The Impact of Social Determinants of Health on Meningococcal Vaccination Awareness, Delivery, and Coverage in Adolescents and Young Adults in the United States: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Screening Strategy
2.3. Data Extraction
2.4. Quality Assessment
2.5. Analysis
3. Results
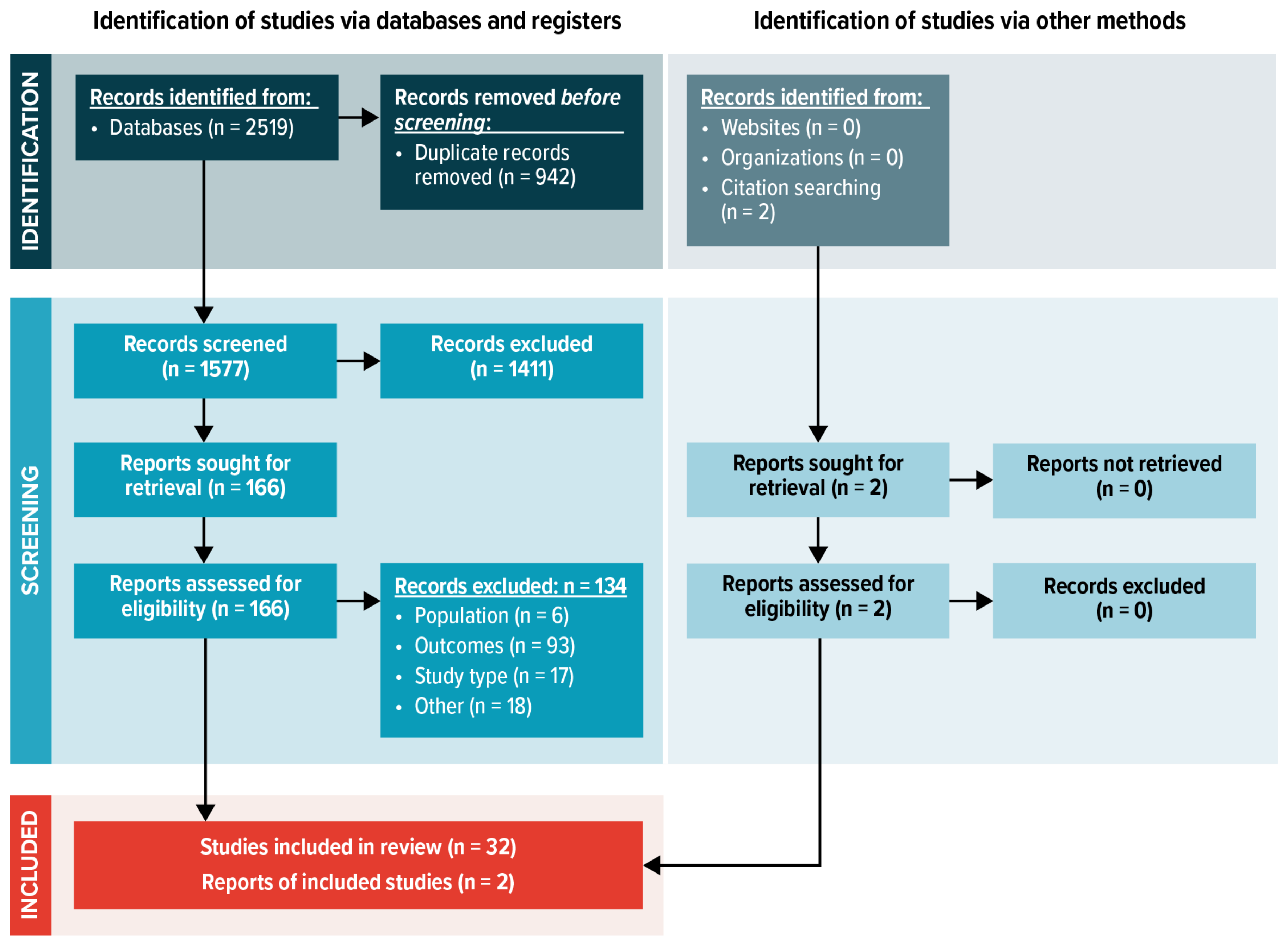
3.1. Search Results
3.2. Description of Included Studies
3.3. Quality Assessment
3.4. Race/Ethnicity, Geography, and Socioeconomic Status and MenACWY and MenB Vaccination Coverage
3.4.1. Race/Ethnicity and MenACWY and MenB Vaccine Coverage
3.4.2. Geographical Factors and MenACWY and MenB Vaccine Coverage
3.4.3. Socioeconomic Status and MenACWY and MenB Vaccine Coverage
3.5. The Impact of Health Insurance on MenACWY and MenB Vaccine Coverage
3.6. The Impact of Healthcare Access on MenACWY and MenB Vaccine Coverage
3.7. Healthcare Professionals’ Knowledge and Awareness of ACIP Meningococcal Vaccine Routine and Shared Decision-Making Recommendations and Disparities in Recommendations for MenACWY and MenB Vaccines
3.8. Parent/Guardian Knowledge and Awareness of Meningococcal Disease and Meningococcal Vaccines and the Impact on MenACWY and MenB Vaccine Coverage
3.9. Lack of Healthcare Professional Recommendations for Meningococcal Vaccination and the Impact on MenACWY and MenB Vaccine Coverage
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mbaeyi, S.A.; Bozio, C.H.; Duffy, J.; Rubin, L.G.; Hariri, S.; Stephens, D.S.; MacNeil, J.R. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–41. [Google Scholar] [CrossRef]
- Wang, B.; Santoreneos, R.; Giles, L.; Haji Ali Afzali, H.; Marshall, H. Case Fatality Rates of Invasive Meningococcal Disease by Serogroup and Age: A Systematic Review and Meta-analysis. Vaccine 2019, 37, 2768–2782. [Google Scholar] [CrossRef]
- CDC. Enhanced Meningococcal Disease Surveillance Report. 2019. Available online: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2019.pdf (accessed on 19 August 2022).
- Brigham, K.S.; Sandora, T.J. Neisseria meningitidis: Epidemiology, treatment and prevention in adolescents. Curr. Opin. Pediatr. 2009, 21, 437–443. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report. 2018. Available online: https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2018.pdf (accessed on 23 September 2022).
- Centers for Disease Control and Prevention. Meningococcal Disease: What Everyone Should Know. 2021. Available online: https://www.cdc.gov/vaccines/vpd/mening/public/index.html (accessed on 10 May 2022).
- BEXSERO. Package Insert. GlaxoSmithKline. 2015. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/bexsero (accessed on 22 November 2022).
- Trumenba. Package Insert. Wyeth Pharmaceuticals, Inc. 2021. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/trumenba (accessed on 22 November 2022).
- Menactra. Package Insert. Sanofi Pasteur, Inc. 2018. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/menactra (accessed on 22 November 2022).
- MENVEO. Package Insert. GlaxoSmithKline Biologicals SA. 2022. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/menveo (accessed on 22 November 2022).
- MenQuadfi. Package Insert. Sanofi Pasteur, Inc. 2022. Available online: https://www.fda.gov/vaccines-blood-biologics/menquadfi (accessed on 22 November 2022).
- Basta, N.E.; Becker, A.B.; Li, Q.; Nederhoff, D. Parental Awareness of Meningococcal B Vaccines and Willingness to Vaccinate Their Teens. Vaccine 2019, 37, 670–676. [Google Scholar] [CrossRef]
- Elwyn, G.; Frosch, D.; Thomson, R.; Joseph-Williams, N.; Lloyd, A.; Kinnersley, P.; Cording, E.; Tomson, D.; Dodd, C.; Rollnick, S. Shared decision making: A model for clinical practice. J. Gen. Intern. Med. 2012, 27, 1361–1367. [Google Scholar] [CrossRef]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Valier, M.R.; Fredua, B.; Crowe, S.J.; Stokley, S.; Singleton, J.A. National Vaccination Coverage among Adolescents Aged 13–17 Years—National Immunization Survey-Teen, United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1101–1108. [Google Scholar] [CrossRef]
- Fergie, J.; Howard, A.; Huang, L.; Srivastava, A. Implementation Experience with Meningococcal Serogroup B Vaccines in the United States: Impact of a Nonroutine Recommendation. Pediatr. Infect. Dis. J. 2021, 40, 269–275. [Google Scholar] [CrossRef]
- Bernstein, H.H.; Bocchini, J.A., Jr.; Committee on Infectious Diseases; Byington, C.L.; Maldonado, Y.A.; Barnett, E.D.; Campbell, J.D.; Davies, H.D.; Lynfield, R.; Munoz, F.M.; et al. The Need to Optimize Adolescent Immunization. Pediatrics 2017, 139, e20164186. [Google Scholar] [CrossRef]
- Marshall, G.S.; Fergie, J.; Presa, J.; Peyrani, P. Rationale for the Development of a Pentavalent Meningococcal Vaccine: A US-Focused Review. Infect. Dis. Ther. 2022, 11, 937–951. [Google Scholar] [CrossRef]
- Coyne-Beasley, T.; Reiter, P.L.; Liberty, A.C.; Ford, C.A.; Miles, D.R.; Brewer, N.T. Awareness Is not Enough: The Need to Increase Meningococcal Vaccine Uptake. Clin. Pediatr. 2013, 52, 441–450. [Google Scholar] [CrossRef]
- Taha, M.-K.; Martinon-Torres, F.; Kollges, R.; Bonanni, P.; Safadi, M.A.P.; Booy, R.; Smith, V.; Garcia, S.; Bekkat-Berkani, R.; Abitbol, V. Equity in Vaccination Policies to Overcome Social Deprivation as a Risk Factor for Invasive Meningococcal Disease. Expert. Rev. Vaccines 2022, 21, 659–674. [Google Scholar] [CrossRef]
- Pruitt, S.L.; Tiro, J.A.; Kepka, D.; Henry, K. Missed Vaccination Opportunities among U.S. Adolescents by Area Characteristics. Am. J. Prev. Med. 2022, 62, 538–547. [Google Scholar] [CrossRef]
- Cochrane Collaboration. Cochrane Methods: Equity. Evidence for Equity (E4E). 2022. Available online: https://methods.cochrane.org/equity/evidence-equity (accessed on 21 October 2022).
- Mbaeyi, S.; Pondo, T.; Blain, A.; Yankey, D.; Potts, C.; Cohn, A.; Hariri, S.; Shang, N.; MacNeil, J.R. Incidence of Meningococcal Disease before and after Implementation of Quadrivalent Meningococcal Conjugate Vaccine in the United States. JAMA Pediatr. 2020, 174, 843–851. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP Case Control Study Checklist. 2022. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 27 July 2022).
- Critical Appraisal Skills Programme. CASP Cohort Study Checklist. 2022. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 27 July 2022).
- Critical Appraisal Skills Programme. CASP Economic Evaluation Study Checklist. 2022. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 27 July 2022).
- Critical Appraisal Skills Programme. CASP Qualitative Studies Checklist. 2022. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 27 July 2022).
- Critical Appraisal Skills Programme. CASP Randomised Controlled Trial Checklist. 2022. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 27 July 2022).
- Critical Appraisal Skills Programme. CASP Systematic Review Checklist. 2022. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 27 July 2022).
- Niccolai, L.M.; Yakely, A.E.; Hansen, C.E. Up-to-Date Coverage with Meningococcal Vaccine among Adolescents Age 17 Years: Patterns and Correlates in the United States, 2017. Vaccine 2019, 37, 5934–5938. [Google Scholar] [CrossRef]
- Phillips, G., 2nd; Johnson, A.K.; Adames, C.N.; Mustanski, B. Meningitis Vaccination, Knowledge, and Awareness among YMSM in Chicago. Health Educ. Behav. 2018, 45, 607–615. [Google Scholar] [CrossRef]
- Kurosky, S.K.; Esterberg, E.; Irwin, D.E.; Trantham, L.; Packnett, E.; Novy, P.; Whelan, J.; Hogea, C. Meningococcal Vaccination Among Adolescents in the United States: A Tale of Two Age Platforms. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2019, 65, 107–115. [Google Scholar] [CrossRef]
- La, E.M.; Garbinsky, D.; Hunter, S.; Poston, S.; Novy, P.; Ghaswalla, P. Meningococcal B Vaccination Coverage among Older Adolescents in the United States. Vaccine 2021, 39, 2660–2667. [Google Scholar] [CrossRef]
- Packnett, E.R.; Zimmerman, N.M.; Kim, G.; Novy, P.; Morgan, L.C.; Chime, N.; Ghaswalla, P. A Real-world Claims Data Analysis of Meningococcal Serogroup B Vaccine Series Completion and Potential Missed Opportunities in the United States. Pediatr. Infect. Dis. J. 2022, 41, e158–e165. [Google Scholar] [CrossRef]
- Bart, S.M.; Eberhart, M.; Feemster, K. Impact of a Category B Recommendation: Meningococcal B (MenB) Vaccine Uptake Among Adolescents in Philadelphia County. 2018. Available online: https://plan.core-apps.com/pas2018/abstract/e5177900e60498db774eaebbee6695f0 (accessed on 19 July 2022).
- Watkins, E.; Feemster, K. Factors Associated with Uptake of Meningococcus B Vaccination after an ACIP Category B Recommendation. Open Forum Infect. Dis. 2018, 5, S737. [Google Scholar]
- Bernstein, S.; North, A.; Schwartz, J.; Niccolai, L.M. State-Level Voting Patterns and Adolescent Vaccination Coverage in the United States, 2014. Am. J. Pub. Health 2016, 106, 1879–1881. [Google Scholar] [CrossRef]
- Gowda, C.; Dong, S.; Potter, R.C.; Dombkowski, K.J.; Dempsey, A.F. A Population-Level Assessment of Factors Associated With Uptake of Adolescent-Targeted Vaccines in Michigan. J. Adolesc. Health 2013, 53, 498–505. [Google Scholar] [CrossRef]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; McNamara, L.A.; Stokley, S.; Singleton, J.A. National, Regional, State, and Selected Local Area Vaccination Coverage among Adolescents Aged 13–17 Years—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1183–1190. [Google Scholar] [CrossRef]
- Singer, D.C.; Davis, M.M.; Gebremariam, A.; Clark, S.J. Underinsurance for Recently Recommended Vaccines in Private Health Plans. J. Community Health 2012, 37, 1164–1167. [Google Scholar] [CrossRef]
- Gowda, C.; Dempsey, A.F. Medicaid Reimbursement and the Uptake of Adolescent Vaccines. Vaccine 2012, 30, 1682–1689. [Google Scholar] [CrossRef]
- Rees-Clayton, E.; Montgomery, J.P.; Enger, K.S.; Boulton, M.L. Trends in Michigan Early Adolescent Immunization: 2006–2008. Am. J. Pub. Health 2012, 102, 1735–1741. [Google Scholar] [CrossRef]
- Seib, K.; Underwood, N.L.; Gargano, L.M.; Sales, J.M.; Morfaw, C.; Weiss, P.; Murray, D.; Vogt, T.M.; DiClemente, R.J.; Hughes, J.M. Preexisting Chronic Health Conditions and Health Insurance Status Associated With Vaccine Receipt Among Adolescents. J. Adolesc. Health 2016, 58, 148–153. [Google Scholar] [CrossRef]
- Lu, P.-J.; Yankey, D.; Jeyarajah, J.; O’Halloran, A.; Fredua, B.; Elam-Evans, L.D.; Reagan-Steiner, S. Association of Health Insurance Status and Vaccination Coverage among Adolescents 13–17 Years of Age. J. Pediatr. 2018, 195, 256–262.e1. [Google Scholar] [CrossRef]
- Hansen, C.E.; Niccolai, L.M. Factors Associated With Receipt of Meningococcal B Vaccine Among United States Adolescents, National Immunization Survey-Teen, 2017–2018. J. Adolesc. Health 2021, 69, 769–773. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Immunization Schedules for 2022. Child and Adolescent Immunization Schedule. Recommendations for Ages 18 Years or Younger, United States. 2022. Available online: https://publications.aap.org/redbook/pages/Immunization-Schedules?_ga=2.14217719.1337953238.1665770613-519092411.1665770612?autologincheck=redirected?nfToken=00000000-0000-0000-0000-000000000000 (accessed on 14 October 2022).
- Cataldi, J.R.; Brewer, S.E.; Perreira, C.; Furniss, A.; Nederveld, A.; Suresh, K.; Williams, C.; O’Leary, S.T.; Dempsey, A.F. Rural Adolescent Immunization: Delivery Practices and Barriers to Uptake. J. Am. Board Fam. Med. 2021, 34, 937–949. [Google Scholar] [CrossRef]
- Ghaswalla, P.K.; Garbinsky, D.; Poston, S.; Hunter, S.; Novy, P.; La, E.M. Correlates of and Disparities in Meningococcal B Vaccination Coverage among 17-year-olds in the United States: A Pooled Analysis of 2016–2018 National Immunization Survey-Teen. Pediatrics 2021, 147, 296–298. [Google Scholar] [CrossRef]
- Kempe, A.; Allison, M.A.; MacNeil, J.R.; O’Leary, S.T.; Crane, L.A.; Beaty, B.L.; Hurley, L.P.; Brtnikova, M.; Lindley, M.C.; Liang, J.L.; et al. Knowledge and Attitudes Regarding Category B ACIP Recommendations among Primary Care Providers for Children. Acad. Pediatr. 2018, 18, 763–768. [Google Scholar] [CrossRef]
- Huang, L.; Goren, A.; Lee, L.K.; Li, V.W.; Dempsey, A.; Srivastava, A. Disparities in Healthcare Providers’ Interpretations and Implementations of ACIP’s Meningococcal Vaccine Recommendations. Hum. Vaccin. Immunother. 2020, 16, 933–944. [Google Scholar] [CrossRef]
- Kempe, A.; Allison, M.A.; MacNeil, J.R.; O’Leary, S.T.; Crane, L.A.; Beaty, B.L.; Hurley, L.P.; Brtnikova, M.; Lindley, M.C.; Albert, A.P. Adoption of Serogroup B Meningococcal Vaccine Recommendations. Pediatrics 2018, 142, e20180344. [Google Scholar] [CrossRef]
- Richardson, E.; Ryan, K.A.; Lawrence, R.M.; Harle, C.A.; Young, A.; Livingston, M.D.; Rawal, A.; Staras, S.A.S. Perceptions and Knowledge about the MenB Vaccine among Parents of High School Students. J. Comm. Health 2021, 46, 808–816. [Google Scholar] [CrossRef]
- Painter, J.E.; Viana De O Mesquita, S.; Jimenez, L.; Avila, A.A.; Sutter, C.J.; Sutter, R. Vaccine-Related Attitudes and Decision-Making Among Uninsured, Latin American Immigrant Mothers of Adolescent Daughters: A Qualitative Study. Hum. Vacc. Immunother 2019, 15, 121–133. [Google Scholar] [CrossRef]
- Srivastava, A.; Dempsey, A.; Galitsky, A.; Fahimi, M.; Huang, L. Parental Awareness and Utilization of Meningococcal Serogroup B Vaccines in the United States. BMC Public Health 2020, 20, 1109. [Google Scholar] [CrossRef]
- Greenfield, L.S.; Page, L.C.; Kay, M.; Li-Vollmer, M.; Breuner, C.C.; Duchin, J.S. Strategies for Increasing Adolescent Immunizations in Diverse Ethnic Communities. J. Adolesc. Health 2015, 56, S47–S53. [Google Scholar] [CrossRef]
- Kricorian, K.; Lopez, D.; Seu, M.; Pham, T.; Kigoonya, R.; Equils, O. The Role of Health Literacy in Vaccination Disparities: Do Patients Understand the Health Messages? Open Forum Infect. Dis. 2020, 7, S44. [Google Scholar] [CrossRef]
- Dorell, C.; Yankey, D.; Kennedy, A.; Stokley, S. Factors That Influence Parental Vaccination Decisions for Adolescents, 13 to 17 Years Old: National Immunization Survey-Teen, 2010. Clin. Pediatr. 2013, 52, 162–170. [Google Scholar] [CrossRef]
- Gargano, L.M.; Herbert, N.L.; Painter, J.E.; Sales, J.M.; Morfaw, C.; Rask, K.; Murray, D.; DiClemente, R.J.; Hughes, J.M. Impact of a Physician Recommendation and Parental Immunization Attitudes on Receipt or Intention to Receive Adolescent Vaccines. Hum. Vacc. Immunother. 2013, 9, 2627–2633. [Google Scholar] [CrossRef]
- Moss, J.L.; Reiter, P.L.; Rimer, B.K.; Brewer, N.T. Collaborative Patient-Provider Communication and Uptake of Adolescent Vaccines. Soc. Sci. Med. 2016, 159, 100–107. [Google Scholar] [CrossRef]
- Darden, P.M.; Thompson, D.M.; Roberts, J.R.; Hale, J.J.; Pope, C.; Naifeh, M.; Jacobson, R.M. Reasons for not Vaccinating Adolescents: National Immunization Survey of Teens, 2008–2010. Pediatrics 2013, 131, 645–651. [Google Scholar] [CrossRef]
- Healy, J.; Rodriguez-Lainz, A.; Elam-Evans, L.D.; Hill, H.A.; Reagan-Steiner, S.; Yankey, D. Vaccination Coverage Among Foreign-Born and U.S.-Born Adolescents in the United States: Successes and Gaps—National Immunization Survey-Teen, 2012–2014. Vaccine 2018, 36, 1743–1750. [Google Scholar] [CrossRef]
- Hogue, M.D.; Foster, S.; Rothholz, M.C. Shared Clinical Decision Making on Vaccines: Nothing has Really Changed for Pharmacists. J. Am. Pharm. Assoc 2020, 60, e91–e94. [Google Scholar]
- C.S. Mott Children’s Hospital. Mott Poll Report. Parents Not Keeping up with Teen Vaccines. Volume 29. Issue 4. 2017. Available online: https://mottpoll.org/sites/default/files/documents/071717_teenvaccines.pdf (accessed on 24 October 2022).
- Johnson, R.; Fairchild, A.; Whittington, D.; Presa, J.; Srivastava, A.; Huang, L. Understanding Patient Preferences for Meningococcal Serogroup B Vaccines in the United States. Open Forum Infect. Dis. 2020, 7, S23. [Google Scholar] [CrossRef]
- Alderfer, J.T.; Moran, M.M.; Srivastava, A.; Isturiz, R.E. Meningococcal Vaccination: A Discussion with all Adolescents, Whether College-Bound or Not. Postgrad Med. 2019, 131, 551–554. [Google Scholar] [CrossRef]
- Soeters, H.M.; McNamara, L.A.; Blain, A.E.; Whaley, M.; MacNeil, J.R.; Hariri, S.; Mbaeyi, S.A.; Serogroup, B.M.D.U.O.G. University-Based Outbreaks of Meningococcal Disease Caused by Serogroup B, United States, 2013–2018. Emerg. Infect. Dis. 2019, 25, 434–440. [Google Scholar] [CrossRef]
- Atkinson, B.; Gandhi, A.; Balmer, P. History of Meningococcal Outbreaks in the United States: Implications for Vaccination and Disease Prevention. Pharmacotherapy 2016, 36, 880–892. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Report Emerging Infections Program Network, Neisseria Meningitidis. 2019. Available online: https://www.cdc.gov/abcs/downloads/NMEN_Surveillance_Report_2019.pdf (accessed on 21 August 2022).
- Mbaeyi, S.A.; Blain, A.; Whaley, M.J.; Wang, X.; Cohn, A.C.; MacNeil, J.R. Epidemiology of Meningococcal Disease Outbreaks in the United States, 2009–2013. Clin. Infect. Dis. 2019, 68, 580–585. [Google Scholar] [CrossRef]
- National Meningitis Association. Meningococcal Disease on U.S. College Campuses, 2013–2019. Available online: https://nmaus.org/wp-content/uploads/2019/04/Alembic_NMA_Map_r28.pdf (accessed on 14 November 2022).
- Talbird, S.E.; Carrico, J.; La, E.M.; Carias, C.; Marshall, G.S.; Roberts, C.S.; Chen, Y.T.; Nyaku, M.K. Impact of Routine Childhood Immunization in Reducing Vaccine-Preventable Diseases in the United States. Pediatrics 2022, 150, e2021056013. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; National Center for Immunization and Respiratory Diseases. NIS-Teen Data—Adolescents/Teens (13–17 years). 2015. Available online: https://www.cdc.gov/vaccines/imz-managers/coverage/nis/teen/index.html (accessed on 19 October 2022).
- Boyd, R.W.; Lindo, E.G.; Weeks, L.D.; McLemore, M.R. On Racism: A New Standard for Publishing on Racial Health Inequities. 2020. Available online: https://www.healthaffairs.org/do/10.1377/forefront.20200630.939347 (accessed on 24 October 2022).
- Rubin, V.; Ngo, D.; Ross, A.; Butler, D.; Balaram, N. Counting a Diverse Nation: Disaggregating Data on Race and Ethnicity to Advance a Culture of Health. 2018. Available online: https://www.policylink.org/resources-tools/counting-a-diverse-nation (accessed on 1 December 2022).
- Agency for Healthcare Research and Quality. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. 2018. Available online: https://www.ahrq.gov/research/findings/final-reports/iomracereport/index.html (accessed on 21 November 2022).
- Williams, D.R.; Lawrence, J.A.; Davis, B.A. Racism and Health: Evidence and Needed Research. Annu. Rev. Public Health 2019, 40, 105–125. [Google Scholar] [CrossRef]
- Smedley, B.D.; Stith, A.Y.; Nelson, A.R. (Eds.) Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Brodie, N.; Metzenberg, G.E.; Silberholz, E.A. A Clinical Update on Vaccines: Focus on Determinants of Under-Immunization and Special Considerations for Adolescents. Curr. Opin. Pediatr. 2020, 32, 328–335. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masaquel, C.; Schley, K.; Wright, K.; Mauskopf, J.; Parrish, R.A.; Presa, J.V.; Hewlett, D., Jr. The Impact of Social Determinants of Health on Meningococcal Vaccination Awareness, Delivery, and Coverage in Adolescents and Young Adults in the United States: A Systematic Review. Vaccines 2023, 11, 256. https://doi.org/10.3390/vaccines11020256
Masaquel C, Schley K, Wright K, Mauskopf J, Parrish RA, Presa JV, Hewlett D Jr. The Impact of Social Determinants of Health on Meningococcal Vaccination Awareness, Delivery, and Coverage in Adolescents and Young Adults in the United States: A Systematic Review. Vaccines. 2023; 11(2):256. https://doi.org/10.3390/vaccines11020256
Chicago/Turabian StyleMasaquel, Catherine, Katharina Schley, Kelly Wright, Josephine Mauskopf, Ronika Alexander Parrish, Jessica Vespa Presa, and Dial Hewlett, Jr. 2023. "The Impact of Social Determinants of Health on Meningococcal Vaccination Awareness, Delivery, and Coverage in Adolescents and Young Adults in the United States: A Systematic Review" Vaccines 11, no. 2: 256. https://doi.org/10.3390/vaccines11020256
APA StyleMasaquel, C., Schley, K., Wright, K., Mauskopf, J., Parrish, R. A., Presa, J. V., & Hewlett, D., Jr. (2023). The Impact of Social Determinants of Health on Meningococcal Vaccination Awareness, Delivery, and Coverage in Adolescents and Young Adults in the United States: A Systematic Review. Vaccines, 11(2), 256. https://doi.org/10.3390/vaccines11020256





