumour heterogeneity | B16-OVA tumour-bearing mice | [94] | ||
| pH-driven interlocked DNA nano-spring (iDNS) to stimulate T-cell activation | Merit: Spring-like shrinking of iDNS leading to antitumour effect Demerit: Challenge to merge functional DNA building blocks | B16F10 tumour-bearing mice | [95] | ||
| DNA tetrahedron to create a nanoplatform for co-delivery of drug doxorubicin and the CpG oligodeoxy-nucleotides | Merit: Synergistic therapeutic effects and pronounced antitumour efficiency Demerit: DNA tetrahedron might not be able to carry long nucleic acids | B16F10 tumour-bearing mouse model | [96] | ||
| 3 | Gene editing | Programmable unlocking nano-matryoshka-CRISPR system (PUN) targeting programmed cell death ligand 1 (PD-L1) and protein tyrosine phosphatase N2 (PTPN2) | Merit: PUN exhibits optimal antitumour efficiency and long-term immune memory Demerit: Xenograft tumour model used | B16 tumour-bearing mouse model | [104] |
| Nanoassembled ribonucleoprotein complexes (NanoRNP), which can efficiently block the PD-L1 immune checkpoint | Merit: Sustained downregulation of PD-L1 | B16F10 tumour-bearing mouse model | [102] | ||
| Lipid nanoparticle complexed to plasmid DNA co-encoding CRISPR-associated protein 9 and LDHA-specific sgRNA, to form the lipoplex, pCas9-sgLDHA/F3 | Merit: Treatment activated the interferon-gamma and granzyme production of T cells in culture Demerit: Transfection mechanism of F3 not known | B16F10 tumour-bearing mouse model | [108] | ||
| Specific promoter-driven CRISPR/Cas9 system, F-PC/pHCP, achieves permanent genomic disruption of PD-L1 | Merit: Disrupts the PD-L1 gene preventing immune escape | B16F10 tumour-bearing mouse model | [105] | ||
| HPT-PFs modified with hyaluronic acid (HA) and tumour microenvironment sensitive peptides (TMSP) | Combined CD47 knockout with IL-12 production, leads to significant inhibition of tumour growth | B16F10 tumour-bearing mouse model | [107] | ||
| 4 | Exosomes | Surface-engineered antigenic exosomes using melanoma tumour peptides | Merit: Induced antigen- specific CD8 T cell proliferation | 2 Pmel 1 transgenic mice | [112] |
| 5 | Engineered cells | Paclitaxel-loaded fake blood cell Eudragit particle (Eu-FBCP/PTX) | Merit: Exhibits better phagocytic and micropinocytic uptake | MC-38 tumour models | [25] |
| Bone marrow-derived mesenchymal stem cells (MSCs) engineered to express the immune stimulating factor LIGHT | Merits: LIGHT- expressing MSCs exhibit potent antitumour immune response; Reverses immunesuppressive TME | TUBO (murine mammary carcinoma) | [117] | ||
| Dibenzocyclooctyne- poly(ethylene glycol)- pheophorbide A conjugated to human mesenchymal stem cell (hMSC-DPP) | Merits: hMSC-DPP recognizes cancer lesions, mediates cell death by irradiation; Immune regulation at the target site | K1735 tumour-bearing mouse model | [116] | ||
| 6 | CAR-T Therapy | Stem cells engineered to stably express various chimeric antigen receptors (CARs) against tumour-associated antigens | Merits: Long-term immune cell generation, sustained tumour-specific effector cells to maintain remission Demerit: T cells in human thymus may not cause immune-tolerance to the mouse host | BLT (Bone, Liver, Thymus) humanized mouse model | [115] |
| 7 | Nano-optogenetics | Pan-T cells (Human Peripheral Blood CD3+ T Cells) transduced using pCDH-OPN4-eGFP and pNFAT-3CK construct exposed to blue light illumination | Merits: Photo- activatable engineered T cells suppressing tumour growth; Cytotoxicity increases with blue light illumination Demerit: Low penetration depth of blue light | Subcutaneous xenografts in hepatocellular carcinoma | [124] |
| Far-red light-controlled immunomodulatory engineered cells (FLICs) loaded into a hydrogel scaffold | Merits: FLICs-loaded hydrogel implants elicit long-term immunological memory; Prevents tumour recurrence Demerit: Determination of T cell response was only carried out ex vivo; In vivo response not known | B16F10 ovalbumin expressing melanoma model | [125] | ||
| Engineered bacteria EcN-pDawn-φx174E/TRAIL | Merits: Both diagnosis and light—controlled cancer therapy Demerit: Poor penetration depth of blue radiation | Colorectal cancer theranostic and therapy | [142] | ||
| 8 | Virus and viral components for Immunotherapy | Papaya mosaic virus nanoparticle (PapMV) | Merits: Synergistically improves the therapeutic effect; PapMV alone induced the development of CD8+ T-cell responses against endogenous tumour epitopes Demerit: Intratumoral imjection for antitumour activity performed and may not be applicable to deep-seated tumours | Subcutaneous (B16-OVA) melanoma model | [130] |
| Cowpea mosaic virus (CPMV) | Merits: In situ vaccine modulates the TME potentiate antitumour immunity; Exhibits excellent antitumour activity when compared to other visruses Demerit: Antitumour response depends on the capsid viral protein recognition | Colon cancer, Melanoma, Ovarian cancer model. | [132] | ||
| Tobacco mosaic virus (TMV) conjugated with toll-like receptor 7 agonist (1V209), and surface- coated with photothermal biopolymer polydopamine (PDA) | Merits: Intratumorally injected and irradiated using an 808 nm near-infrared laser enhances antitumour activity; Inhibition of tumour recurrence Demerit: Long-term effects due to irradiation not known | B16F10 dermal melanoma mice | [134] | ||
| SeV (sendai virus) + aCD47)@PLGA nanoparticles | Merits: Nano-composite strategy enhances antitumour efficacy by TME; Immuno- modulation suppresses tumour metastasis and recurrence Demerit: Intratumoral injection performed; May not be applicable to deep seated tumours | 4T1 murine mouse model | [129] | ||
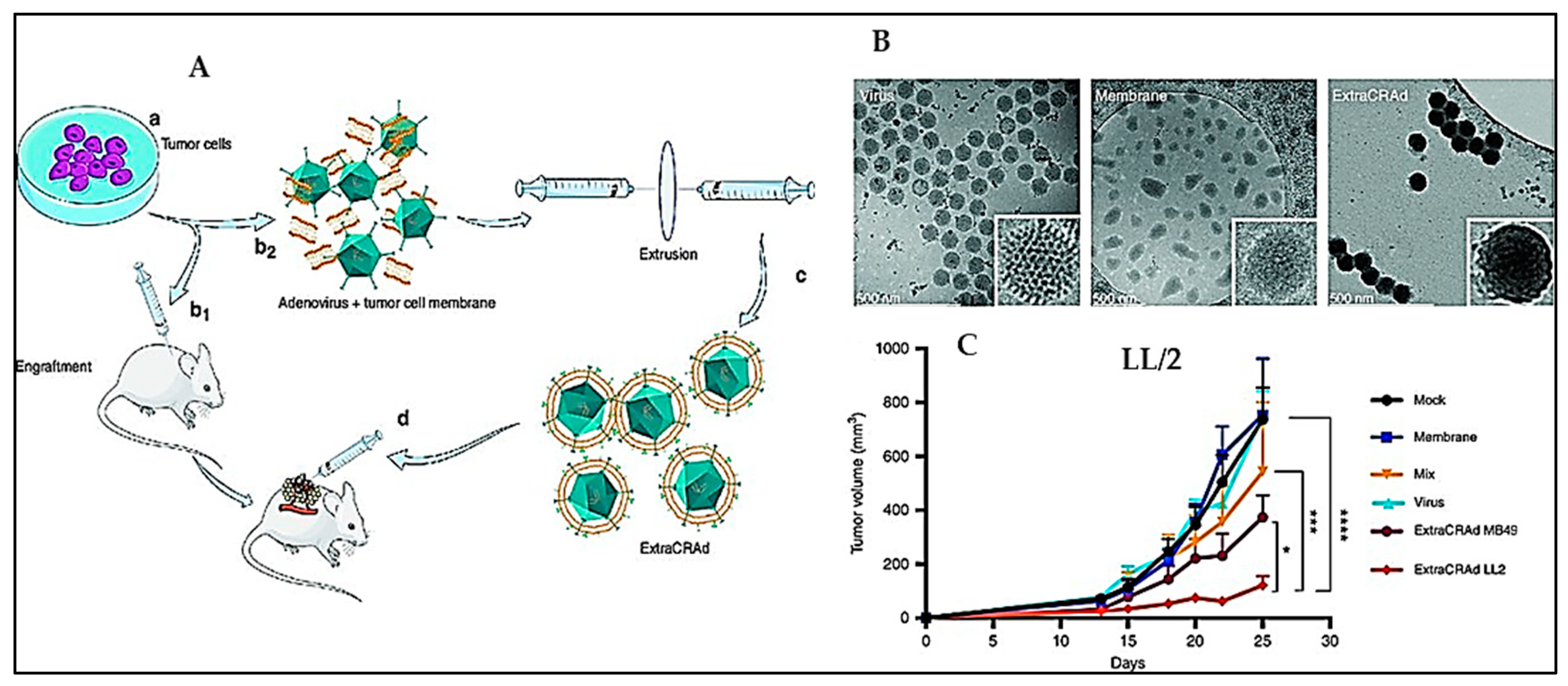
| 9 | Oncolytic virus based immunotherapy | Virus artificially wrapped with tumour cancer membranes carrying tumour antigens | Merits: Increased infectivity and oncolytic effect; controls the growth of aggressive melanoma and lung tumours | Subcutaneous murine model of melanoma and lung cancer | [138] |
| 10 | Bacterial Immuno- therapeutics | Hybrid vaccine platform (HMP@Ag) using hybrid M13 phage and personal tumour antigens | Merits: Activation of antigen-presenting cells (APCs) through the Toll-like receptor 9 (TLR9) signaling pathway; Uses personalised antigens Demerit: Pathogenicity of bacteria might induce immune-related adverse events | B16-OVA melanoma model | [135] |
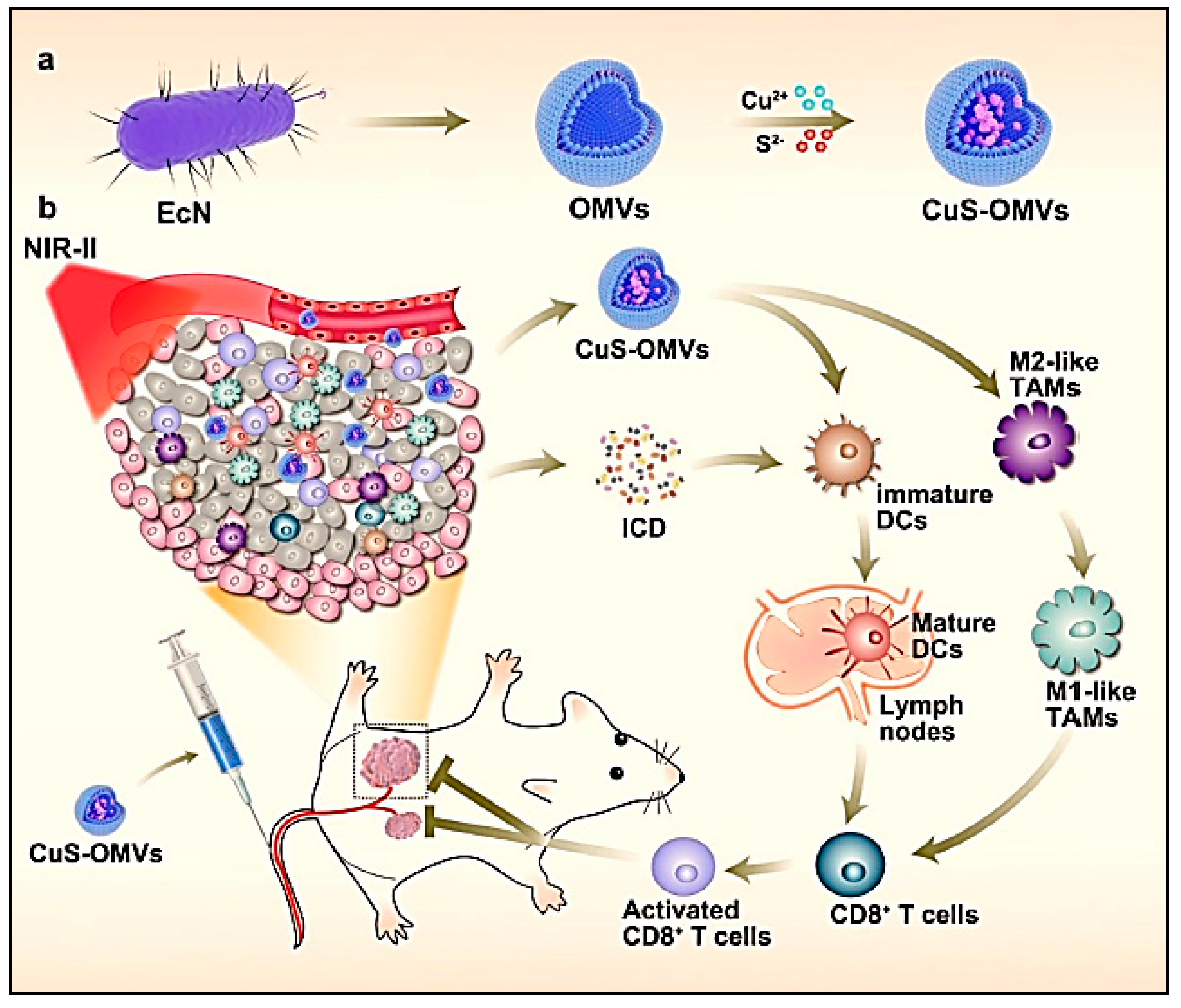
| Outer membrane vesicles (OMVs) from Escherichia colbiomimetic containing copper sulfide nanoparticles are fabricated (CuS-OMVs). | Merits: Induced strong immunogenic cell death (ICD) of tumour cells; Acts as immune adjuvant and causes repolarisation of TAMs Demerit: Long-term safety of CuS not known | Murine 4T1 breast cancer model | [146] | ||
| Gold nanoparticles (AuNPs) cloaked within the outer-membrane vesicles (OMVs) from E. coli | Merits: Cytotoxic effect on GL261 glioma cells; low—dose combination radiotherapy Demerit: Need to explore the affinity of AuNPs and OMVs | Subcutaneous tumour model and In situ brain tumour model | [145] | ||
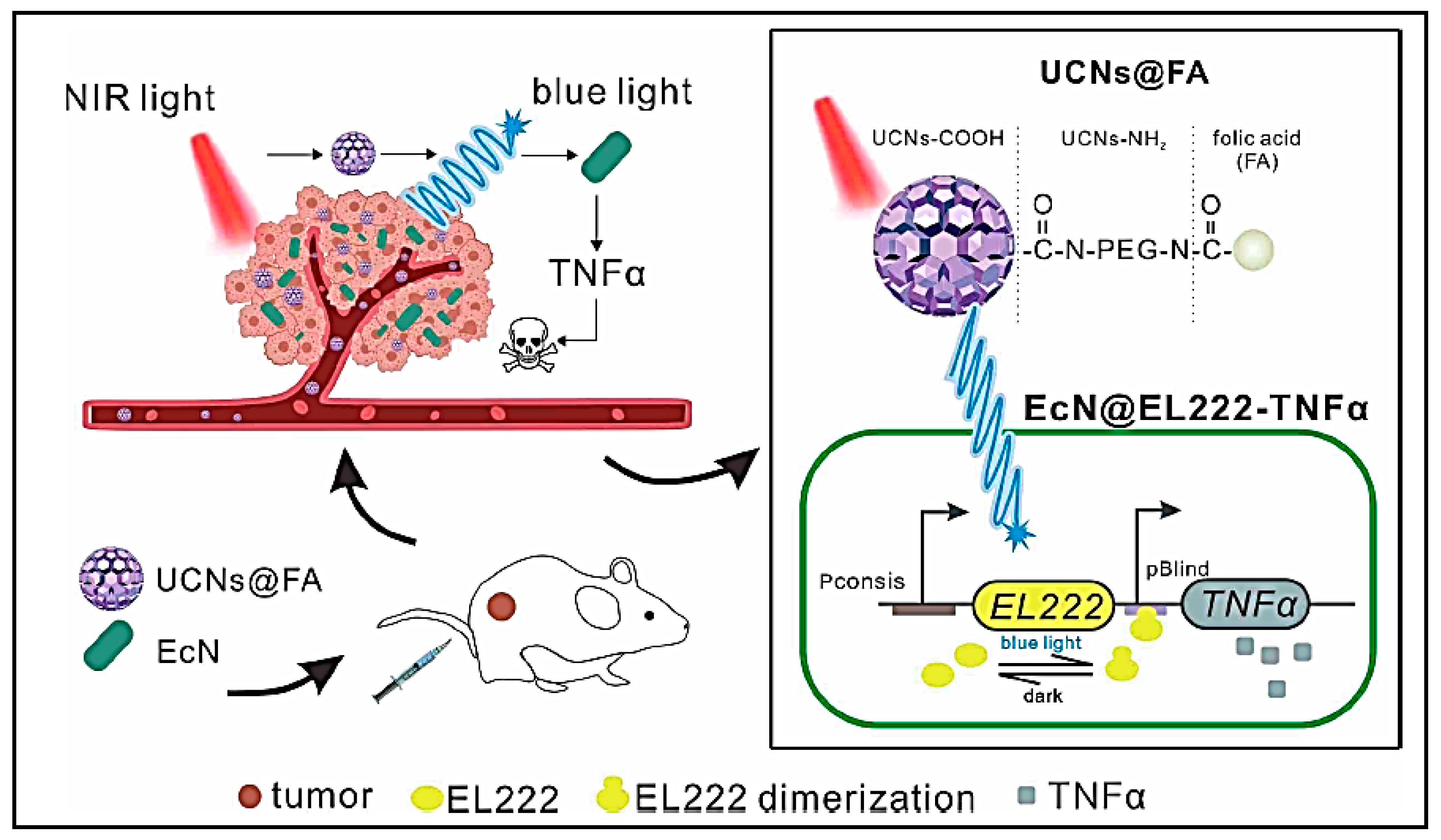
| Engineered biotherapeutic platform using EcN-EL222-TNFα and UCNs@FA | Merits: Upconversion optogenetic strategy enhances anti—tumour efficacy; Controllability and biocompatibility, deeper penetrability Demerit: Long-term effects of therapy unknown | 4T1—tumour-bearing mouse model | [141] | ||
| 11 | Fungal based Immunotherapeutic | Fungal beta-glucans | Merit: Stimulates both innate and adaptive immune responses Merit:.Direct cytotoxic effect Demerits: Toxicity studies are not performed; Orthotopic models not studied | Xenograft colon cancer | [148] |
| Nanoparticle incorporated polysaccharide mannan structure from Saccharomyces cerevisiae | Merit: Strongly induces T helper 17 (TH17) responseDemerit: Intratumoral injection carried out; May not be applicable to deep seated tumours | Colon and melanoma cancer | [149] | ||
| Immuno modualting polysaccharides from Inonotus obliquus | Merit: Transforms TAMs into proinflammatory phenotype Demerit: Systemic characterisation of immunological properties is lacking | In vitro studies by co-culture of using mouse lung cancer cell lines and macrophages | [147] | ||
| 12 | Herbal interventions for Immunotherapy | Enzyme-sensitive tumour-targeting nano drug delivery system AP-PP-DOX (Polysaccharides from Angelica sinensis (AP)) | Merit: Restores Th1/Th2 immune balance in tumour microenvironment. Demerit: In vivo study has not been reported | - | [167] |
| Innate immune activator Astragaloside III (As) photosensitizer chlorine e6 (Ce6) ((As + Ce6)@MSNs-PEG) | Merits: Effectively activates NK cells and inhibits the proliferation of tumour cells in vitro; Induces infiltration of immune cells into the tumour; Enhances the cytotoxicity of natural killer cells and CD8+ T cells in vivo | CT26 tumour-bearing model | [152] | ||
| 13 | 3D matrix architecture for Immunotherapy | Three-dimensional (3D) poly(ethylene)glycol (PEG) hydrogels covalently combined with low molecular weight heparin | Merits: PEG provides structural and mechanical property, anchoring of CCL21 to heparin influences cell migration and proliferation; T cells reproduce in large number Demerit: In vivo study has not been reported | - | [163] |
| S. No | Clinical Trial | Cancer Type | Therapeutic Intervention | Clinical Trial ID |
|---|---|---|---|---|
| 1 | A study to evaluate/tolerability of immune-therapy combinations in participation with TNBC or gynaecologic malignancies (Completed) | TNBC and Ovarian cancer | Etrumadenant (antagonist of immunomodulatory checkpoint molecules adenosine A2A and A2B receptors), Eganelisib (IPI-549, phosphoinositide 3-kinase inhibitor), PEGylated liposomal doxorubicin (PLD), albumin nanoparticle-bound paclitaxel (NP) | NCT03719326 |
| 2 | Neoadjuvant LDRT combined with Durvalumab in potentially resectable stage III NSCLC (Ongoing) | Stage III NSCLC | Durvalumab (immune checkpoint inhibitor antibody), Albumin nanoparticle-bound paclitaxel along with low-dose radiation therapy | NCT05157542 |
| 3 | Dose escalation study of immunomodulatory nanoparticles (Ongoing) | Advanced solid tumours | PRECIOUS-01 (invariant natural killer T cell activator threitolceramide-6 and New York Esophageal Squamous Cell Carcinoma-1 cancer-testis antigen peptides encapsulated in PLGA nanoparticle) | NCT04751786 |
| 4 | A pilot study of neoadjuvant chemotherapy with or without Camrelizumab for locally advanced gastric cancer (Ongoing) | Gastric cancer | Camrelizumab (anti-PD1) and chemotherapy with albumin nanoparticle-bound paclitaxel and oxaliplatin | NCT05101616 |
| 5 | NBTXR3, Radiation Therapy, and Pembrolizumab for the treatment of recurrent or metastatic head and neck squamous cell cancer (Ongoing) | Metastatic head and neck squamous cell carcinoma and recurrent head and neck squamous cell carcinoma | Hafnium oxide containing nano- particles (NBTXR3) with hypo-fractionated radiation therapy and Pembrolizumab (anti-PD1 humanized antibody) with Stereotactic body radiation therapy | NCT04862455 |
| 6 | Radiation therapy to the usual treatment (Immunotherapy with or without chemotherapy) for Stage IV non-small cell lung cancer patients who are PD-L1 negative (Ongoing) | Advanced lung adenocarcinoma, Advanced lung adenosquamous carcinoma, Advanced and metastatic lung NSCC (Stages IIIB/IIIC/IV/IVA/IVB), Metastatic lung adeno-carcinoma, Metastatic lung adeno-squamous carcinoma, lung cancer AJCC v8 | Carboplatin, Ipilimumab (CTLA4 targeting antibody), albumin nanoparticle-bound paclitaxel, Nivolumab (anti-PD1), Paclitaxel, Pembrolizumab, Pemetrexed along with Stereotactic body radiation therapy | NCT04929041 |
| 7 | Gemcitabine, Nab-paclitaxel, Durvalumab, and Oleclumab before surgery for the treatment of in resectable/borderline resectable primary pancreatic cancer (Ongoing) | Borderline resectable pancreatic adeno-carcinoma, Resectable pancreatic adeno-carcinoma (IA/IB/IIA/IIB) pancreatic cancer AJCC v8 | Durvalumab, Gemcitabine, albumin nanoparticle-bound paclitaxel, Oleclumab (anti-CD73) | NCT04940286 |
| 8 | Combination with chemotherapy for the treatment of advanced solid tumours involving the abdomen or thorax (Ongoing) | Advanced breast carcinoma, Advanced endometrial carcinoma, Advanced fallopian tube carcinoma, Advanced hepatocellular carcinoma, Advanced malignant abdominal neoplasm, Advanced malignant female reproductive system neoplasm, Advanced malignant thoracic neoplasm, Advanced ovarian carcinoma, Advanced primary peritoneal carcinoma, Advanced renal cell carcinoma | Atezolizumab (anti-PD1), Cabozantinib S-malate (tyrosine kinase inhibitor), albumin nanoparticle-bound paclitaxel | NCT05092373 |
| 9 | Durvalumab in combination with chemotherapy in treating patients with advanced solid tumours (Ongoing) | Locally advanced malignant solid neoplasm, Metastatic malignant solid neoplasm, Unresectable malignant solid neoplasm | Capecitabine, Carboplatin, Durvalumab, Gemcitabine hydrochloride, Paclitaxel, albumin nanoparticle-bound paclitaxel, PEGylated liposomal doxorubicin hydrochloride | NCT03907475 |
| 10 | Addition of anticancer drug, ZEN003694 (ZEN-3694) and PD-1 inhibitor (Pembrolizumab) to standard chemo-therapy (Nab-Paclitaxel) treatment in patients with advanced Triple-Negative Breast Cancer (TNBC) (Ongoing) | Anatomic stage III/IV breast cancer AJCC, Locally advanced TNBC, Metastatic TNBC, Unresectable TNBC | BET Bromodomain inhibitor ZEN-3694, albumin nanoparticle-bound paclitaxel, Pembrolizumab | NCT05422794 |
| 11 | Pembro + Chemo in brain mets (Ongoing) | Lung cancer, Lung cancer metastatic, Brain cancer, Cancer | Pembrolizumab, Paclitaxel, Pemetrexed, Carboplatin, albumin nanoparticle-bound paclitaxel | NCT04964960 |
| 12 | Atezolizumab with chemotherapy in treating patients with anaplastic or poorly differentiated thyroid cancer (Ongoing) | Metastatic thyroid gland carcinoma, Poorly differentiated thyroid gland carcinoma, Stage IVA/IVB/IVC thyroid gland anaplastic carcinoma AJCC v8, Gland anaplastic carcinoma, Unresectable thyroid gland carcinoma | Atezolizumab (anti-PDL1), Bevacizumab (anti-VEGFA), Cobimetinib (MEK inhibitor), Paclitaxel, Vemurafenib (B-Raf inhibitor), albumin nanoparticle-bound paclitaxel | NCT03181100 |
| 13 | Local consolidative therapy and Durvalumab for oligoprogressive and polyprogressive stage III NSCLC after chemoradiation and anti-PD-L1 therapy (Ongoing) | Stage III/IIIA/IIIB lung cancer AJCC v8 Stage III/IIIA/IIIB lung Non-Small Cell Cancer AJCC v7 | Carboplatin, Durvalumab, Gemcitabine, Paclitaxel, Pemetrexed, albumin nanoparticle-bound paclitaxel | NCT04892953 |
| 14 | NBTXR3, Radiation therapy, Ipilimumab, and Nivolumab for the treatment of lung and/or liver metastases from solid malignancy (Ongoing) | Advanced malignant solid neoplasm, Meta- static malignant neo- plasm in the liver, Metastatic malignant neoplasm in the lung, Metastatic malignant solid neoplasm | Hafnium oxide-containing nanoparticles (NBTXR3), Ipilimumab (anti-CTLA4), Nivolumab along with radiation therapy | NCT05039632 |
| 15 | Durvalumab and Tremelimumab in combination with propranolol and chemotherapy for treatment of advanced hepato-pancreabiliary tumours (Ongoing) | Pancreatic Cancer, Hepatocellular Cancer, Biliary Tract Cancer, Cholangiocarcinoma | Durvalumab, Gemcitabine, Tremelimumab, Propranolol, Cisplatin, albumin nanoparticle-bound paclitaxel | NCT05451043 |
| 16 | Novel RNA-nanoparticle vaccine for the treatment of early melanoma recurrence following adjuvant anti-PD-1 antibody therapy (Ongoing) | Melanoma | Autologous total tumour mRNA loaded DOTAP liposome vaccine | NCT05264974 |
| 17 | CAR-T cell therapy in relapsed/refractory myeloma with extramedullary disease—an in vivo imaging and molecular monitoring study (CARAMEL) (Ongoing) | Extramedullary Myeloma | JNJ-68284528 (Cilta-cel)—B cell maturation antigen (BCMA) and 64Cu SPION dual PET-MR imaging agent | NCT05666700 |
| 18 | Split course adaptive radiation therapy with Pembrolizumab with/without chemotherapy for treating stage IV lung cancer (Ongoing) | Lung Non-Small Cell Carcinoma, Stage IV lung cancer AJCC v8 | Carboplatin, Fludeoxyglucose F-18, Pembrolizumab, Pemetrexed, albumin nanoparticle-bound paclitaxel along with radiation therapy, [18-F] (fluoropropyl)-L-glutamate (FSPG) PET scan | NCT05501665 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandasamy, G.; Karuppasamy, Y.; Krishnan, U.M. Emerging Trends in Nano-Driven Immunotherapy for Treatment of Cancer. Vaccines 2023, 11, 458. https://doi.org/10.3390/vaccines11020458
Kandasamy G, Karuppasamy Y, Krishnan UM. Emerging Trends in Nano-Driven Immunotherapy for Treatment of Cancer. Vaccines. 2023; 11(2):458. https://doi.org/10.3390/vaccines11020458
Chicago/Turabian StyleKandasamy, Gayathri, Yugeshwaran Karuppasamy, and Uma Maheswari Krishnan. 2023. "Emerging Trends in Nano-Driven Immunotherapy for Treatment of Cancer" Vaccines 11, no. 2: 458. https://doi.org/10.3390/vaccines11020458
APA StyleKandasamy, G., Karuppasamy, Y., & Krishnan, U. M. (2023). Emerging Trends in Nano-Driven Immunotherapy for Treatment of Cancer. Vaccines, 11(2), 458. https://doi.org/10.3390/vaccines11020458








