Immunogenicity and Safety of a Combined Intramuscular/Intranasal Recombinant Spike Protein COVID-19 Vaccine (RCP) in Healthy Adults Aged 18 to 55 Years Old: A Randomized, Double-Blind, Placebo-Controlled, Phase I Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Randomization and Masking
2.4. Procedures
2.5. Outcomes
2.6. Statistical Analysis
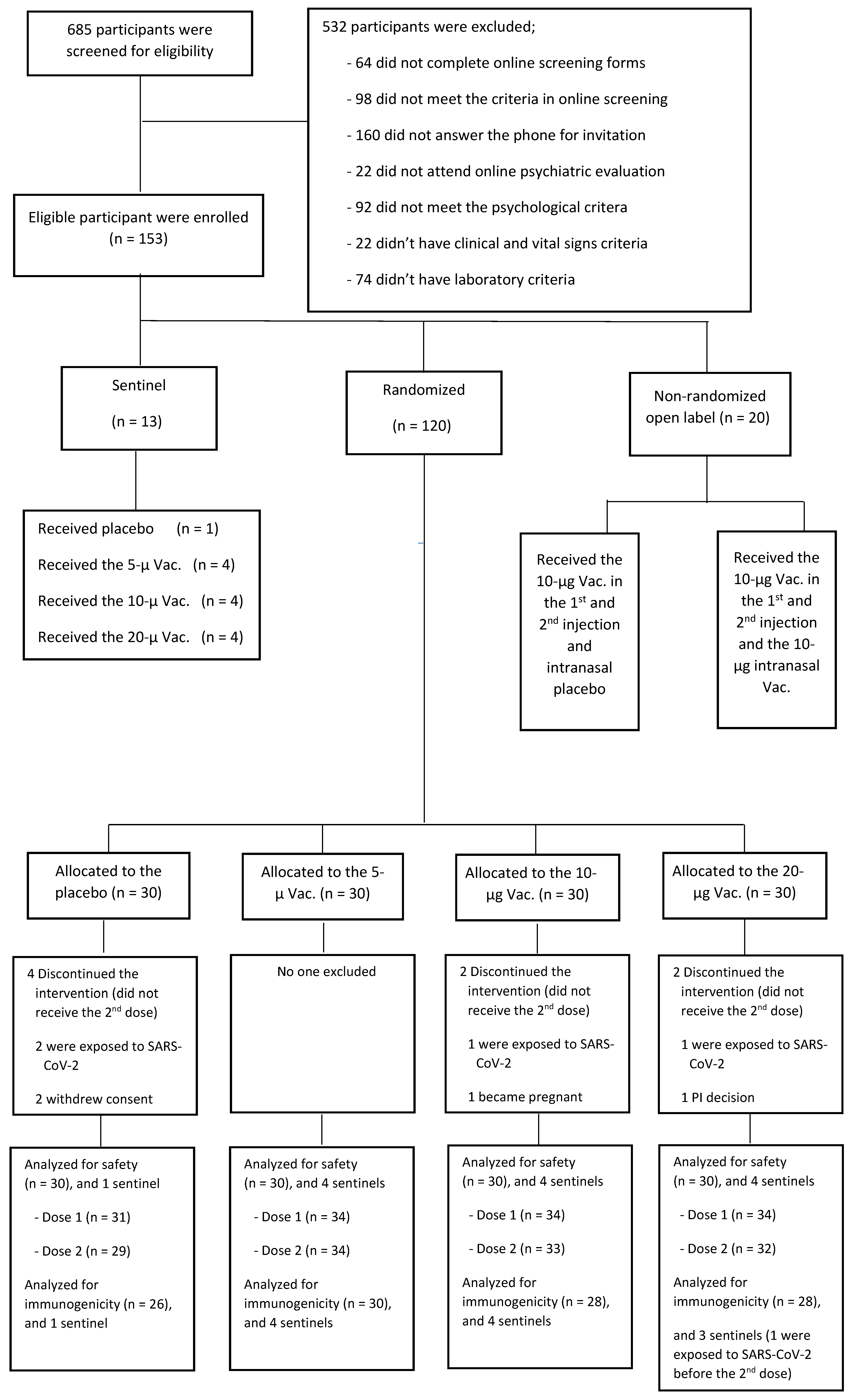
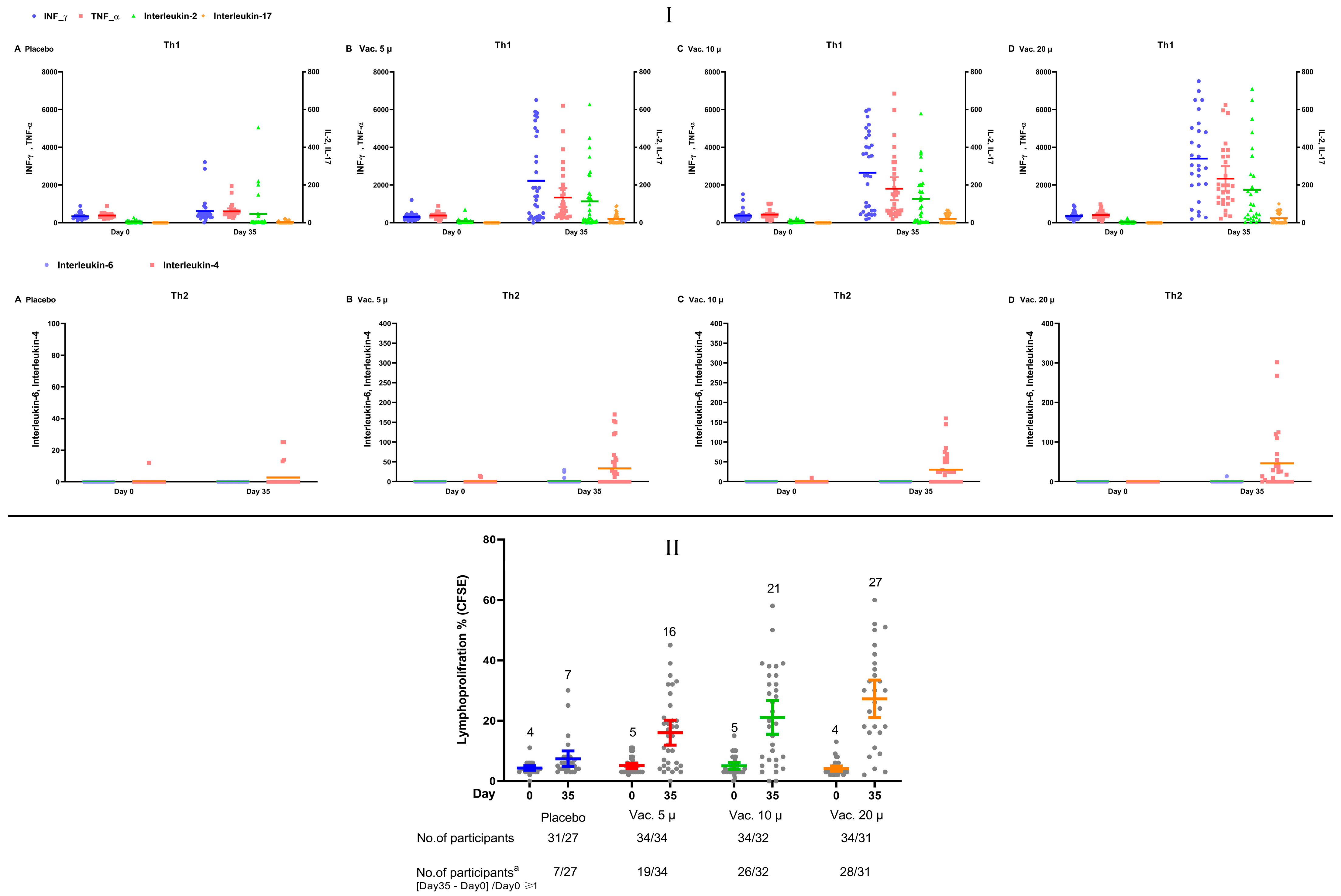
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soraci, L.; Lattanzio, F.; Soraci, G.; Gambuzza, M.E.; Pulvirenti, C.; Cozza, A.; Corsonello, A.; Luciani, F.; Rezza, G. COVID-19 Vaccines: Current and Future Perspectives. Vaccines 2022, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 vaccine development: Milestones, lessons and prospects. Signal Transduct. Target. Ther. 2022, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Richmond, P.; Hatchuel, L.; Dong, M.; Ma, B.; Hu, B.; Smolenov, I.; Li, P.; Liang, P.; Han, H.H.; Liang, J.; et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 682–694. [Google Scholar] [CrossRef]
- Pack, S.M.; Peters, P.J. SARS-CoV-2-Specific Vaccine Candidates; the Contribution of Structural Vaccinology. Vaccines 2022, 10, 236. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Cao, H.; Liu, C. SARS-CoV-2 S1 is superior to the RBD as a COVID-19 subunit vaccine antigen. J. Med. Virol. 2021, 93, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 vaccines: From bench to bed. eBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]
- Bleier, B.S.; Ramanathan, M.; Lane, A.P. COVID-19 Vaccines May Not Prevent Nasal SARS-CoV-2 Infection and Asymptomatic Transmission. Otolaryngol. Head Neck Surg. 2021, 164, 305–307. [Google Scholar] [CrossRef]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.-L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. Med. 2021, 2, 100230. [Google Scholar] [CrossRef]
- Du, Y.; Xu, Y.; Feng, J.; Hu, L.; Zhang, Y.; Zhang, B.; Guo, W.; Mai, R.; Chen, L.; Fang, J.; et al. Intranasal administration of a recombinant RBD vaccine induced protective immunity against SARS-CoV-2 in mouse. Vaccine 2021, 39, 2280–2287. [Google Scholar] [CrossRef]
- Ku, M.-W.; Bourgine, M.; Authié, P.; Lopez, J.; Nemirov, K.; Moncoq, F.; Noirat, A.; Vesin, B.; Nevo, F.; Blanc, C.; et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe 2021, 29, 236–249.e236. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Purushotham, J.N.; Schulz, J.E.; Holbrook, M.G.; Bushmaker, T.; Carmody, A.; Port, J.R.; Yinda, C.K.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 2021, 13, eabh0755. [Google Scholar] [CrossRef]
- Dhama, K.; Dhawan, M.; Tiwari, R.; Emran, T.B.; Mitra, S.; Rabaan, A.A.; Alhumaid, S.; Alawi, Z.A.; Al Mutair, A. COVID-19 intranasal vaccines: Current progress, advantages, prospects, and challenges. Hum. Vaccines Immunother. 2022, 18, 2045853. [Google Scholar] [CrossRef] [PubMed]
- Banihashemi, S.R.; Es-Haghi, A.; Mehrabadi, M.H.F.; Nofeli, M.; Mokarram, A.R.; Ranjbar, A.; Salman, M.; Moradi, H.; Razaz, S.H.; Taghdiri, M.; et al. Safety and efficacy of combined intramuscular/intranasal RAZI-COV PARS vaccine candidate against SARS-CoV-2: A Preclinical Study in several animal models. Front. Immunol. 2022, 13, 836745. [Google Scholar] [CrossRef]
- Kovalenko, A.; Ryabchevskaya, E.; Evtushenko, E.; Nikitin, N.; Karpova, O. Recombinant Protein Vaccines against Human Betacoronaviruses: Strategies, Approaches and Progress. Int. J. Mol. Sci. 2023, 24, 1701. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.-J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, X.; Lu, X.; Wu, L.; Yin, L.; Zhu, L.; Liang, H.; Xu, F.; Zhou, Q. A spike protein S2 antibody efficiently neutralizes the Omicron variant. Cell. Mol. Immunol. 2022, 19, 644–646. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Finsterbusch, K.; Wu, M.; Harvey, R.; Hussain, S.; Greco, M.; Liu, Y.; Kjaer, S.; Swanton, C.; et al. SARS-CoV-2 S2-targeted vaccination elicits broadly neutralizing antibodies. Sci. Transl. Med. 2022, 14, eabn3715. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Canziani, G.A.; Carter, E.P.; Chaiken, I. The Case for S2: The Potential Benefits of the S2 Subunit of the SARS-CoV-2 Spike Protein as an Immunogen in Fighting the COVID-19 Pandemic. Front. Immunol. 2021, 12, 637651. [Google Scholar] [CrossRef]
- Zaccaro, D.J.; Wagener, D.K.; Whisnant, C.C.; Staats, H.F. Evaluation of vaccine-induced antibody responses: Impact of new technologies. Vaccine 2013, 31, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.; He, J.; Liao, Y.; Li, Y.; Pei, R.; Fang, X.; Zeng, P.; Fan, R.; Ou, Z.; et al. Safety and immunogenicity of a recombinant interferon-armed RBD dimer vaccine (V-01) for COVID-19 in healthy adults: A randomized, double-blind, placebo-controlled, Phase I trial. Emerg. Microbes Infect. 2021, 10, 1589–1597. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Hsieh, S.-M.; Liu, W.-D.; Huang, Y.-S.; Lin, Y.-J.; Hsieh, E.-F.; Lian, W.-C.; Chen, C.; Janssen, R.; Shih, S.-R.; Huang, C.-G. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVCCOV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. eClinicalMedicine 2021, 38, 100989. [Google Scholar] [CrossRef] [PubMed]
- Vajdy, M.; Baudner, B.; Del Giudice, G.; O’Hagan, D. A vaccination strategy to enhance mucosal and systemic antibody and T cell responses against influenza. Clin. Immunol. 2007, 123, 166–175. [Google Scholar] [CrossRef]
- Zhou, F.; Goodsell, A.; Uematsu, Y.; Vajdy, M. Prolonged Protection against Intranasal Challenge with Influenza Virus following Systemic Immunization or Combinations of Mucosal and Systemic Immunizations with a Heat-Labile Toxin Mutant. Clin. Vaccine Immunol. 2009, 16, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Vajdy, M.; Singh, M.; Kazzaz, J.; Soenawan, E.; Ugozzoli, M.; Zhou, F.; Srivastava, I.; Bin, Q.; Barnett, S.; Donnelly, J.; et al. Mucosal and systemic anti-HIV responses in rhesus macaques following combinations of intranasal and parenteral immunizations. AIDS Res. Hum. Retrovir. 2004, 20, 1269–1281. [Google Scholar] [CrossRef]
- Brown, M.A.; Hural, J. Functions of IL-4 and control of its expression. Crit. Rev. Immunol. 1997, 17, 1–32. [Google Scholar] [CrossRef]
- Carcaboso, A.M.; Hernández, R.M.; Igartua, M.; Rosas, J.E.; Patarroyo, M.E.; Pedraz, J.L. Potent, long lasting systemic antibody levels and mixed Th1/Th2 immune response after nasal immunization with malaria antigen loaded PLGA microparticles. Vaccine 2004, 22, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.A. The role of cytokines in determining the Th1/Th2 phenotype of an immune response: Coherence of the T cell response and the Cytokine Implementation Hypothesis. Scand. J. Immunol. 2022, 95, e13110. [Google Scholar] [CrossRef]
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nature Human Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Purushotham, J.; Schulz, J.; Holbrook, M.; Bushmaker, T.; Carmody, A.; Port, J.R.; Yinda, C.K.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. BioRxiv 2021. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Y.; Liu, S.; Kou, Z.; Li, W.; Farzan, M.; Jiang, S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: Implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004, 324, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Yang, C.L.; Ge, M.R.; Liu, Y.; Zhang, P.; Li, H.; Li, X.L.; Li, T.; Liu, Y.D.; Dou, Y.C.; et al. M1 Macrophage Derived Exosomes Aggravate Experimental Autoimmune Neuritis via Modulating Th1 Response. Front Immunol. 2020, 11, 1603. [Google Scholar] [CrossRef]
- Miao, Q.; Zhang, X.X.; Han, Q.X.; Ren, S.S.; Sui, R.X.; Yu, J.W.; Wang, J.; Wang, Q.; Yu, J.Z.; Cao, L.; et al. The therapeutic potential of bilobalide on experimental autoimmune encephalomyelitis (EAE) mice. Metab. Brain Dis. 2020, 35, 793–807. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials; Food and Drug Administration, US Department of Health and Human Services: Silver Spring, MA, USA, 2007.


| Screening | 0 | 7 | 14 | 21 | 28 | 35 | 51 | 58 | 65 | 150 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasal swab for COVID-19 PCR test a | × | ||||||||||
| Visit to the study center and physical examination | × | × | × | × | × | × | × | × | × | × | × |
| Psychological assessment | × | ||||||||||
| Blood sample: screening b | × | ||||||||||
| Vaccination | × | × | × | ||||||||
| Blood sample: safety c | × | × | × | ||||||||
| Blood sample: humoral immunogenicity | × | × | × | × | × | × | × | × | |||
| Blood sample: cellular immunogenicity | × | × | × | × | |||||||
| Blood sample: VNT | × | × | × | × | |||||||
| Immediate and solicited local and systemic reactions |  |  |  | ||||||||
| Unsolicited adverse events |  | ||||||||||
| Medically attended adverse events |  | ||||||||||
| Placebo n = 30 | Vac. 5 µ n = 30 | Vac. 10 µ n = 30 | Vac. 20 µ n = 30 | Total n = 120 | |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 20 (66.67) | 26 (86.67) | 24 (80.0) | 21 (70.0) | 91 (75.83) |
| Female | 10 (33.33) | 4 (13.33) | 6 (20.0) | 9 (30.0) | 29 (24.17) |
| Age | |||||
| Mean (SD) | 35.76 (6.69) | 36.16 (8.03) | 37.3 (6.94) | 35.03 (6.65) | 36.06 (7.06) |
| Median (min–max) | 36.5 (21–54) | 36 (21–49) | 36.5 (23–55) | 35.5 (23–55) | 36.0 (21–55) |
| Body-mass index | |||||
| Mean (SD) | 26.18(3.52) | 25.99 (3.81) | 25.89 (3.46) | 25.72 (4.65) | 25.94 (3.84) |
| Median (min–max) | 26 (18.5–33) | 25.5 (19.2–34.2) | 25.2 (18.7–32.4) | 25.85 (18.7–34.7) | 25.7 (18.5–34.7) |
| Smoking, n (%) | |||||
| Current Smoking | 5 (16.67) | 8 (26.67) | 8 (26.67) | 8 (26.67) | 29 (24.17) |
| Past Smoking | 3 (10.0) | 2 (6.67) | 6 (20.07) | 2 (6.67) | 13 (10.83) |
| Never Smoking | 22 (73.33) | 20 (66.67) | 16 (53.33) | 20 (66.67) | 78 (65.0) |
| Education, n (%) | |||||
| Diploma | 5 (16.67) | 5 (16.67) | 4 (13.33) | 4 (13.33) | 18 (15.0) |
| Diploma plus | 3 (10.0) | 2 (6.67) | 3 (10.0) | 0 (0.0) | 8 (6.67) |
| Bachelor | 8 (26.67) | 11 (36.67) | 10 (33.33) | 12 (40.0) | 41 (34.17) |
| Master | 9 (30.0) | 10 (33.33) | 10 (33.33) | 10 (33.3) | 39 (32.50) |
| Doctoral and above | 5 (16.67) | 2 (6.67) | 3 (10.0) | 4 (13.33) | 14 (11.67) |
| Job, n (%) | |||||
| Unemployed/Retired | 2 (6.67) | 4 (13.33) | 0 (0.0) | 1 (3.33) | 7 (5.83) |
| Government employee | 9 (30.0) | 9 (30.0) | 9 (30.0) | 10 (33.33) | 37 (30.83) |
| Private employee | 7 (23.33) | 7 (23.33) | 12 (40.0) | 9 (30.0) | 35 (29.17) |
| Private work | 7 (23.33) | 8 (26.67) | 7 (23.33) | 8 (26.67) | 30 (25.0) |
| Housewife | 5 (16.67) | 2 (6.67) | 2 (6.67) | 2 (6.67) | 11 (9.17) |
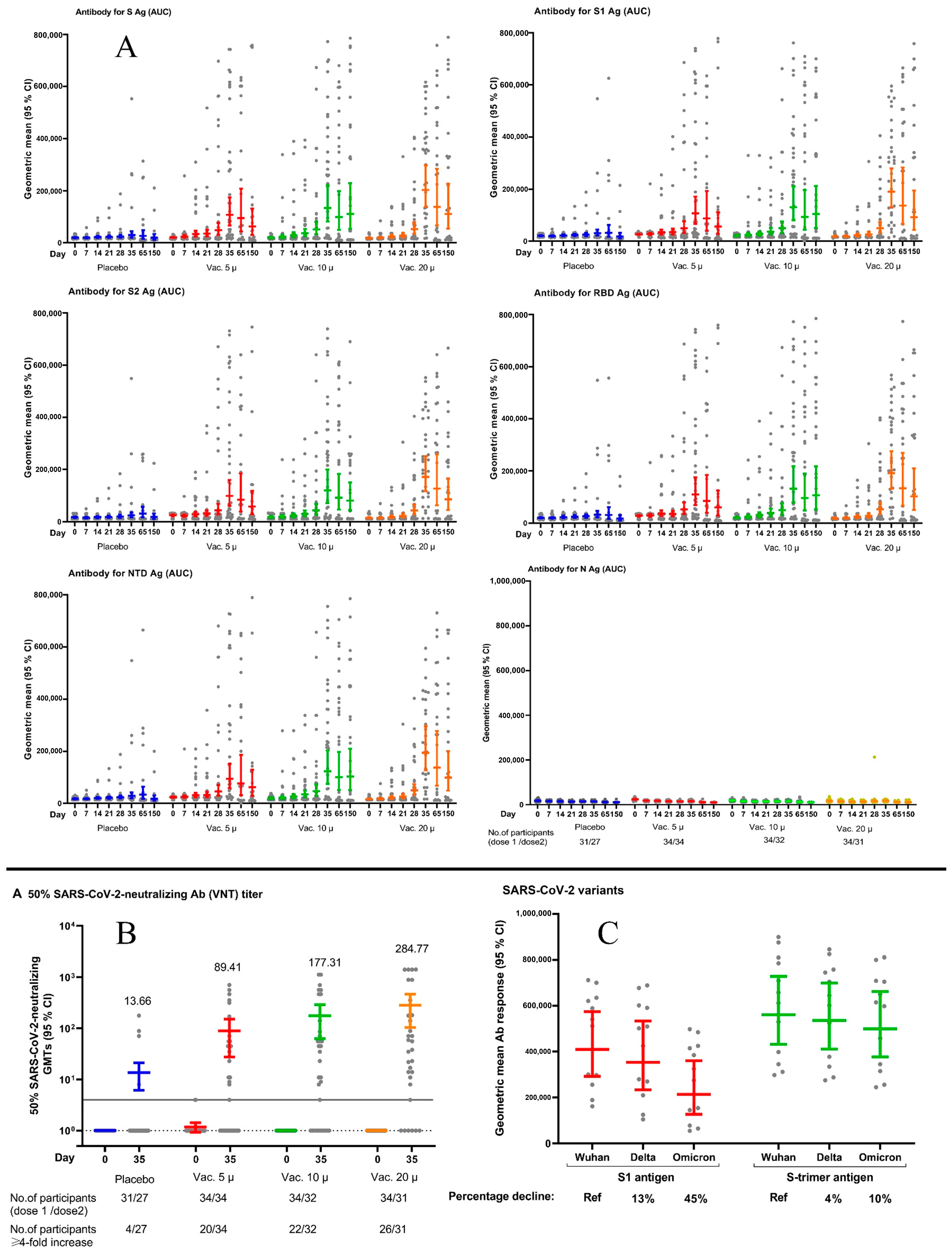
| Baseline a | Day 7 | Day 14 | Day 21 a | Day 28 | Day 35 | Day 65 | Day 150 | Seroconversion Rate b on Day 35 (%) and 95% CI c | ||
|---|---|---|---|---|---|---|---|---|---|---|
| GMRAUC (95% CI) | ||||||||||
| Anti S antibody | Placebo | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14.8 (4.2–33.7) |
| Vac. 5 µg | 1.28 (1.08–1.52) | 1.36 (1.00–1.84) | 1.50 (0.99–2.28) | 1.50 (0.92–2.45) | 1.96 (1.03–3.73) | 3.44 (1.66–7.14) | 3.55 (1.69–11.25) | 3.20 (1.70–9.03) | 55.9 (37.9–72.8) | |
| Vac. 10 µg | 1.01 (0.85–1.20) | 1.21 (0.90–1.64) | 1.29 (0.85–1.96) | 1.57 (0.96–2.57) | 1.97 (1.03–3.78) | 4.19 (2.00–8.79) | 3.68 (1.61–15.33) | 5.60 (1.68–27.39) | 65.6 (46.8–81.4) | |
| Vac. 20 µg | 0.90 (0.75–1.07) | 0.93 (0.69–1.26) | 1.03 (0.68–1.56) | 1.15 (0.70–1.89) | 1.96 (1.02–3.78) | 6.12 (2.90–12.89) | 5.15 (1.66–25.79) | 5.61 (1.70–25.28) | 80.0 (61.4–92.3) | |
| Anti S1 antibody | Placebo | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14.8 (4.2–33.7) |
| Vac. 5 µg | 1.28 (1.08–1.52) | 1.36 (1.0–1.84) | 1.50 (0.99–2.28) | 1.50 (0.92–2.45) | 1.96 (1.03–3.73) | 3.46 (1.67–7.18) | 2.70 (0.73–9.97) | 3.04 (0.87–10.59) | 47.0 (29.8–64.9) | |
| Vac. 10 µg | 1.01 (1.08–1.20) | 1.21 (1.14–1.64) | 1.24 (0.82–1.88) | 1.57 (0.96–2.57) | 1.97 (1.03–3.78) | 4.22 (2.01–8.84) | 2.87 (0.87–9.52) | 5.61 (1.64–19.17) | 62.5 (43.7–78.9) | |
| Vac. 20 µg | 0.90 (0.75 -1.07) | 0.93 (0.69–1.26) | 1.03 (0.68–1.56) | 1.15 (0.70–1.89) | 1.96 (1.02–3.78) | 6.15 (2.92–12.97) | 4.20 (1.19–14.84) | 4.95 (1.40–17.45) | 80.0 (61.4–92.3) | |
| Anti S2 antibody | Placebo | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 11.1 (2.3–29.1) |
| Vac. 5 µg | 1.46 (1.09–1.95) | 1.49 (1.05–2.10) | 1.65 (1.05–2.61) | 1.73 (1.02–2.93) | 2.17 (1.10–4.30) | 4.04 (1.90–8.59) | 2.69 (1.67–6.82) | 3.17 (1.62–10.91) | 47.0 (29.8–64.9) | |
| Vac. 10 µg | 1.07 (0.80–1.44) | 1.26 (0.89–1.77) | 1.39 (0.88–2.20) | 1.67 (0.99–2.84) | 2.17 (1.09–4.34) | 4.89 (2.28–10.50) | 2.94 (1.60–9.78) | 4.40 (1.61–22.65) | 65.6 (46.8–81.4) | |
| Vac. 20 µg | 0.87 (0.65–1.16) | 0.95 (0.67–1.34) | 1.07 (0.68–1.68) | 1.25 (0.73–2.12) | 2.14 (1.06–4.30) | 6.98 (3.23–15.08) | 4.05 (1.64–16.61) | 4.68 (1.63–23.81) | 83.3 (65.3–94.3) | |
| Anti RBD antibody | Placebo | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14.8 (4.2–33.7) |
| Vac. 5 µg | 1.38 (1.13–1.68) | 1.45 (1.06–1.98) | 1.57 (1.00–2.47) | 1.43 (0.87–2.34) | 1.99 (1.06–3.76) | 3.40 (1.63–7.07) | 2.69 (1.68–6.82) | 3.38 (1.68–10.38) | 55.9 (37.9–72.8) | |
| Vac. 10 µg | 1.05 (0.86–1.27) | 1.21 (0.89–1.64) | 1.30 (0.83–2.05) | 1.57 (0.96–2.57) | 1.88 (0.99–3.58) | 4.07 (1.94–8.55) | 3.04 (1.60–10.49) | 5.98 (1.67–32.79) | 62.5 (43.7–78.9) | |
| Vac. 20 µg | 0.91 (0.75–1.11) | 1.01 (0.74–1.37) | 1.05 (0.67–1.65) | 1.13 (0.69–1.86) | 2.01 (1.05–3.84) | 5.88 (2.78–12.44) | 4.22 (1.65–17.99) | 5.75 (1.69–27.94) | 83.3 (65.3–94.3) | |
| Anti NTD antibody | Placebo | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14.8 (4.2–33.7) |
| Vac. 5 µg | 1.31 (1.08–1.60) | 1.40 (1.03–1.91) | 1.54 (1.01–2.37) | 1.50 (0.91–2.47) | 1.93 (1.01–3.67) | 3.24 (1.51–6.93) | 2.30 (1.71–4.71) | 3.44 (1.68–10.70) | 50.0 (32.4–67.6) | |
| Vac. 10 µg | 1.06 (0.87–1.29) | 1.21 (0.89–1.64) | 1.31 (0.85–2.00) | 1.62 (0.98–2.66) | 1.98 (1.03–3.81) | 4.23 (1.95–9.15) | 3.01 (1.63–9.49) | 5.75 (1.67–30.27) | 65.6 (46.8–81.4) | |
| Vac. 20 µg | 0.93 (0.76–1.13) | 1.01 (0.74–1.37) | 1.12 (0.73–1.71) | 1.24 (0.75–2.05) | 2.11 (1.09–4.09) | 6.67 (3.07–14.52) | 4.12 (1.68–15.49) | 5.52 (1.69–25.79) | 83.3 (65.3–94.3) | |
| Neutralyzing antibody cVNT | Placebo | 1 | - | - | - | - | 1 | 1 | 1 | 14.8 (4.2–33.7) |
| Vac. 5 µg | 1.08 (0.98–1.20) | - | - | - | - | 5.82 (1.46–23.13) | 0.95 (0.03–27.05) | 0.84 (0.02–28.22) | 58.8 (40.7–75.3) | |
| Vac. 10 µg | 1.00 (0.91–1.10) | - | - | - | - | 11.12 (2.74–45.09) | 31.11 (1.46–663.67) | 6.58 (0.17–259.11) | 68.7 (49.9–83.9) | |
| Vac. 20 µg | 1.00 (0.91–1.10) | - | - | - | - | 20.70 (5.05–84.76) | 17.46 (0.47–652.38) | 1.68 (0.03–82.75) | 83.3 (65.3–94.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodaran, M.S.; Banihashemi, S.R.; Es-haghi, A.; Mehrabadi, M.H.F.; Nofeli, M.; Mokarram, A.R.; Mokhberalsafa, L.; Sadeghi, F.; Ranjbar, A.; Ansarifar, A.; et al. Immunogenicity and Safety of a Combined Intramuscular/Intranasal Recombinant Spike Protein COVID-19 Vaccine (RCP) in Healthy Adults Aged 18 to 55 Years Old: A Randomized, Double-Blind, Placebo-Controlled, Phase I Trial. Vaccines 2023, 11, 455. https://doi.org/10.3390/vaccines11020455
Dodaran MS, Banihashemi SR, Es-haghi A, Mehrabadi MHF, Nofeli M, Mokarram AR, Mokhberalsafa L, Sadeghi F, Ranjbar A, Ansarifar A, et al. Immunogenicity and Safety of a Combined Intramuscular/Intranasal Recombinant Spike Protein COVID-19 Vaccine (RCP) in Healthy Adults Aged 18 to 55 Years Old: A Randomized, Double-Blind, Placebo-Controlled, Phase I Trial. Vaccines. 2023; 11(2):455. https://doi.org/10.3390/vaccines11020455
Chicago/Turabian StyleDodaran, Masoud Solaymani, Seyed Reza Banihashemi, Ali Es-haghi, Mohammad Hossein Fallah Mehrabadi, Mojtaba Nofeli, Ali Rezaei Mokarram, Ladan Mokhberalsafa, Fariba Sadeghi, Alireza Ranjbar, Akram Ansarifar, and et al. 2023. "Immunogenicity and Safety of a Combined Intramuscular/Intranasal Recombinant Spike Protein COVID-19 Vaccine (RCP) in Healthy Adults Aged 18 to 55 Years Old: A Randomized, Double-Blind, Placebo-Controlled, Phase I Trial" Vaccines 11, no. 2: 455. https://doi.org/10.3390/vaccines11020455
APA StyleDodaran, M. S., Banihashemi, S. R., Es-haghi, A., Mehrabadi, M. H. F., Nofeli, M., Mokarram, A. R., Mokhberalsafa, L., Sadeghi, F., Ranjbar, A., Ansarifar, A., Mohazzab, A., Setarehdan, S. A., Bagheri Amiri, F., Mohseni, V., Hajimoradi, M., Ghahremanzadeh, N., Razzaz, S. H., Masoomi, S., Taghdiri, M., ... Kalantari, S. (2023). Immunogenicity and Safety of a Combined Intramuscular/Intranasal Recombinant Spike Protein COVID-19 Vaccine (RCP) in Healthy Adults Aged 18 to 55 Years Old: A Randomized, Double-Blind, Placebo-Controlled, Phase I Trial. Vaccines, 11(2), 455. https://doi.org/10.3390/vaccines11020455





