Abstract
Glycoconjugate vaccines have proven their worth in the protection and prevention of infectious diseases. The introduction of the Haemophilus influenzae type b vaccine is the prime example, followed by other glycoconjugate vaccines. Glycoconjugate vaccines consist of two components: the carrier protein and the carbohydrate antigen. Current carrier proteins are tetanus toxoid, diphtheria toxoid, CRM197, Haemophilus protein D and the outer membrane protein complex of serogroup B meningococcus. Carbohydrate antigens have been produced mainly by extraction and purification from the original host. However, current efforts show great advances in the development of synthetically produced oligosaccharides and bioconjugation. This review evaluates the advances of glycoconjugate vaccines in the last five years. We focus on developments regarding both new carriers and antigens. Innovative developments regarding carriers are outer membrane vesicles, glycoengineered proteins, new carrier proteins, virus-like particles, protein nanocages and peptides. With regard to conjugated antigens, we describe recent developments in the field of antimicrobial resistance (AMR) and ESKAPE pathogens.
1. Introduction
Vaccines have proven to be one of the most effective applications to prevent and protect against infectious diseases. As such, society relies heavily on existing vaccines, but also on the development of new vaccines and vaccine platforms. It has already been more than five centuries since the first strategies to battle infectious diseases were implemented [1]. However, it was not until the late 18th century that Jenner came up with his strategy to battle smallpox, utilizing live-attenuated viruses [1]. In the 19th century, Pasteur developed a successor in the form of a live-attenuated vaccine protecting against rabies. New and more efficient production platforms were established when viruses, such as influenza, mumps, measles, rubella, and polio, were propagated on cells and bacteria, such as Clostridium tetani and Corynebacterium diphtheriae, were isolated and grown in a culture medium for the production of toxoid vaccines (tetanus and diphtheria). The very first concepts for glycoconjugates were already evaluated in the early 1930s by Avery and Goebel [2]. However, the first conjugate vaccine targeting Haemophilus influenzae type b (Hib) was developed around 1985 [3,4], based on extracted polysaccharides (PS). Glycoconjugate vaccines appeared to be an important vaccine technology platform. Utilizing the PS is important since they aid bacteria in their ability to infect individuals and to evade the immune system and trigger an inflammatory response. Glycoconjugate vaccines consist of the extracted and purified PS alone or covalent attachment (conjugate) of these PS to carrier proteins. Vaccines containing purified PS alone do work, but they can only stimulate B-cells to produce antibodies and no immunological memory response. There are certain benefits in using a carrier protein with the PS conjugated to a carrier. Conjugation to a carrier protein significantly improves the immunogenicity towards a T-cell-dependent response to the PS [5,6]. This has been shown for PS originating from the capsular structures of Hib, Streptococcus pneumoniae, Neisseria meningitidis and Salmonella Typhi [7,8]. For children under the age of two, these conjugate vaccines are extremely effective, which is fortunate since the disease burden of encapsulated bacteria at that age is high [9,10]. Long-lasting immunity is induced by the conjugation to a carrier protein, triggering the immune system towards a T-cell-dependent immune response [11].
The use of conjugate vaccines has had some undesirable effects. Pichichero (2013) [12] and Broker (2017) [13] describe the interference of immunogenicity thoroughly and refer to two underlying mechanisms: antigen competition and carrier-induced epitope suppression (CIES). Antigen competition is caused by the processing of antigens in combination vaccines. On these specific occasions, a protein (e.g., tetanus toxoid) is co-administered as an independent vaccine constituent (against tetanus) in combination with a conjugate vaccine using the same protein, but now as a carrier (e.g., DaKTP-Hib-HepB). CIES is attributed to a pre-existing vaccination using the carrier protein as the single vaccine component as well as the carrier for the conjugate vaccine or simply co-administration. It became apparent that there was a diminished immune response observed towards the conjugated polysaccharide antigen [14,15,16,17]. CIES is difficult to predict for any particular glycoconjugate vaccine. Based on immunological data from pre-clinical animal studies, it is still difficult to estimate the outcome of a clinical study. The introduction of new and different carriers originating from the same species as the immunogen could potentially circumvent poor immunogenicity. It could also induce a broader application for targeting multiple serotypes [18] and would at least have the potential to partially solve these issues attributed to antigen competition and CIES. The selection and development of carriers and PS have led to exciting combinations and innovative approaches. In this review article, we provide an overview of recent innovations in the development of novel conjugate vaccines. The vaccine carriers include new proteins, virus-like particles (VLP), (glycoengineered) Outer Membrane Vesicles (OMVs) and proteins nanocages and peptides. Furthermore, this review discusses the latest development of the antigenic parts of a conjugate vaccine, aimed to protect against AMR-designated ESKAPE pathogens.
2. Conjugate Vaccine Carriers
The choice of a carrier for conjugate vaccines has to take certain criteria into account, of which a proven track and safety record is the most important. The first traditional carrier proteins utilized were, in fact, toxoids produced from tetanus and diphtheria toxins. Even though chemical detoxification of these toxins can lead to potential heterogeneity and unwanted modifications, they have proven to be safe and efficacious. A second selection criterium was that these carriers could be produced at a large scale under GMP conditions. Several other additional criteria cannot be disregarded. New carriers will also have to be evaluated for their immunogenicity after conjugation, reproducible manufacturing and sufficient surface exposure of reactive amino acids for conjugation, solubility, and stability. Extensive physicochemical and immunological characterization is important for the selection of a carrier. Moreover, immunological responses of new carrier proteins have to be evaluated.
This information will be assessed by regulatory agencies, such as WHO, EMA or FDA. Additionally, when the carrier is also used as an antigen itself, it is key to evaluate at which site conjugation is performed. Here, the conjugation of antigens to potential B-cell epitopes can interfere with or reduce the immune response towards the carrier antigen. With the previously described CIES, the selection and development of new carrier proteins beyond the traditional currently licensed carriers seems inevitable. In the next sections, we discuss the traditional carrier proteins for glycoconjugate vaccines followed by a detailed evaluation of new carrier proteins.
2.1. Traditional Protein Carriers
The currently used carrier proteins in licensed vaccines are tetanus toxoid (TT), diphtheria toxoid (DT), CRM197, Haemophilus protein D (PD) and the outer membrane protein complex of serogroup B meningococcus (OMPC). TT and DT are the first protein carriers used for Hib conjugate vaccines due to their safety profile. The Hib conjugate vaccines were administered as stand-alone vaccines. They are derived from the chemical detoxification of bacterial toxins. CRM197 is a non-toxic mutant due to the detoxification of diphtheria toxin by means of genetic mutation. A single glycine to glutamic amino acid substitution at position 52 resulted in a non-toxic mutant [19]. There are multiple licensed vaccines using CRM197, of which Hib, multivalent meningococcal and pneumococcal conjugate vaccines are prime examples [20,21]. PD (cell–surface protein) are applied in a multivalent pneumococcal vaccine [22,23] and in an OMPC-based vaccine for both Hib and pneumococcal vaccines [24,25].
2.2. Other Protein Carriers
The groups of Broker et al. (2017) [13] and Micoli et al. (2018) [26] have given an overview of investigated carrier proteins actively applied in the field including analytical characterization criteria. A prime example was the recombinant non-toxic form of Pseudomonas aeruginosa exotoxin A (rEPA) used as a carrier for Escherichia coli [27,28], Shigella O-antigens, Staphylococcus aureus type 5 and 8 capsular PS and the S. Typhi Vi antigen [29,30,31,32]. Other examples included the pneumococcal recombinant proteins spr96/2021 and spr1875 [33]; proteins originating from the extra-intestinal pathogenic E. coli-derived Upec-5211 and Orf3526 [33]; a recombinant protein comprising of filaments of promiscuous human CD4+ T-cell epitopes [34,35]; and the recombinant tetanus toxin HC fragment [36]. Furthermore, they discuss several examples of synthetic peptide-based carriers, including the best-known example, a 13 amino acids non-natural pan DR (PADRE) universal helper T-lymphocyte epitope [37,38,39,40,41,42,43]. Lastly, they provide data on the use of proteins with the dual role of both carrier and antigen (detoxified pneumococcal-based pneumolysin [44], S. aureus-derived recombinant proteins [45], Clostridioides difficile toxin fragments [46], Group B Streptococcus pili proteins GBS80 and GBS67 [47,48] and flagellin as the carrier protein for Salmonella enteritidis O-antigen [49]) and the application of nanoparticles (KLH [50], Qβ virus-like particles [51] and glycoengineered outer membrane vesicles [52]).
2.3. Recent Developments Regarding Protein Carriers
In this section, we provide an overview of developments with respect to the application of different carrier proteins in the field of prophylactic vaccines in the last five years. A multitude of different proteins and other types of carriers have been investigated. Even though the traditional carrier proteins and other potential carriers (liposomes, polymers, inorganic gold particles, dendrimers and nanodiscs) are still very much applicable in the development of conjugate vaccines, here we have focused on six new and different types of carriers including: (1) Outer membrane vesicles (OMV) and generalized modules for membrane antigens (GMMA), (2) glycoengineered proteins and OMVs, (3) proteins, (4) virus-like particles (VLP), (5) protein nanocages and (6) peptides (Figure 1).
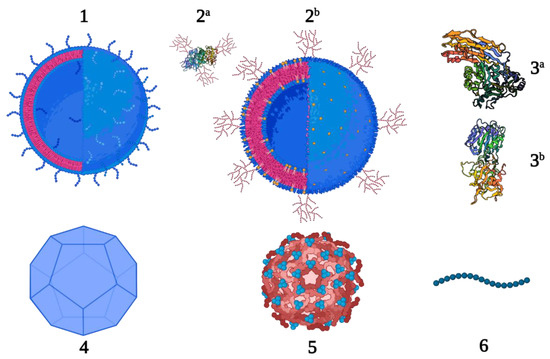
Figure 1.
New carrier proteins for conjugate vaccines; OMV and GMMA (1), glycoengineered proteins (2a P. aeruginosa exotoxin A, PDB 1IKQ) and OMV (2b), proteins (3a Streptococcus pyogenes streptolysin O, PDB 4HSC and 3b receptor-binding fragment HC of tetanus neurotoxin, PDB 1AF9), VLP (4), protein nanocages (5) and peptides (6).
2.3.1. Outer Membrane Vesicles/Generalized Modules for Membrane Antigens
The use of outer membrane vesicles (OMV) or generalized modules for membrane antigens (GMMA) provides a versatile and flexible platform for the development of monovalent or multivalent conjugate vaccines [53]. This platform is an excellent basis as a carrier and can be produced from a multitude of different bacteria [54]. The safety profiles of OMVs and GMMAs have been studied in several clinical studies [55,56,57]. Additionally, they are of particular interest for their self-adjuvant properties, governed by the presence of pathogen-associated molecular patterns (PAMPS), such as lipooligosaccharide (LOS) and lipoproteins [58,59,60]. Due to these characteristics, there is less need for the addition of adjuvants, e.g., aluminum hydroxide. In comparison to smaller carries, the sheer size of OMVs and GMMAs (50–200 nm) with their large surface area bearing a variety of properties, such as hydrophobicity, charge and the potential for receptor interaction, provide a significant constructive effect on the uptake by APCs [61]. Finally, a major benefit is that they can be efficiently manufactured using high-yielding production platforms [62,63]. Several groups provide ample data on the successful application of OMV, and GMMA as a carrier (Table 1). The group of Micoli et al. has reported extensively on their GMMA carriers originating from different pathogens and conjugating various polysaccharide and protein antigens [53,64]. Palmieri et al. have investigated conjugates using GMMAs originating from S. enterica and conjugating Group A Streptococcus cell wall oligosaccharide [65], whereas Scaria et al. describe the application of OMV, a membrane vesicle derived from N. meningitidis as the carrier for a malaria transmission-blocking antigen.

Table 1.
OMV/GMMA conjugation strategies.
2.3.2. Glycoengineered OMVs and Proteins
Where traditional glycoconjugate vaccines rely on the extracted or synthetically designed saccharides being chemically conjugated to a carrier protein, in vivo protein glycan coupling technology (PGCT) is achieved through recombinant enzymatic construction of a glycoconjugate directly in bacteria [69]. These glycoconjugates consist of a carrier protein and a carbohydrate moiety originating from the bacterial capsule or the O-antigen polysaccharide (O-PS) [70]. A prerequisite here is that the O-PS structure has been identified, including the gene cluster responsible for its production. PGCT is based on the Campylobacter jejuni N-linked glycosylation system, which can be expressed in E. coli. Moreover, the E. coli mutant is capable of producing heterologous polysaccharides on its glycosyl carrier lipid [71]. The final construction of the glycoconjugate requires the presence of genome clusters of the bacterial polysaccharide, which is achieved by a plasmid encoding for the carrier protein, including oligosaccharyl transferase (OTase; characteristically originating from PglB from C. jejuni [71]. Bioconjugation in general leads to heterogeneously expressed glycoproteins. The polysaccharides themselves show polydispersity, and the number of carbohydrate repeat units can vary significantly. Additionally, the conjugation rate, or quantity of polysaccharides conjugated to one single carrier protein, varies as well. For these reasons, the structure elucidation of the conjugate is not evident and requires several methods [72,73]. Next to this, there are some other challenges, such as OTases, that are not fully compatible with most bacterial O-SP, resulting in even more heterogeneous bioconjugates. Nevertheless, PGCT does not need intermediate steps, such as glycan synthesis, purification, conjugation, and process controls. As such, PGCT is potentially more cost-effective. This new and exciting field of conjugate vaccines is already well studied (Table 2). Nicolardi et al. describe the development of a protein-based glycoengineered vaccine using the exotoxin A from P. aeruginosa as a carrier targeting E. coli serotypes O2, O6A and O25B [72]. Harding et al. report (1) on a polyvalent pneumococcal bioconjugate vaccine using the natural acceptor protein ComP as a vaccine carrier decorated with S. pneumoniae CPSs [74] and (2) K. pneumoniae K1 and K2 serotypes produced in glycoengineered E. coli.

Table 2.
Glycoengineered conjugation strategies.
2.3.3. Protein-Based Carriers
Antigen competition and CIES pose a problem for the inclusion of antigens with respect to their use of vaccines. For this reason, the introduction of new protein carriers that improve upon the traditional carriers is much needed. Recent developments in conjugate vaccine design include proteins from both homologues and heterologous origin as carriers of conjugated antigens. From this, either a monovalent or bivalent (combining two target antigens) approach is used to target specific pathogens (Table 3). Wang et al. targeted S. pyogenes (Group A Streptococcus, GAS) cell wall oligosaccharides (GAC) conjugated to the streptococcal C5a peptidase carrier as a bivalent conjugate vaccine [82,83]. Furthermore, Kapoor et al. also target the GAC oligosaccharides from GAS. However, they found an application in the use of the secreted toxin antigen streptolysin O (SLO) as the protein carrier [84]. Additionally, Romero-Saavedra et al. described the use of two enterococcal proteins (secreted antigen A and the peptidyl-prolyl cis-trans isomerase) as a carrier for the Enterococcus faecalis polysaccharide di-heteroglycan [85].

Table 3.
Protein-based conjugation strategies.
2.3.4. Virus-Like Particles
Virus-like particles (VLPs) are part of the family of subunit vaccines. VLPs are formed by means of viral capsid protein self-assembly. The formed particles mimic the original virus but cannot replicate or induce an infection. This is an advantage with respect to the risks associated with live-attenuated vaccines [98]. Next to serving as a vaccine component themselves, VLPs also provide a platform as a carrier. Similar to chemical conjugation, glycoengineered proteins and OMVs, protein display can be used for VLPs to present heterologous protein antigens (Table 4). An example is reported by Basu et al., who in one study, conjugated two Zika virus proteins (MS2 and PP7) and, in another study, coupled multiple synthetic peptides mimicking Chikungunya virus B-cell epitopes to a canonical ssRNA phage Qβ VLP (Qβ) [99,100]. Warner et al. used the same strategy, but conjugated synthetic peptides originating from the Dengue virus to the Qβ VLP carrier [101]. More recently, Zha et al. demonstrated that a recombinantly expressed SARS-CoV-2 receptor-binding domain (RBD) can be conjugated to VLPs, derived from the cucumber mosaic virus [102]. Carbral-Miranda et al. used the cucumber mosaic virus VLP carrier to successfully conjugate a Zika virus E-DIII protein [103].

Table 4.
VLP conjugation strategies.
2.3.5. Protein Nanocages
A comprehensive overview of the application of nanocages is given by Curley et al. [109]. Nanocages are constructed from non-viral protein subunits and have similarities to VLPs. A number of these subunits can self-assemble into nanocages. They form symmetrical structures that differ from VLPs in terms of shape and size [110]. Nanocages contain repetitive structures recognized by B-cell receptors. One specific example where nanocages are utilized as a carrier for vaccine development is ferritin-based nanocages. These ferritin modules derived from Helicobacter pylori assemble into a 12 nm diameter spherical cage and are composed of 24 subunits with a hollow core [111]. An interesting aspect here is the application of the innovative SpyCatcher-SpyTag conjugation approach as described by Chen et al. [112] and Wang et al. [113]. This method of protein ligation is based on the modified domain of the S. pyogenes surface protein (SpyCatcher) that specifically recognizes a 13-amino-acid peptide (SpyTag). A covalent isopeptide bond is formed between the side chain of lysine in the SpyCatcher and aspartate in the SpyTag. This results in covalently linked protein complexes [114]. Several vaccine targets have been investigated that use protein carrier nanocages (Table 5), including hemagglutinin from the influenza virus [115] and the preS1 protein from hepatitis B [113].

Table 5.
Protein nanocages conjugation strategies.
2.3.6. Peptides
Peptide-based vaccines are a type of subunit vaccine predominantly comprised of short (single-epitope) sequences that can be produced synthetically. These types of vaccines have several advantages, such as their good safety profile, fast and simple manufacturability at a large-scale and high purity [117]. Additionally, designing peptide vaccines combining multiple single epitopes and creating synthetic long peptides into recombinant overlapping peptide vaccines could broaden their applications and effectiveness [118,119]. In most cases, the antigenic peptide has to be equipped with an adjuvant, e.g., the universal T-cell epitope PADRE, and also with polyleucine as a hydrophobic solubilizing moiety in order for them to be useable. Several exemplary applications are described in Table 6.

Table 6.
Peptide conjugation strategies.
3. Conjugate Vaccine Antigens
Since the introduction of the first carbohydrate conjugate vaccine targeting H. influenzae type b [4], conjugate vaccines have been developed for many different applications in the prophylactic domain to prevent infectious diseases, such as pneumonia, meningitis and sepsis. Currently, there are close to twenty conjugate vaccines on the market, licensed by the FDA, EMA and WHO. These vaccines are all based on the chemical conjugation of capsular polysaccharides targeted against Hib, N. meningitidis serogroup ACWY, S. pneumoniae or S. Typhi. Recent developments show conjugate vaccines targeting other pathogens such as a multivalent Extraintestinal pathogenic E. coli bioconjugate (ExPEC), Klebsiella O-antigen based, Group B Streptococcus, other serotypes of S. pneumoniae, PNAG (N. meningitidis, S. aureus, Neisseria gonorrhea, Klebsiella pneumoniae, E. coli and S. pneumoniae), Shigella flexneri 2a and others [124,125].
Next to the area of infectious diseases, carbohydrate-based conjugate vaccines find their application in the therapeutic domain targeting different forms of cancer. Often these vaccines are based on synthetic moieties mimicking tumor-associated carbohydrate antigens (TACAs) [126] including protein-linked Tn, Sialyl-Tn (STn) and TF (conjugated to the -OH group of serine) and glycolipid-based gangliosides GM2, GD2, GD3, fucosyl-GM1, Globo-H and Lewisy. The advantages of vaccination and targeting these TACAs include the significant reduction in toxicity and invasiveness compared to cytotoxic therapies such as radio- and chemotherapy [127]. Soriaul et al., Shivatare et al. and Hossain et al. gave an excellent overview of the current field of carbohydrate-based cancer vaccines. This included potential carrier proteins, adjuvants, and modifications of TACA antigens to improve stability, reduce hydrolytic cleavage and the cost of large-scale chemical or enzymatical synthesis [128,129,130]. Another area where carbohydrate-based conjugate vaccines find their application is the field of neurodegenerative diseases such as Alzheimer’s disease [131]. Overall, it cannot be denied that conjugate vaccines find their use in the broadest sense stretching multiple disease areas.
Despite the broad potential, the importance of the battle against antimicrobial resistance pathogens (AMR) cannot be overstated. Resistance to antibiotics can occur naturally in bacteria. However, the main reason for AMR is related to incompliance with respect to dosage and in adherence with the duration of the treatment. If antibiotic resistance would confine itself to a single pathogenic species or antibiotic, it would potentially still be manageable. At this moment, several bacteria show multidrug resistance (MDR), meaning they are resistant to multiple antibiotics. MDR is even more problematic specifically due to bacteria’s abilities to transfer AMR-related genes between different species (Table 7).

Table 7.
AMR for each of the ESKAPE pathogens.
The so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) have developed MDR against macrolides, lipopeptides, fluoroquinolones, oxazolidinones, β-lactams, tetracyclines, β-lactam–β-lactamase inhibitor combinations and against the last line of defense antibiotics such as glycopeptides, carbapenems and polymyxins [132]. Murray et al. (2019) estimated that the global burden of AMR included 23 pathogens and 88 pathogen–drug combinations spreading across 204 territories and countries. They attributed 1.27 million deaths directly to AMR and even a staggering 4.95 million deaths associated with AMR. This makes AMR the third largest global killer due to an infectious agent after COVID-19 and tuberculosis [133]. Now, looking past the COVID-19 pandemic, AMR can be classified as the new overlooked pandemic [134,135]. WHO and CDC have produced priority lists on which AMR targets have been classified (Figure 2 [136,137]).
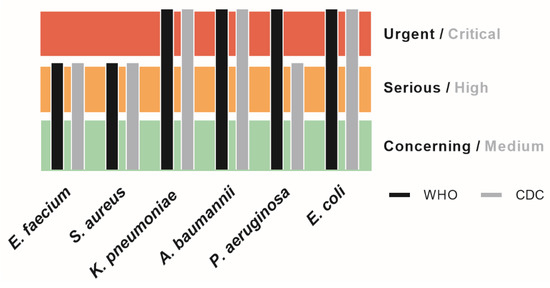
Figure 2.
Priority list scaling for the ESKAPE pathogens according to both CDC and WHO.
A collaborative study performed in 2019 (European Antimicrobial Resistance Collaborators, EARC) provided confirmational data that six of the ESKAPE pathogens that are at the top of AMR list, are each responsible for more than 25.000 associated deaths in Europe in 2019 alone (Figure 3 [138]). While the list extends much further than the six ESKAPE pathogens alone, both agencies rate all the ESKAPE pathogens at the top of their priority list.
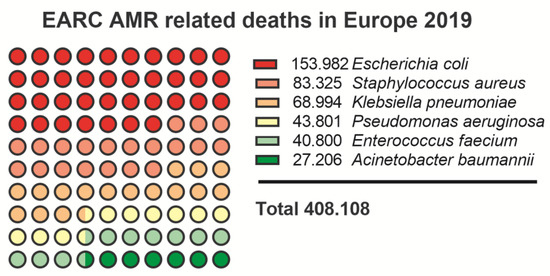
Figure 3.
EARC nr. of deaths caused by ESKAPE pathogens in 2019 [138].
Glycoconjugate vaccines have proven to be successful in the battle against infectious diseases and potential AMR targets. Current developments show that substantial progress is made in the development of conjugate vaccines applying complex glycans obtained by either extraction, chemical synthesis or through bioconjugation. Here, we provide an overview of the individual AMR challenges for each of the ESKAPE pathogens and the recent applications of carbohydrate conjugate vaccines targeting AMR in the last 5 years.
3.1. Enterococcus faecium
E. faecium is a Gram-positive commensal bacterium that is associated with severe infections in immunocompromised populations. Infections are persistent in the hospital environment with common hospital-acquired infections (bloodstream and urinary tract infections) in patients suffering from other underlying conditions. Up to 30% of all healthcare-related enterococcal infections are resistant to vancomycin, with increasing resistance towards other antibiotics [136,137]. Primary targets for vaccine development include capsular polysaccharide, cell wall teichoic acid and lipoteichoic acid. Another polysaccharide which is anchored to the peptidoglycan layer with possible immunogenic properties is the enterococcal polysaccharide antigen [139].
Kalfopoulou et al. provided data on two highly specific antibodies directed against the polysaccharide and carrier protein. Both antibodies showed good opsonic killing against target bacterial strains in upwards of 40% for E. faecium and 90% for E. faecalis respectively [140]. Romero-Saavedra et al. showed similar results with antibodies against the corresponding conjugate, as well as against the respective protein and carbohydrate antigens. This included effective opsonophagocytic killing against different E. faecalis and E. faecium strains and recognition of 22 enterococcal strains [85]. Zhou et al. took a different approach with a synthetic cell wall teichoic acid (Figure 4), and also demonstrated a robust immune response and high antibody titers [141]. All three described examples provided successful data and are promising vaccine candidates against E. faecium targeting different antigens. They also included homologues carrier proteins, thus providing potential broader protection among multiple serotypes (Table 8).
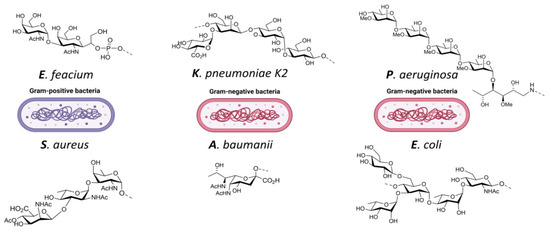
Figure 4.
ESKAPE pathogens and examples of target antigens: E. feacium: cell wall teichoic acid [141]; S. aureus: trisaccharide type 8 capsular polysaccharide [142]; K. pneumoniae: capsular polysaccharide K2 [143]; A. baumanii: pseudaminic-acid [144]; P. aeruginosa: methyl rhamnan pentasaccharide [145]; E. coli: serotype O25B [146].

Table 8.
Carbohydrate conjugate vaccines targeting Enterococcus faecium.
3.2. Staphylococcus aureus
S. aureus is a commensal Gram-positive bacterium associated with methicillin resistance (MRSA) and not only hospital-acquired infections in patients suffering from other underlying conditions, but also as a communal acquired infection of the skin and soft tissue. MRSA results in increased morbidity, mortality, length of hospitalization of patients and incurred costs [136,137]. Vancomycin is the first line antibiotic in the treatment of MRSA infections [147]. However, both intermediate and complete resistance to vancomycin has developed among clinical isolates within the past decades [148]. While the incidence of vancomycin resistance is still rare, it has become a serious public health concern [149]. The capsular polysaccharide from S. aureus has 13 different serotypes identified from clinical isolates of which types 5 and 8 are the most abundant [150]. Furthermore, the broadly associated poly-(1→6)-N-acetyl-D-glucosamine exopolysaccharide (PNAG) is also a potential target for vaccine development.
Gening et al. describe the successful development of a PNAG glycoconjugate including passive and active protection against different pathogens. Their conjugate 5GlcNH2-TT is being produced under GMP conditions and has entered safety and effectiveness testing in humans and economically important animals [151]. Zhao et al. provide data on the successful development of a synthetic capsular polysaccharide type 8 trisaccharide (Figure 4) conjugated to CRM197. They report a robust immune response including monoclonal antibodies recognizing the S. aureus strain [142]. While the prior two groups employ more traditional conjugation using carriers and antigens, the group of Stevenson et al. provided data on an OMV bioconjugate. They first showed the successful decoration of the E. coli OMV with PNAG followed by the introduction of an S. aureus enzyme responsible for PNAG deacetylation. Antibodies that were generated by vaccinating with this candidate provided efficient in vitro killing of S. aureus and Francisella tularensis as well [79]. All three described groups provided results that reveal the potential for effective vaccines targeting S. aureus utilizing different platforms (Table 9).

Table 9.
Carbohydrate conjugate vaccines targeting Staphylococcus aureus.
3.3. Klebsiella pneumoniae
K. pneumoniae is a commensal Gram-negative bacterium. It is associated with persistent urinary tract, soft tissue, and ventilator-associated pneumonia infections. Furthermore, it affects the elderly and immunocompromised patients causing pneumonia and sepsis. Infections can also be found in communal settings and are widespread. Resistance is found for third-generation cephalosporins and carbapenems. This resistance is conferred through extended-spectrum beta-lactamase (ESBL) or Amp-C beta-lactamase production. Last resort options to treat ESBL-producing Enterobacteriaceae infections are limited and can include the use of intravenous (IV) carbapenem antibiotics [136,137]. K. pneumoniae polysaccharides offer a wide array of targets for vaccine development, of which the capsular polysaccharide-derived K-antigen (77 serotypes) and lipopolysaccharide-derived O-antigen are the most promising [152]. Early developments of a 24-valent vaccine (1990s) have been shown to be effective in humans, but further development has been stopped due to overly complicated manufacturing [153].
Lin et al. suggest that traditional depolymerization of the CPS results in the loss of immunogenicity. They circumvent this by first extracting the polysaccharides from K. pneumoniae before applying CPS depolymerases identified from phages to cleave K1 and K2 (Figure 4) CPS, resulting in intact structural units of oligosaccharides without modifications. Conjugate vaccines employing TT as a carrier and using both K1 and K2 oligosaccharides were successful in protecting mice in a challenging study post-vaccination [143]. Feldman et al. reported on the recombinant production of a bioconjugate vaccine produced in glycoengineered E. coli targeting serotypes K1 and K2. The bioconjugates proved to be immunogenic and provided protection in mice when subjected to a lethal infection using two hypervirulent K. pneumoniae strains [77]. Ravinder et al. designed synthetic saccharides mimicking the K2 oligosaccharide. After investigating different quantities in repeat units they concluded that there was an optimum at five repeat units of the saccharide. Their glycoconjugate vaccine equipped with the hepta-saccharide exhibited good bactericidal activity [154]. There are multiple strategies being investigated in the field to design new vaccines targeting K. pneumoniae that include traditionally extracted polysaccharides, synthetic strategies and bioconjugates. This will yield multiple vaccine candidates with great potential (Table 10).

Table 10.
Carbohydrate conjugate vaccines targeting Klebsiella pneumoniae.
3.4. Acinetobacter baumannii
Acinetobacter baumannii is a commensal Gram-negative bacterium with great capability to spread in hospital settings. A particular challenge lies in the fact that it is frequently found at healthcare facilities. It is the leading cause of ventilator-associated pneumonia, and bloodstream, wound and urinary tract infections while being inherently resistant to various classes of antibiotics and easily acquiring resistance. It shows high mortality rates for invasive infections, particularly in carbapenem-resistant strains. Some Acinetobacter strains have built resistance to practically all antibiotics. Therefore, they are designated at the top of all AMR priority lists. While overall carbapenem-resistant Acinetobacter rates have decreased, gene transfer between other species, making them carbapenemases competent, is an increasing problem [136,137]. A. baumannii shows great biodiversity in polysaccharide structures, of which more than 40 different serotypes are described [159].
Rudenko et al. provided data on glycoconjugates using extracted K9 CPS fragments. They report on a substantial cross-reactive antibody response, including protective effectiveness demonstrated by a challenge of immunized mice with a lethal dose of A. baumannii K9 [160]. Li et al. demonstrated that their bioconjugate vaccine candidate elicited an efficient immune response and provided protection against an A. baumannii infection in a murine sepsis model [161]. Wei et al. employed a synthetically produced pseudaminic acid (Figure 4) conjugated to CRM197. Antibodies raised to their vaccine candidate were able to recognize pseudaminic acid [144]. Among the different research groups, the three different conjugation strategies all provided the ability to come up with effective vaccine designs targeting A. baumanii yielding multiple vaccine candidates that show great potential (Table 11).

Table 11.
Carbohydrate conjugate vaccines targeting Acinetobacter baumanii.
3.5. Pseudomonas aeruginosa
P. aeruginosa is a Gram-negative bacterium and primarily opportunistic nosocomial pathogen. It is rated as one of the most common causes of pneumonia in immunocompromised patients, including those with lung disease. Typically cystic fibrosis, HIV-1 and cancer patients often suffer from opportunistic P. aeruginosa infections in conjunction with chronic lung infections [163]. Between 2% and 3% of P. aeruginosa strains carry the carbapenemase enzyme making them carbapenem-resistant. This resistance particularly increases the risk of mortality among patients with bloodstream infections and leaves very few treatment options [136,137]. Pseudomonas aeruginosa carries O-polysaccharide, part of the LPS, as an important variable region and virulence factor. Further characterization of serotyping can be done by applying the International Antigenic Scheme. P. aeruginosa can be subdivided into 20 standard O serotypes (O1–O20) based on the structure of O-polysaccharide [164]. Serotypes O5, O6 and O11 are most prevalent in burn wound infections [165], whereas serotypes O6 and O11 are most common in pneumonia [166].
Jamshidi et al. reported on the successful development of a pentasaccharide conjugate from P. aeruginosa’s A-Band polysaccharide (Figure 4). Evaluation of the immunogenicity showed that antibodies were able to recognize P. aeruginosa LPS [145]. Hegerle et al. investigated a dual-use conjugate vaccine targeting both P. aeruginosa (rFlaA and rFlaB, carrier protein) and K. pneumoniae (O-polysaccharide O1, O2, O3 and O5, antigens). A quadrivalent conjugate vaccine formulation for the four antigens provided passive protection in both rabbits and mice when challenged [157]. Taken together, these vaccine candidates provide promising preclinical results and provide proof-of-concept for P. aeruginosa vaccines (Table 12).

Table 12.
Carbohydrate conjugate vaccines targeting Pseudomonas aeruginosa.
3.6. Escherichia coli
Part of the group of Enterobacter spp, E. coli is a Gram-negative commensal bacterium. It is associated with increasing reports of resistance in most countries and high mortality rates. Resistance has been observed towards third-generation cephalosporins through ESBL or Amp-C beta-lactamase production. E. coli is widespread and responsible for community-acquired and hospital-acquired urinary tract infections and bloodstream infections, ventilator-associated pneumonia infections and diarrheal disease as well. Resistance leaves very limited treatment options [136,137]. Serotyping of E. coli is based on the capsular polysaccharides, part of the protective structure on their surface. There is significant diversity where more than 180 different O-antigens and approximately 80 K-antigens have been reported [168].
Both Nicolardi et al. and Kowarik et al. (Figure 4) successfully constructed their bioconjugate via the traditional pathways and provide extensive data on the characterization. However, no immunogenicity data was provided making it impossible to assess the effectiveness [72,146]. Both Stevenson et al. and Gening et al. have provided successful immunogenicity data which was discussed for S. aureus already regarding the PNAG antigen and which could be applied towards E. coli as well [79,151] (Table 13).

Table 13.
Carbohydrate conjugate vaccines targeting Escherichia coli.
4. Discussion
Recent developments regarding six selected carriers and their applications are described in the field of conjugate vaccines. OMVs provide a very diverse and flexible platform for the construction of new conjugate vaccines (Table 1). For OMV specifically, we find that there are only a few pathogens used in current approaches for the extraction of OMVs; Salmonella, Shigella and Neisseria. For the future, it is important, with respect to CIES, to include the plethora of other pathogens from which OMVs can be produced [62]: e.g., A. baumannii [170], Bordetella pertussis [171], Borrelia burgdorferi [172], H. pylori [173], K. pneumoniae [174] and P. aeruginosa [175]. Glycoengineered OMVs and proteins go beyond the traditionally extracted OMVs as a carrier. The in vivo conjugation of specific antigens elaborates more on combinations of different pathogens and targets for vaccination (Table 2). The well-known PGCT and OTase-combined expression systems have great potential for new conjugate vaccine development. Whereas less unit operations are required for processing and conjugation of carrier and antigen, heterogeneity of these bioconjugates remains a challenge [72,73]. One direct solution for preventing such heterogeneity would be the more elaborate production of conjugates from individual carrier proteins and synthetically produced oligosaccharides (Table 3). In the field, a lot of progress is made towards synthetically designed oligosaccharides as a replacement for extracted oligosaccharides, aiding in better-controlled production processes [176]. The trade-off between bioconjugation and synthetic oligosaccharide conjugation lies between bioconjugation-related heterogeneity and sometimes longer development times for synthetic oligosaccharides. Other nanoparticles, such as VLPs and protein nanocages, are also studied in the field (Table 4 and Table 5). VLPs can be decorated with different antigens in the form of peptides, proteins, or CPS either through protein display or chemical conjugation. There is a clear advantage for VLPs with the absence of infectivity compared to traditional inactivated viruses and associated risks. Both the full potential of VLPs and protein nanocages as carriers are not yet obvious, with limited variation in the carrier–antigen combinations actively pursued in the field at this time. Synthetically manufactured peptides are finding their application in the cancer field, but also for infectious diseases (Table 6). The main advantage of peptides is that they can be easily designed and manufactured at a large scale and at very high purities with conjugation-ready reactive groups. Additionally, they can be employed both as a carrier and an antigen, used as an adjuvant and provide a specific B- or T-cell-mediated immune response. Peptides might be considered one of the highest potentials in fast vaccine design which could aid in new developments.
With the exciting developments in new carrier proteins, we looked further into the field of vaccination and current and future challenges. With increasing multidrug-resistant strains in the field of AMR, it cannot be overstated that this can be classified as the new (silent) pandemic [134,135]. With the WHO- and CDC-listed ESKAPE pathogens (Table 7), one would urge the development of new vaccines for each of these specific bacteria. Concentrating on recent developments it shows that more examples of the design of synthetic oligosaccharides against the ESKAPE pathogens are found than conjugate vaccines as a whole. Nevertheless, there are, however limited, multiple examples for each of the six pathogens (Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13). While other new carrier proteins are described in this review, their application in the field of ESKAPE pathogens is not obvious. The bioconjugates excluded, it is striking to see that most of the carriers still consist of CRM197 and also TT, which will not be beneficial towards CIES and the effectiveness of newly designed vaccines. When looking at those examples provided, it can be concluded that there are multiple promising vaccine candidates that show favorable preclinical results and provide proof-of-concept for all six ESKAPE pathogens.
5. Conclusions
A large influx of new vaccines has been achieved through the use of conjugation strategies. Many conjugate vaccines are developed to prevent infections and diseases. Because of carrier-induced epitope suppression and many AMR targets, traditional carrier proteins, such as TT, DT, CRM197, PD and OMPC, cannot always be used [177]. New carriers will help the development of conjugate vaccines. These carriers can be derived from many different organisms or be produced synthetically and contribute to increased protection against diseases. These carriers will have to be evaluated extensively by physicochemical and immunological methods in combination with the antigen. Looking at recent developments and the data provided in this review, we conclude that all six types of carriers have promising potential. With respect to conjugate vaccines, it was not surprising to find a wide array of new carriers and vaccine candidates to battle different diseases including ESKAPE pathogens. With the lessons learned from COVID-19 and the introduction of mRNA vaccines, we would expect that both vaccine platforms (conjugate and mRNA vaccines) co-exist with conjugate vaccines in the developments to battle ESKAPE pathogens. New vaccine candidates are needed soon to combat infectious diseases.
6. Perspective
Conjugate vaccines have proven their application in preventing disease and providing protection through eliciting functional antibodies against infectious diseases. The introduction of new carriers is crucial to provide ample versatility and choice to target particular diseases and AMR targets specifically. It can be expected that the new carriers currently under investigation find their way to clinical trials. Reliable and large-scale production processes of these carriers will be developed within the coming years.
Improvements in the availability and quality of existing polysaccharide antigens for vaccination will largely depend on two platforms. Both bioconjugation and synthetically manufactured polysaccharides show the most potential. Bioconjugation and synthetic routes have their downsides but both platforms eliminate the need for tedious extraction and purification of polysaccharides. As such more reliable and consistently produced bioconjugates will become available. Additionally, new routes for the large-scale production of synthetic oligosaccharides will become available but will have to be financially evaluated in terms of cost-effectiveness. Recent advances in carbohydrate-based antigens and new carriers are currently available. They will enable the swift design of new glycoconjugates targeting AMR and other vaccine-preventable diseases. Lastly, we expect the development of conjugate vaccines in the fight against cancer and neurodegenerative diseases.
Author Contributions
Conceptualization, R.M.v.d.P., B.M. and R.J.P.; investigation, R.M.v.d.P.; writing—original draft preparation, R.M.v.d.P.; writing—review and editing, R.M.v.d.P., B.M. and R.J.P.; supervision, B.M. and R.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
References
- CDC. History of Smallpox. Available online: https://www.cdc.gov/smallpox/history/history.html (accessed on 29 September 2022).
- Avery, O.T.; Goebel, W.F. Chemo-Immunological Studies on Conjugated Carbohydrate-Proteins: Ii. Immunological Specificity of Synthetic Sugar-Protein Antigens. J. Exp. Med. 1929, 50, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Schneerson, R.; Robbins, J.B.; Parke, J.C., Jr.; Bell, C.; Schlesselman, J.J.; Sutton, A.; Wang, Z.; Schiffman, G.; Karpas, A.; Shiloach, J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect. Immun. 1986, 52, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Schneerson, R.; Robbins, J.B.; Barrera, O.; Sutton, A.; Habig, W.B.; Hardegree, M.C.; Chaimovich, J. Haemophilus influenzae type B polysaccharide-protein conjugates: Model for a new generation of capsular polysaccharide vaccines. Prog. Clin. Biol. Res. 1980, 47, 77–94. [Google Scholar] [PubMed]
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.F.; Pollard, A.J.; Moxon, E.R. Immunological memory: The role of B cells in long-term protection against invasive bacterial pathogens. JAMA 2005, 294, 3019–3023. [Google Scholar] [CrossRef] [PubMed]
- Frasch, C.E. Preparation of bacterial polysaccharide-protein conjugates: Analytical and manufacturing challenges. Vaccine 2009, 27, 6468–6470. [Google Scholar] [CrossRef]
- Knuf, M.; Kowalzik, F.; Kieninger, D. Comparative effects of carrier proteins on vaccine-induced immune response. Vaccine 2011, 29, 4881–4890. [Google Scholar] [CrossRef]
- Peltola, H.; Kayhty, H.; Sivonen, A.; Makela, H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: A double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics 1977, 60, 730–737. [Google Scholar] [CrossRef]
- Koskela, M.; Leinonen, M.; Haiva, V.M.; Timonen, M.; Makela, P.H. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr. Infect. Dis. 1986, 5, 45–50. [Google Scholar] [CrossRef]
- Costantino, P.; Rappuoli, R.; Berti, F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 2011, 6, 1045–1066. [Google Scholar] [CrossRef]
- Pichichero, M.E. Protein carriers of conjugate vaccines: Characteristics, development, and clinical trials. Hum. Vaccin. Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef] [PubMed]
- Broker, M.; Berti, F.; Schneider, J.; Vojtek, I. Polysaccharide conjugate vaccine protein carriers as a "neglected valency" - Potential and limitations. Vaccine 2017, 35, 3286–3294. [Google Scholar] [CrossRef] [PubMed]
- Schutze, M.P.; Leclerc, C.; Vogel, F.R.; Chedid, L. Epitopic suppression in synthetic vaccine models: Analysis of the effector mechanisms. Cell. Immunol. 1987, 104, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Herzenberg, L.A.; Tokuhisa, T.; Herzenberg, L.A. Carrier-priming leads to hapten-specific suppression. Nature 1980, 285, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Barington, T.; Kristensen, K.; Henrichsen, J.; Heilmann, C. Influence of prevaccination immunity on the human B-lymphocyte response to a Haemophilus influenzae type b conjugate vaccine. Infect. Immun. 1991, 59, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Eskola, J.; Leclerc, C.; Leroy, O. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect Immun 1998, 66, 2093–2098. [Google Scholar] [CrossRef]
- Dagan, R.; Poolman, J.; Siegrist, C.A. Glycoconjugate vaccines and immune interference: A review. Vaccine 2010, 28, 5513–5523. [Google Scholar] [CrossRef]
- Giannini, G.; Rappuoli, R.; Ratti, G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 1984, 12, 4063–4069. [Google Scholar] [CrossRef]
- Broker, M.; Costantino, P.; DeTora, L.; McIntosh, E.D.; Rappuoli, R. Biochemical and biological characteristics of cross-reacting material 197 CRM197, a non-toxic mutant of diphtheria toxin: Use as a conjugation protein in vaccines and other potential clinical applications. Biologicals 2011, 39, 195–204. [Google Scholar] [CrossRef]
- Shinefield, H.R. Overview of the development and current use of CRM(197) conjugate vaccines for pediatric use. Vaccine 2010, 28, 4335–4339. [Google Scholar] [CrossRef]
- Forsgren, A.; Riesbeck, K.; Janson, H. Protein D of Haemophilus influenzae: A protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin. Infect. Dis. 2008, 46, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Prymula, R.; Peeters, P.; Chrobok, V.; Kriz, P.; Novakova, E.; Kaliskova, E.; Kohl, I.; Lommel, P.; Poolman, J.; Prieels, J.P.; et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: A randomised double-blind efficacy study. Lancet 2006, 367, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.J.; Deck, R.R.; Liu, M.A. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J. Immunol. 1990, 145, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Kilpi, T.; Ahman, H.; Jokinen, J.; Lankinen, K.S.; Palmu, A.; Savolainen, H.; Gronholm, M.; Leinonen, M.; Hovi, T.; Eskola, J.; et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: Randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 2003, 37, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Adamo, R.; Costantino, P. Protein Carriers for Glycoconjugate Vaccines: History, Selection Criteria, Characterization and New Trends. Molecules 2018, 23, 1451. [Google Scholar] [CrossRef] [PubMed]
- Cryz, S.J., Jr.; Cross, A.S.; Sadoff, J.C.; Wegmann, A.; Que, J.U.; Furer, E. Safety and immunogenicity of Escherichia coli O18 O-specific polysaccharide (O-PS)-toxin A and O-PS-cholera toxin conjugate vaccines in humans. J. Infect. Dis. 1991, 163, 1040–1045. [Google Scholar] [CrossRef]
- Cross, A.; Artenstein, A.; Que, J.; Fredeking, T.; Furer, E.; Sadoff, J.C.; Cryz, S.J., Jr. Safety and immunogenicity of a polyvalent Escherichia coli vaccine in human volunteers. J. Infect. Dis. 1994, 170, 834–840. [Google Scholar] [CrossRef]
- Kossaczka, Z.; Lin, F.Y.; Ho, V.A.; Thuy, N.T.; Van Bay, P.; Thanh, T.C.; Khiem, H.B.; Trach, D.D.; Karpas, A.; Hunt, S.; et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect. Immun. 1999, 67, 5806–5810. [Google Scholar] [CrossRef]
- Fattom, A.; Schneerson, R.; Watson, D.C.; Karakawa, W.W.; Fitzgerald, D.; Pastan, I.; Li, X.; Shiloach, J.; Bryla, D.A.; Robbins, J.B. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect. Immun. 1993, 61, 1023–1032. [Google Scholar] [CrossRef]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Szu, S.C.; Stone, A.L.; Robbins, J.D.; Schneerson, R.; Robbins, J.B. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J. Exp. Med. 1987, 166, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Tontini, M.; Romano, M.R.; Proietti, D.; Balducci, E.; Micoli, F.; Balocchi, C.; Santini, L.; Masignani, V.; Berti, F.; Costantino, P. Preclinical studies on new proteins as carrier for glycoconjugate vaccines. Vaccine 2016, 34, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Falugi, F.; Petracca, R.; Mariani, M.; Luzzi, E.; Mancianti, S.; Carinci, V.; Melli, M.L.; Finco, O.; Wack, A.; Di Tommaso, A.; et al. Rationally designed strings of promiscuous CD4(+) T cell epitopes provide help to Haemophilus influenzae type b oligosaccharide: A model for new conjugate vaccines. Eur. J. Immunol. 2001, 31, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Baraldo, K.; Mori, E.; Bartoloni, A.; Norelli, F.; Grandi, G.; Rappuoli, R.; Finco, O.; Del Giudice, G. Combined conjugate vaccines: Enhanced immunogenicity with the N19 polyepitope as a carrier protein. Infect. Immun. 2005, 73, 5835–5841. [Google Scholar] [CrossRef]
- Bongat, A.F.; Saksena, R.; Adamo, R.; Fujimoto, Y.; Shiokawa, Z.; Peterson, D.C.; Fukase, K.; Vann, W.F.; Kovac, P. Multimeric bivalent immunogens from recombinant tetanus toxin HC fragment, synthetic hexasaccharides, and a glycopeptide adjuvant. Glycoconj. J. 2010, 27, 69–77. [Google Scholar] [CrossRef]
- Xin, H.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13526–13531. [Google Scholar] [CrossRef]
- Rothbard, J.B.; Taylor, W.R. A sequence pattern common to T cell epitopes. EMBO J. 1988, 7, 93–100. [Google Scholar] [CrossRef]
- Ingale, S.; Wolfert, M.A.; Gaekwad, J.; Buskas, T.; Boons, G.J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 2007, 3, 663–667. [Google Scholar] [CrossRef]
- Bixler, G.S., Jr.; Eby, R.; Dermody, K.M.; Woods, R.M.; Seid, R.C.; Pillai, S. Synthetic peptide representing a T-cell epitope of CRM197 substitutes as carrier molecule in a Haemophilus influenzae type B (Hib) conjugate vaccine. Adv. Exp. Med. Biol. 1989, 251, 175–180. [Google Scholar] [CrossRef]
- Belot, F.; Guerreiro, C.; Baleux, F.; Mulard, L.A. Synthesis of two linear PADRE conjugates bearing a deca- or pentadecasaccharide B epitope as potential synthetic vaccines against Shigella flexneri serotype 2a infection. Chemistry 2005, 11, 1625–1635. [Google Scholar] [CrossRef]
- Alexander, J.; del Guercio, M.F.; Maewal, A.; Qiao, L.; Fikes, J.; Chesnut, R.W.; Paulson, J.; Bundle, D.R.; DeFrees, S.; Sette, A. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 2000, 164, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; del Guercio, M.F.; Frame, B.; Maewal, A.; Sette, A.; Nahm, M.H.; Newman, M.J. Development of experimental carbohydrate-conjugate vaccines composed of Streptococcus pneumoniae capsular polysaccharides and the universal helper T-lymphocyte epitope (PADRE). Vaccine 2004, 22, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Michon, F.; Fusco, P.C.; Minetti, C.A.; Laude-Sharp, M.; Uitz, C.; Huang, C.H.; D’Ambra, A.J.; Moore, S.; Remeta, D.P.; Heron, I.; et al. Multivalent pneumococcal capsular polysaccharide conjugate vaccines employing genetically detoxified pneumolysin as a carrier protein. Vaccine 1998, 16, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Wilk, K.; Lee, J.C.; Gening, M.; Nifantiev, N.; Pier, G.B. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS ONE 2012, 7, e46648. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Leuzzi, R.; Cappelletti, E.; Tontini, M.; Nilo, A.; Proietti, D.; Berti, F.; Costantino, P.; Adamo, R.; Scarselli, M. Recombinant Clostridium difficile toxin fragments as carrier protein for PSII surface polysaccharide preserve their neutralizing activity. Toxins 2014, 6, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Nilo, A.; Morelli, L.; Passalacqua, I.; Brogioni, B.; Allan, M.; Carboni, F.; Pezzicoli, A.; Zerbini, F.; Maione, D.; Fabbrini, M.; et al. Anti-Group B Streptococcus Glycan-Conjugate Vaccines Using Pilus Protein GBS80 As Carrier and Antigen: Comparing Lysine and Tyrosine-directed Conjugation. ACS Chem. Biol. 2015, 10, 1737–1746. [Google Scholar] [CrossRef]
- Nilo, A.; Passalacqua, I.; Fabbrini, M.; Allan, M.; Usera, A.; Carboni, F.; Brogioni, B.; Pezzicoli, A.; Cobb, J.; Romano, M.R.; et al. Exploring the Effect of Conjugation Site and Chemistry on the Immunogenicity of an anti-Group B Streptococcus Glycoconjugate Vaccine Based on GBS67 Pilus Protein and Type V Polysaccharide. Bioconjug. Chem. 2015, 26, 1839–1849. [Google Scholar] [CrossRef]
- Simon, R.; Tennant, S.M.; Wang, J.Y.; Schmidlein, P.J.; Lees, A.; Ernst, R.K.; Pasetti, M.F.; Galen, J.E.; Levine, M.M. Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect. Immun. 2011, 79, 4240–4249. [Google Scholar] [CrossRef]
- Wang, L.X. Synthetic carbohydrate antigens for HIV vaccine design. Curr. Opin. Chem. Biol. 2013, 17, 997–1005. [Google Scholar] [CrossRef]
- Polonskaya, Z.; Deng, S.; Sarkar, A.; Kain, L.; Comellas-Aragones, M.; McKay, C.S.; Kaczanowska, K.; Holt, M.; McBride, R.; Palomo, V.; et al. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J. Clin. Investig. 2017, 127, 1491–1504. [Google Scholar] [CrossRef]
- Valguarnera, E.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles as a Platform for Vaccine Development. Methods Enzymol. 2017, 597, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G.; et al. GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines. Vaccines 2020, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Launay, O.; Lewis, D.J.M.; Anemona, A.; Loulergue, P.; Leahy, J.; Scire, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine Against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results From Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef]
- de Kleijn, E.D.; de Groot, R.; Labadie, J.; Lafeber, A.B.; van den Dobbelsteen, G.; van Alphen, L.; van Dijken, H.; Kuipers, B.; van Omme, G.W.; Wala, M.; et al. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 2000, 18, 1456–1466. [Google Scholar] [CrossRef]
- Cartwright, K.; Morris, R.; Rumke, H.; Fox, A.; Borrow, R.; Begg, N.; Richmond, P.; Poolman, J. Immunogenicity and reactogenicity in UK infants of a novel meningococcal vesicle vaccine containing multiple class 1 (PorA) outer membrane proteins. Vaccine 1999, 17, 2612–2619. [Google Scholar] [CrossRef]
- Skidmore, B.J.; Chiller, J.M.; Morrison, D.C.; Weigle, W.O. Immunologic properties of bacterial lipopolysaccharide (LPS): Correlation between the mitogenic, adjuvant, and immunogenic activities. J. Immunol. 1975, 114, 770–775. [Google Scholar] [CrossRef]
- Liu, Y.; Hammer, L.A.; Liu, W.; Hobbs, M.M.; Zielke, R.A.; Sikora, A.E.; Jerse, A.E.; Egilmez, N.K.; Russell, M.W. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 2017, 10, 1594–1608. [Google Scholar] [CrossRef]
- Lehmann, A.K.; Halstensen, A.; Aaberge, I.S.; Holst, J.; Michaelsen, T.E.; Sornes, S.; Wetzler, L.M.; Guttormsen, H. Human opsonins induced during meningococcal disease recognize outer membrane proteins PorA and PorB. Infect. Immun. 1999, 67, 2552–2560. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- van der Pol, L.; Stork, M.; van der Ley, P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef] [PubMed]
- Gerritzen, M.J.H.; Salverda, M.L.M.; Martens, D.E.; Wijffels, R.H.; Stork, M. Spontaneously released Neisseria meningitidis outer membrane vesicles as vaccine platform: Production and purification. Vaccine 2019, 37, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Oldrini, D.; Pitirollo, O.; Gasperini, G.; et al. Generalized Modules for Membrane Antigens as Carrier for Polysaccharides: Impact of Sugar Length, Density, and Attachment Site on the Immune Response Elicited in Animal Models. Front. Immunol. 2021, 12, 719315. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, E.; Kis, Z.; Ozanne, J.; Di Benedetto, R.; Ricchetti, B.; Massai, L.; Carducci, M.; Oldrini, D.; Gasperini, G.; Aruta, M.G.; et al. GMMA as an Alternative Carrier for a Glycoconjugate Vaccine against Group A Streptococcus. Vaccines 2022, 10, 1034. [Google Scholar] [CrossRef]
- Jiang, L.; Driedonks, T.A.P.; Jong, W.S.P.; Dhakal, S.; Bart van den Berg van Saparoea, H.; Sitaras, I.; Zhou, R.; Caputo, C.; Littlefield, K.; Lowman, M.; et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J. Extracell. Vesicles 2022, 11, e12192. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, R.; Alfini, R.; Carducci, M.; Aruta, M.G.; Lanzilao, L.; Acquaviva, A.; Palmieri, E.; Giannelli, C.; Necchi, F.; Saul, A.; et al. Novel Simple Conjugation Chemistries for Decoration of GMMA with Heterologous Antigens. Int. J. Mol. Sci. 2021, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Scaria, P.V.; Rowe, C.G.; Chen, B.B.; Muratova, O.V.; Fischer, E.R.; Barnafo, E.K.; Anderson, C.F.; Zaidi, I.U.; Lambert, L.E.; Lucas, B.J.; et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. NPJ Vaccines 2019, 4, 24. [Google Scholar] [CrossRef]
- Langdon, R.H.; Cuccui, J.; Wren, B.W. N-linked glycosylation in bacteria: An unexpected application. Future Microbiol. 2009, 4, 401–412. [Google Scholar] [CrossRef]
- Kay, E.; Cuccui, J.; Wren, B.W. Recent advances in the production of recombinant glycoconjugate vaccines. NPJ Vaccines 2019, 4, 16. [Google Scholar] [CrossRef]
- Wacker, M.; Linton, D.; Hitchen, P.G.; Nita-Lazar, M.; Haslam, S.M.; North, S.J.; Panico, M.; Morris, H.R.; Dell, A.; Wren, B.W.; et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science 2002, 298, 1790–1793. [Google Scholar] [CrossRef]
- Nicolardi, S.; Danuser, R.; Dotz, V.; Dominguez-Vega, E.; Al Kaabi, A.; Beurret, M.; Anish, C.; Wuhrer, M. Glycan and Protein Analysis of Glycoengineered Bacterial E. coli Vaccines by MALDI-in-Source Decay FT-ICR Mass Spectrometry. Anal. Chem. 2022, 94, 4979–4987. [Google Scholar] [CrossRef] [PubMed]
- MacCalman, T.E.; Phillips-Jones, M.K.; Harding, S.E. Glycoconjugate vaccines: Some observations on carrier and production methods. Biotechnol. Genet. Eng. Rev. 2019, 35, 93–125. [Google Scholar] [CrossRef]
- Harding, C.M.; Nasr, M.A.; Scott, N.E.; Goyette-Desjardins, G.; Nothaft, H.; Mayer, A.E.; Chavez, S.M.; Huynh, J.P.; Kinsella, R.L.; Szymanski, C.M.; et al. A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a host. Nat. Commun. 2019, 10, 891. [Google Scholar] [CrossRef]
- Martin, P.; Alaimo, C. The Ongoing Journey of a Shigella Bioconjugate Vaccine. Vaccines 2022, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, N.; Braun, M.; Schneider, J.; Dreyer, A.M.; Wetter, M.; Haeuptle, M.A.; Kemmler, S.; Steffen, M.; Sirena, D.; Herwig, S.; et al. Characterization and immunogenicity of a Shigella flexneri 2a O-antigen bioconjugate vaccine candidate. Glycobiology 2019, 29, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.F.; Mayer Bridwell, A.E.; Scott, N.E.; Vinogradov, E.; McKee, S.R.; Chavez, S.M.; Twentyman, J.; Stallings, C.L.; Rosen, D.A.; Harding, C.M. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2019, 116, 18655–18663. [Google Scholar] [CrossRef]
- Reglinski, M.; Ercoli, G.; Plumptre, C.; Kay, E.; Petersen, F.C.; Paton, J.C.; Wren, B.W.; Brown, J.S. A recombinant conjugated pneumococcal vaccine that protects against murine infections with a similar efficacy to Prevnar-13. NPJ Vaccines 2018, 3, 53. [Google Scholar] [CrossRef]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, E3106–E3115. [Google Scholar] [CrossRef]
- Sun, P.; Pan, C.; Zeng, M.; Liu, B.; Liang, H.; Wang, D.; Liu, X.; Wang, B.; Lyu, Y.; Wu, J.; et al. Design and production of conjugate vaccines against S. Paratyphi A using an O-linked glycosylation system in vivo. NPJ Vaccines 2018, 3, 4. [Google Scholar] [CrossRef]
- Marshall, L.E.; Nelson, M.; Davies, C.H.; Whelan, A.O.; Jenner, D.C.; Moule, M.G.; Denman, C.; Cuccui, J.; Atkins, T.P.; Wren, B.W.; et al. An O-Antigen Glycoconjugate Vaccine Produced Using Protein Glycan Coupling Technology Is Protective in an Inhalational Rat Model of Tularemia. J. Immunol. Res. 2018, 2018, 8087916. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Wang, G.; Feng, S.; Guo, Z.; Gu, G. Group A Streptococcus Cell Wall Oligosaccharide-Streptococcal C5a Peptidase Conjugates as Effective Antibacterial Vaccines. ACS Infect. Dis. 2020, 6, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, J.; Zhao, Y.; Wang, S.; Feng, S.; Gu, G. Immunogenicity Assessment of Different Segments and Domains of Group a Streptococcal C5a Peptidase and Their Application Potential as Carrier Protein for Glycoconjugate Vaccine Development. Vaccines 2021, 9, 139. [Google Scholar] [CrossRef]
- Kapoor, N.; Uchiyama, S.; Pill, L.; Bautista, L.; Sedra, A.; Yin, L.; Regan, M.; Chu, E.; Rabara, T.; Wong, M.; et al. Non-Native Amino Acid Click Chemistry-Based Technology for Site-Specific Polysaccharide Conjugation to a Bacterial Protein Serving as Both Carrier and Vaccine Antigen. ACS Omega 2022, 7, 24111–24120. [Google Scholar] [CrossRef] [PubMed]
- Romero-Saavedra, F.; Laverde, D.; Kalfopoulou, E.; Martini, C.; Torelli, R.; Martinez-Matamoros, D.; Sanguinetti, M.; Huebner, J. Conjugation of Different Immunogenic Enterococcal Vaccine Target Antigens Leads to Extended Strain Coverage. J. Infect. Dis. 2019, 220, 1589–1598. [Google Scholar] [CrossRef]
- Chang, M.J.; Ollivault-Shiflett, M.; Schuman, R.; Ngoc Nguyen, S.; Kaltashov, I.A.; Bobst, C.; Rajagopal, S.P.; Przedpelski, A.; Barbieri, J.T.; Lees, A. Genetically detoxified tetanus toxin as a vaccine and conjugate carrier protein. Vaccine 2022, 40, 5103–5113. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, J.; Sun, C.; Chi, H.; Luo, D.; Wang, R.; Qiu, H.; Zhang, Y.; Wu, M.; Zhang, X.; et al. Rational Development of a Polysaccharide-Protein-Conjugated Nanoparticle Vaccine Against SARS-CoV-2 Variants and Streptococcus pneumoniae. Adv. Mater. 2022, 34, e2200443. [Google Scholar] [CrossRef]
- Park, W.J.; Yoon, Y.K.; Park, J.S.; Pansuriya, R.; Seok, Y.J.; Ganapathy, R. Rotavirus spike protein DeltaVP8* as a novel carrier protein for conjugate vaccine platform with demonstrated antigenic potential for use as bivalent vaccine. Sci. Rep. 2021, 11, 22037. [Google Scholar] [CrossRef]
- Ahmadi, K.; Aslani, M.M.; Pouladfar, G.; Faezi, S.; Kalani, M.; Pourmand, M.R.; Ghaedi, T.; Havaei, S.A.; Mahdavi, M. Preparation and preclinical evaluation of two novel Staphylococcus aureus capsular polysaccharide 5 and 8-fusion protein (Hla-MntC-SACOL0723) immunoconjugates. IUBMB Life 2020, 72, 226–236. [Google Scholar] [CrossRef]
- Chiu, T.W.; Peng, C.J.; Chen, M.C.; Hsu, M.H.; Liang, Y.H.; Chiu, C.H.; Fang, J.M.; Lee, Y.C. Constructing conjugate vaccine against Salmonella Typhimurium using lipid-A free lipopolysaccharide. J. Biomed Sci. 2020, 27, 89. [Google Scholar] [CrossRef]
- Ou, L.; Kong, W.P.; Chuang, G.Y.; Ghosh, M.; Gulla, K.; O’Dell, S.; Varriale, J.; Barefoot, N.; Changela, A.; Chao, C.W.; et al. Preclinical Development of a Fusion Peptide Conjugate as an HIV Vaccine Immunogen. Sci. Rep. 2020, 10, 3032. [Google Scholar] [CrossRef]
- Qian, W.; Huang, Z.; Chen, Y.; Yang, J.; Wang, L.; Wu, K.; Chen, M.; Chen, N.; Duan, Y.; Shi, J.; et al. Elicitation of integrated immunity in mice by a novel pneumococcal polysaccharide vaccine conjugated with HBV surface antigen. Sci. Rep. 2020, 10, 6470. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Yang, Y.; Shaddeau, A.; Cai, C.X.; Li, Y.; Gulla, K.; Zhang, Y.; Ou, L.; Cooper, J.W.; Lei, Q.P. A unique algorithm for the determination of peptide-carrier protein conjugation ratio by amino acid analysis using intrinsic internal standard. Vaccine 2020, 38, 4507–4511. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Scaria, P.V.; Chen, B.; Barnafo, E.; Muratova, O.; Anderson, C.; Lambert, L.; Chae, M.H.; Yang, J.S.; Duffy, P.E. Development of a bivalent conjugate vaccine candidate against malaria transmission and typhoid fever. Vaccine 2018, 36, 2978–2984. [Google Scholar] [CrossRef] [PubMed]
- Baliban, S.M.; Curtis, B.; Toema, D.; Tennant, S.M.; Levine, M.M.; Pasetti, M.F.; Simon, R. Immunogenicity and efficacy following sequential parenterally-administered doses of Salmonella Enteritidis COPS:FliC glycoconjugates in infant and adult mice. PLoS Negl. Trop Dis. 2018, 12, e0006522. [Google Scholar] [CrossRef]
- Baruffaldi, F.; Kelcher, A.H.; Laudenbach, M.; Gradinati, V.; Limkar, A.; Roslawski, M.; Birnbaum, A.; Lees, A.; Hassler, C.; Runyon, S.; et al. Preclinical Efficacy and Characterization of Candidate Vaccines for Treatment of Opioid Use Disorders Using Clinically Viable Carrier Proteins. Mol. Pharm. 2018, 15, 4947–4962. [Google Scholar] [CrossRef]
- Laird, R.M.; Ma, Z.; Dorabawila, N.; Pequegnat, B.; Omari, E.; Liu, Y.; Maue, A.C.; Poole, S.T.; Maciel, M.; Satish, K.; et al. Evaluation of a conjugate vaccine platform against enterotoxigenic Escherichia coli (ETEC), Campylobacter jejuni and Shigella. Vaccine 2018, 36, 6695–6702. [Google Scholar] [CrossRef]
- Fries, C.N.; Curvino, E.J.; Chen, J.L.; Permar, S.R.; Fouda, G.G.; Collier, J.H. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat. Nanotechnol. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Basu, R.; Zhai, L.; Contreras, A.; Tumban, E. Immunization with phage virus-like particles displaying Zika virus potential B-cell epitopes neutralizes Zika virus infection of monkey kidney cells. Vaccine 2018, 36, 1256–1264. [Google Scholar] [CrossRef]
- Basu, R.; Zhai, L.; Rosso, B.; Tumban, E. Bacteriophage Qbeta virus-like particles displaying Chikungunya virus B-cell epitopes elicit high-titer E2 protein antibodies but fail to neutralize a Thailand strain of Chikungunya virus. Vaccine 2020, 38, 2542–2550. [Google Scholar] [CrossRef]
- Warner, N.L.; Frietze, K.M. Development of Bacteriophage Virus-Like Particle Vaccines Displaying Conserved Epitopes of Dengue Virus Non-Structural Protein 1. Vaccines 2021, 9, 726. [Google Scholar] [CrossRef]
- Zha, L.; Chang, X.; Zhao, H.; Mohsen, M.O.; Hong, L.; Zhou, Y.; Chen, H.; Liu, X.; Zhang, J.; Li, D.; et al. Development of a Vaccine against SARS-CoV-2 Based on the Receptor-Binding Domain Displayed on Virus-Like Particles. Vaccines 2021, 9, 395. [Google Scholar] [CrossRef]
- Cabral-Miranda, G.; Lim, S.M.; Mohsen, M.O.; Pobelov, I.V.; Roesti, E.S.; Heath, M.D.; Skinner, M.A.; Kramer, M.F.; Martina, B.E.E.; Bachmann, M.F. Zika Virus-Derived E-DIII Protein Displayed on Immunologically Optimized VLPs Induces Neutralizing Antibodies without Causing Enhancement of Dengue Virus Infection. Vaccines 2019, 7, 72. [Google Scholar] [CrossRef]
- Sungsuwan, S.; Wu, X.; Shaw, V.; Kavunja, H.; McFall-Boegeman, H.; Rashidijahanabad, Z.; Tan, Z.; Lang, S.; Tahmasebi Nick, S.; Lin, P.H.; et al. Structure Guided Design of Bacteriophage Qbeta Mutants as Next Generation Carriers for Conjugate Vaccines. ACS Chem. Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, M.; Podsiadla-Bialoskorska, M.; Mielecki, D.; Ruffier, N.; Fateh, A.; Lambert, A.; Fanuel, M.; Camberlein, E.; Szolajska, E.; Grandjean, C. On the use of adenovirus dodecahedron as a carrier for glycoconjugate vaccines. Glycoconj. J. 2021, 38, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Toonstra, C.; Yang, Q.; Zhang, R.; Wang, L.X. Chemoenzymatic Synthesis and Antibody Binding of HIV-1 V1/V2 Glycopeptide-Bacteriophage Qbeta Conjugates as a Vaccine Candidate. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huo, C.X.; Lang, S.; Caution, K.; Nick, S.T.; Dubey, P.; Deora, R.; Huang, X. Chemical Synthesis and Immunological Evaluation of a Pentasaccharide Bearing Multiple Rare Sugars as a Potential Anti-pertussis Vaccine. Angew. Chem. Int. Ed. Engl. 2020, 59, 6451–6458. [Google Scholar] [CrossRef]
- Xu, L.; Li, Z.; Su, Z.; Yang, Y.; Ma, G.; Yu, R.; Zhang, S. Development of meningococcal polysaccharide conjugate vaccine that can elicit long-lasting and strong cellular immune response with hepatitis B core antigen virus-like particles as a novel carrier protein. Vaccine 2019, 37, 956–964. [Google Scholar] [CrossRef]
- Curley, S.M.; Putnam, D. Biological Nanoparticles in Vaccine Development. Front. Bioeng. Biotechnol. 2022, 10, 867119. [Google Scholar] [CrossRef]
- Flenniken, M.L.; Uchida, M.; Liepold, L.O.; Kang, S.; Young, M.J.; Douglas, T. A library of protein cage architectures as nanomaterials. Curr. Top Microbiol. Immunol. 2009, 327, 71–93. [Google Scholar] [CrossRef]
- Chakraborti, S.; Chakrabarti, P. Self-Assembly of Ferritin: Structure, Biological Function and Potential Applications in Nanotechnology. Adv. Exp. Med. Biol. 2019, 1174, 313–329. [Google Scholar] [CrossRef]
- Chen, R.R.R. Development of Receptor Binding Domain (RBD)-Conjugated Nanoparticle Vaccines with Broad Neutralization against SARS-CoV-2 Delta and Other Variants. Adv. Sci. 2022, 9, 5378. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, H.; Yan, X.; et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Z.; Yang, Y.; Ma, G.; Su, Z.; Zhang, S. An Apoferritin-Hemagglutinin Conjugate Vaccine with Encapsulated Nucleoprotein Antigen Peptide from Influenza Virus Confers Enhanced Cross Protection. Bioconjug. Chem. 2020, 31, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.Y.Y. Apoferritin nanoparticle based dual-antigen influenza conjugate vaccine with potential cross-protective efficacy against heterosubtypic influenza virus. Particuology 2022, 64, 56–64. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef]
- Jiang, S.; Song, R.; Popov, S.; Mirshahidi, S.; Ruprecht, R.M. Overlapping synthetic peptides as vaccines. Vaccine 2006, 24, 6356–6365. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, H.; Li, D.; Ma, S.; Di, Y.; Stoten, A.; Haig, N.; Di Gleria, K.; Yu, Z.; Xu, X.N.; et al. Comparing pooled peptides with intact protein for accessing cross-presentation pathways for protective CD8+ and CD4+ T cells. J. Biol. Chem. 2009, 284, 9184–9191. [Google Scholar] [CrossRef]
- He, Y.; Yu, W.; Shen, L.; Yan, W.; Xiao, L.; Qi, J.; Hu, T. A SARS-CoV-2 vaccine based on conjugation of SARS-CoV-2 RBD with IC28 peptide and mannan. Int. J. Biol. Macromol. 2022, 222, 661–670. [Google Scholar] [CrossRef]
- Azuar, A.; Shibu, M.A.; Adilbish, N.; Marasini, N.; Hung, H.; Yang, J.; Luo, Y.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; et al. Poly(hydrophobic amino acid) Conjugates for the Delivery of Multiepitope Vaccine against Group A Streptococcus. Bioconjug. Chem. 2021, 32, 2307–2317. [Google Scholar] [CrossRef]
- Shalash, A.O.; Becker, L.; Yang, J.; Giacomin, P.; Pearson, M.; Hussein, W.M.; Loukas, A.; Skwarczynski, M.; Toth, I. Oral Peptide Vaccine against Hookworm Infection: Correlation of Antibody Titers with Protective Efficacy. Vaccines 2021, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Sengupta, S.; Gupta, D.; Bhan, M.K.; Kumar, R.; Khan, A.; Jailkhani, B.S. Typhi derived OmpC peptide conjugated with Vi-polysaccharide evokes better immune response than free Vi-polysaccharide in mice. Biologicals 2019, 62, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Costantino, P.; Adamo, R. Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, L.; Osterlid, K.E.; Wu, D.Y.; Nonne, F.; Romano, M.R.; Codee, J.; Adamo, R. Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance. Chem. Rev. 2022, 122, 15672–15716. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent Advance in Tumor-associated Carbohydrate Antigens (TACAs)-based Antitumor Vaccines. ACS Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.Y. Therapeutic cancer vaccines: Past, present, and future. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [CrossRef]
- Sorieul, C.; Papi, F.; Carboni, F.; Pecetta, S.; Phogat, S.; Adamo, R. Recent advances and future perspectives on carbohydrate-based cancer vaccines and therapeutics. Pharmacol. Ther. 2022, 235, 108158. [Google Scholar] [CrossRef]
- Shivatare, S.S.; Shivatare, V.S.; Wong, C.H. Glycoconjugates: Synthesis, Functional Studies, and Therapeutic Developments. Chem. Rev. 2022, 122, 15603–15671. [Google Scholar] [CrossRef]
- Hossain, F.; Andreana, P.R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 84. [Google Scholar] [CrossRef]
- Bajad, N.G.; Swetha, R.; Gutti, G.; Singh, M.; Kumar, A.; Singh, S.K. A systematic review of carbohydrate-based bioactive molecules for Alzheimer’s disease. Future Med. Chem. 2021, 13, 1695–1711. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Laxminarayan, R. The overlooked pandemic of antimicrobial resistance. Lancet 2022, 399, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A. Antimicrobial Resistance: The Next Probable Pandemic. JNMA J. Nepal. Med. Assoc. 2022, 60, 225–228. [Google Scholar] [CrossRef] [PubMed]
- WHO. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 11 November 2022).
- CDC. 2019 AR Threats Report. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html#carp (accessed on 11 November 2022).
- European Antimicrobial Resistance, C. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: A cross-country systematic analysis. Lancet Public Health 2022, 7, e897–e913. [Google Scholar] [CrossRef]
- Teng, F.; Singh, K.V.; Bourgogne, A.; Zeng, J.; Murray, B.E. Further characterization of the epa gene cluster and Epa polysaccharides of Enterococcus faecalis. Infect. Immun. 2009, 77, 3759–3767. [Google Scholar] [CrossRef]
- Kalfopoulou, E.; Laverde, D.; Miklic, K.; Romero-Saavedra, F.; Malic, S.; Carboni, F.; Adamo, R.; Lenac Rovis, T.; Jonjic, S.; Huebner, J. Development of Opsonic Mouse Monoclonal Antibodies against Multidrug-Resistant Enterococci. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Zhou, Z.; Ding, W.; Li, C.; Wu, Z. Synthesis and immunological study of a wall teichoic acid-based vaccine against E. faecium U0317. J. Carbohydr. Chem. 2017, 36, 205–219. [Google Scholar] [CrossRef]
- Zhao, M.; Qin, C.; Li, L.; Xie, H.; Ma, B.; Zhou, Z.; Yin, J.; Hu, J. Conjugation of Synthetic Trisaccharide of Staphylococcus aureus Type 8 Capsular Polysaccharide Elicits Antibodies Recognizing Intact Bacterium. Front. Chem. 2020, 8, 258. [Google Scholar] [CrossRef]
- Lin, T.L.; Yang, F.L.; Ren, C.T.; Pan, Y.J.; Liao, K.S.; Tu, I.F.; Chang, Y.P.; Cheng, Y.Y.; Wu, C.Y.; Wu, S.H.; et al. Development of Klebsiella pneumoniae Capsule Polysaccharide-Conjugated Vaccine Candidates Using Phage Depolymerases. Front. Immunol. 2022, 13, 843183. [Google Scholar] [CrossRef]
- Wei, R.; Yang, X.; Liu, H.; Wei, T.; Chen, S.; Li, X. Synthetic Pseudaminic-Acid-Based Antibacterial Vaccine Confers Effective Protection against Acinetobacter baumannii Infection. ACS Cent. Sci. 2021, 7, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.P.; Cairns, C.; Chong, S.; St Michael, F.; Vinogradov, E.V.; Cox, A.D.; Sauvageau, J. Synthesis and Immunogenicity of a Methyl Rhamnan Pentasaccharide Conjugate from Pseudomonas aeruginosa A-Band Polysaccharide. ACS Infect. Dis. 2022, 8, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, M.; Wetter, M.; Haeuptle, M.A.; Braun, M.; Steffen, M.; Kemmler, S.; Ravenscroft, N.; De Benedetto, G.; Zuppiger, M.; Sirena, D.; et al. The development and characterization of an E. coli O25B bioconjugate vaccine. Glycoconj. J. 2021, 38, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, T.C.; Packham, D.R.; Shanker, S.; Foldes, M.; Munro, R. Vancomycin therapy for methicillin-resistant Staphylococcus aureus. Ann. Intern. Med. 1982, 97, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Sievert, D.M.; Hageman, J.C.; Boulton, M.L.; Tenover, F.C.; Downes, F.P.; Shah, S.; Rudrik, J.T.; Pupp, G.R.; Brown, W.J.; et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 2003, 348, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar]
- O’Riordan, K.; Lee, J.C. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef]
- Gening, M.L.; Pier, G.B.; Nifantiev, N.E. Broadly protective semi-synthetic glycoconjugate vaccine against pathogens capable of producing poly-beta-(1-->6)-N-acetyl-d-glucosamine exopolysaccharide. Drug Discov. Today Technol. 2020, 35-36, 13–21. [Google Scholar] [CrossRef]
- Choi, M.; Hegerle, N.; Nkeze, J.; Sen, S.; Jamindar, S.; Nasrin, S.; Sen, S.; Permala-Booth, J.; Sinclair, J.; Tapia, M.D.; et al. The Diversity of Lipopolysaccharide (O) and Capsular Polysaccharide (K) Antigens of Invasive Klebsiella pneumoniae in a Multi-Country Collection. Front. Microbiol. 2020, 11, 1249. [Google Scholar] [CrossRef]
- Cryz, S.J. Progress in immunization against Klebsiella infections. Eur. J. Clin. Microbiol. 1983, 2, 523–528. [Google Scholar] [CrossRef]
- Ravinder, M.; Liao, K.S.; Cheng, Y.Y.; Pawar, S.; Lin, T.L.; Wang, J.T.; Wu, C.Y. A Synthetic Carbohydrate-Protein Conjugate Vaccine Candidate against Klebsiella pneumoniae Serotype K2. J. Org. Chem. 2020, 85, 15964–15997. [Google Scholar] [CrossRef] [PubMed]
- Ghaderinia, P.; Shapouri, R.; Rostamizadeh, K.; Khodavandi, A.; Mahdavi, M. Capsular K-antigen-PLGA Nano conjugated Vaccine against Klebsiella pneumoniea pneumoniae K2O1 Infection. J. Adv. Med. Biomed. Res. 2022, 30, 73–74. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, J.; Wang, K.; Li, X.; Sun, P.; Zhang, L.; Huang, J.; Liu, Y.; Hua, X.; Yu, Y.; et al. Production of a Promising Biosynthetic Self-Assembled Nanoconjugate Vaccine against Klebsiella Pneumoniae Serotype O2 in a General Escherichia Coli Host. Adv. Sci. (Weinh) 2021, 8, e2100549. [Google Scholar] [CrossRef] [PubMed]
- Hegerle, N.; Choi, M.; Sinclair, J.; Amin, M.N.; Ollivault-Shiflett, M.; Curtis, B.; Laufer, R.S.; Shridhar, S.; Brammer, J.; Toapanta, F.R.; et al. Development of a broad spectrum glycoconjugate vaccine to prevent wound and disseminated infections with Klebsiella pneumoniae and Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0203143. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Pereira, C.L.; Khan, N.; Xiao, G.; Diago-Navarro, E.; Reppe, K.; Opitz, B.; Fries, B.C.; Witzenrath, M. A Semi-Synthetic Glycoconjugate Vaccine Candidate for Carbapenem-Resistant Klebsiella pneumoniae. Angew. Chem. Int. Ed. Engl. 2017, 56, 13973–13978. [Google Scholar] [CrossRef] [PubMed]
- Giguere, D. Surface polysaccharides from Acinetobacter baumannii: Structures and syntheses. Carbohydr. Res. 2015, 418, 29–43. [Google Scholar] [CrossRef]
- Rudenko, N.; Karatovskaya, A.; Zamyatina, A.; Shepelyakovskaya, A.; Semushina, S.; Brovko, F.; Shpirt, A.; Torgov, V.; Kolotyrkina, N.; Zinin, A.; et al. Immune Response to Conjugates of Fragments of the Type K9 Capsular Polysaccharide of Acinetobacter baumannii with Carrier Proteins. Microbiol. Spectr. 2022, 10, e0167422. [Google Scholar] [CrossRef]
- Li, X.; Pan, C.; Liu, Z.; Sun, P.; Hua, X.; Feng, E.; Yu, Y.; Wu, J.; Zhu, L.; Wang, H. Safety and immunogenicity of a new glycoengineered vaccine against Acinetobacter baumannii in mice. Microb. Biotechnol. 2022, 15, 703–716. [Google Scholar] [CrossRef]
- Lee, I.M.; Yang, F.L.; Chen, T.L.; Liao, K.S.; Ren, C.T.; Lin, N.T.; Chang, Y.P.; Wu, C.Y.; Wu, S.H. Pseudaminic Acid on Exopolysaccharide of Acinetobacter baumannii Plays a Critical Role in Phage-Assisted Preparation of Glycoconjugate Vaccine with High Antigenicity. J. Am. Chem. Soc. 2018, 140, 8639–8643. [Google Scholar] [CrossRef]
- Pier, G.B. The challenges and promises of new therapies for cystic fibrosis. J. Exp. Med. 2012, 209, 1235–1239. [Google Scholar] [CrossRef]
- Liu, P.V. Comparison of the Chinese schema and the International Antigenic Typing System for serotyping Pseudomonas aeruginosa. J. Clin. Microbiol. 1987, 25, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Estahbanati, H.K.; Kashani, P.P.; Ghanaatpisheh, F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns 2002, 28, 340–348. [Google Scholar] [CrossRef]
- Lu, Q.; Eggimann, P.; Luyt, C.E.; Wolff, M.; Tamm, M.; Francois, B.; Mercier, E.; Garbino, J.; Laterre, P.F.; Koch, H.; et al. Pseudomonas aeruginosa serotypes in nosocomial pneumonia: Prevalence and clinical outcomes. Crit. Care 2014, 18, R17. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Azimi, S.; Salouti, M. Protective effect of two new nanovaccines against Pseudomonas aeruginosa based on LPS and OPS: A comparison study. Immunobiology 2022, 227, 152278. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef]
- Saade, E.; Gravenstein, S.; Donskey, C.J.; Wilson, B.; Spiessens, B.; Abbanat, D.; Poolman, J.; de Palacios, P.I.; Hermans, P. Characterization of Escherichia coli isolates potentially covered by ExPEC4V and ExPEC10V, that were collected from post-transrectal ultrasound-guided prostate needle biopsy invasive urinary tract and bloodstream infections. Vaccine 2020, 38, 5100–5104. [Google Scholar] [CrossRef]
- McConnell, M.J.; Rumbo, C.; Bou, G.; Pachon, J. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine 2011, 29, 5705–5710. [Google Scholar] [CrossRef]
- Roberts, R.; Moreno, G.; Bottero, D.; Gaillard, M.E.; Fingermann, M.; Graieb, A.; Rumbo, M.; Hozbor, D. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 2008, 26, 4639–4646. [Google Scholar] [CrossRef]
- Shang, E.S.; Champion, C.I.; Wu, X.Y.; Skare, J.T.; Blanco, D.R.; Miller, J.N.; Lovett, M.A. Comparison of protection in rabbits against host-adapted and cultivated Borrelia burgdorferi following infection-derived immunity or immunization with outer membrane vesicles or outer surface protein A. Infect. Immun. 2000, 68, 4189–4199. [Google Scholar] [CrossRef]
- Keenan, J.; Day, T.; Neal, S.; Cook, B.; Perez-Perez, G.; Allardyce, R.; Bagshaw, P. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol. Lett. 2000, 182, 259–264. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, E.J.; Lee, J.H.; Jun, S.H.; Choi, C.W.; Kim, S.I.; Kang, S.S.; Hyun, S. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol. Lett. 2012, 331, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.N.; Leiman, S.A.; Kuehn, M.J. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 2010, 78, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- van der Put, R.M.F.; Smitsman, C.; de Haan, A.; Hamzink, M.; Timmermans, H.; Uittenbogaard, J.; Westdijk, J.; Stork, M.; Ophorst, O.; Thouron, F.; et al. The First-in-Human Synthetic Glycan-Based Conjugate Vaccine Candidate against Shigella. ACS Cent. Sci. 2022, 8, 449–460. [Google Scholar] [CrossRef] [PubMed]
- FDA. Vaccines Licensed for Use in the United States. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states (accessed on 17 November 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).