Maturation of Aluminium Adsorbed Antigens Contributes to the Creation of Homogeneous Vaccine Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Antigens
2.2. Adjuvants, Buffers and Excipients
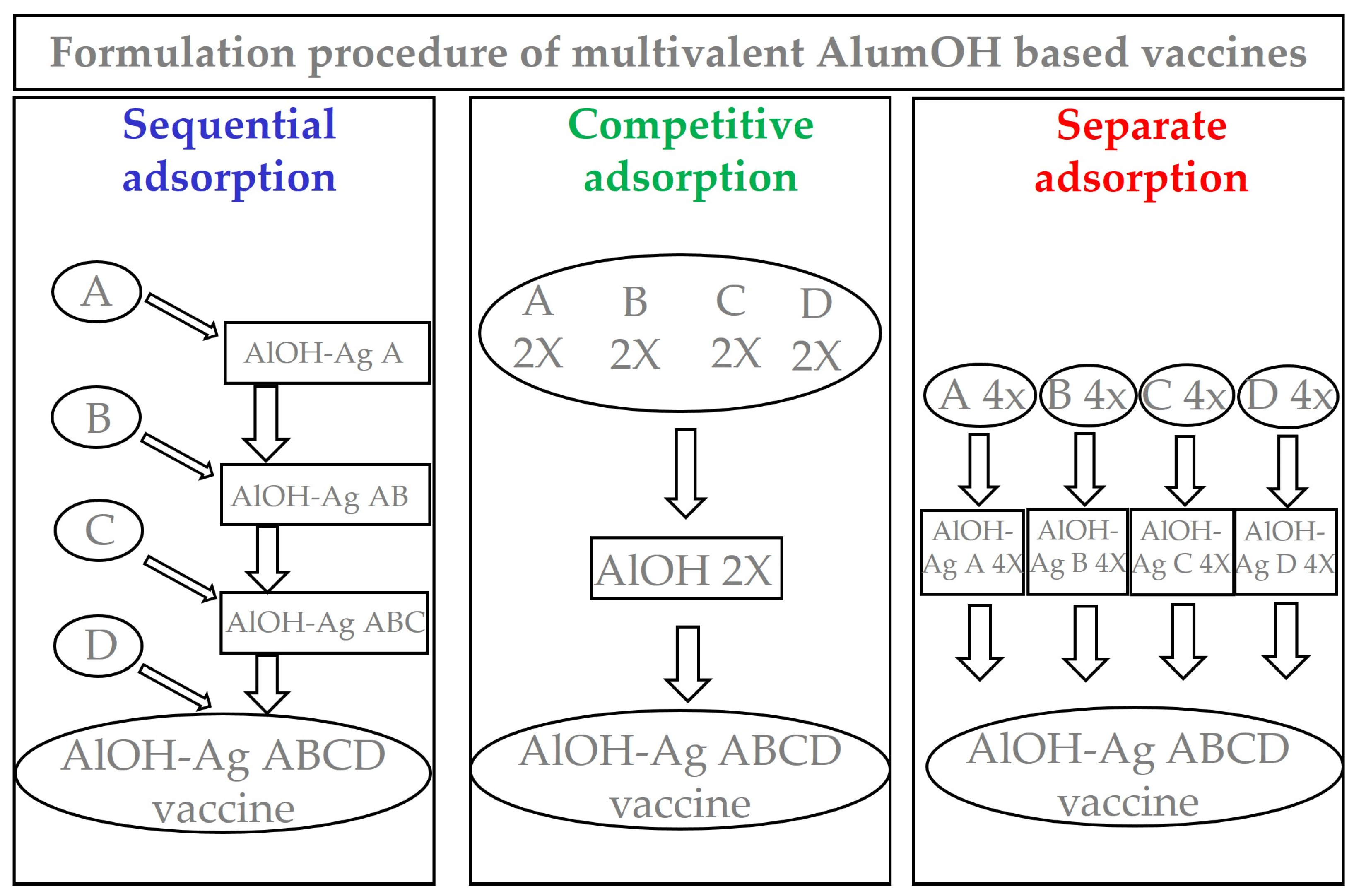
2.3. Preparation of Formulation Samples
2.3.1. Sequential Adsorption Approach
2.3.2. Competitive Adsorption Approach
2.3.3. Separate Adsorption Approach
2.4. pH-Metry
2.5. Sodium Dodecyl-Sulfate PolyAcrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Static Light Scattering (SLS)
2.7. Zeta Potential (ZP)
2.8. Flow Cytometry (FC)
2.9. Separate Adsorption Sample Sorting and Western Blot Analysis
3. Results
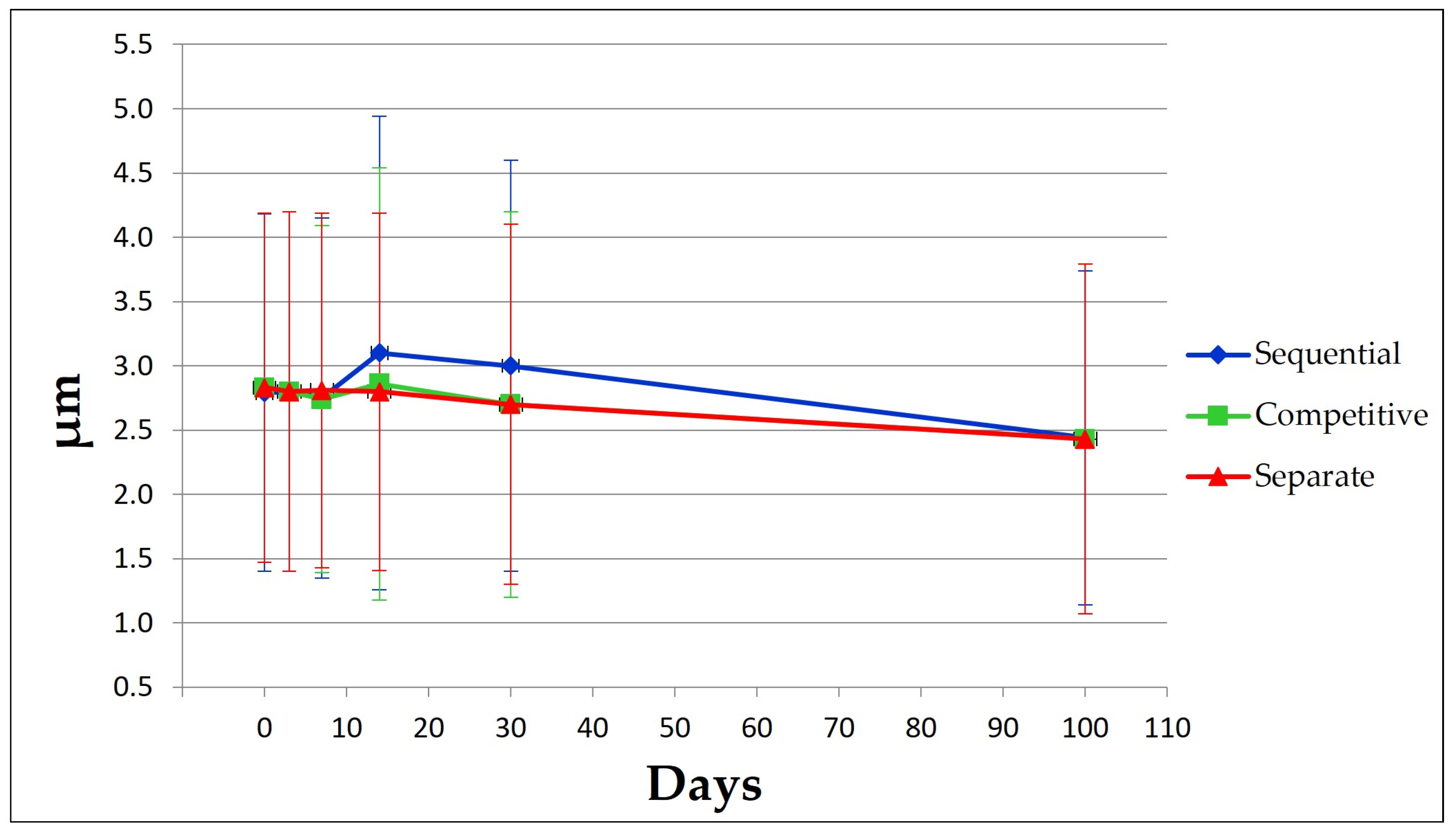
3.1. pH Values and Particle Size Distribution of Formulation Samples
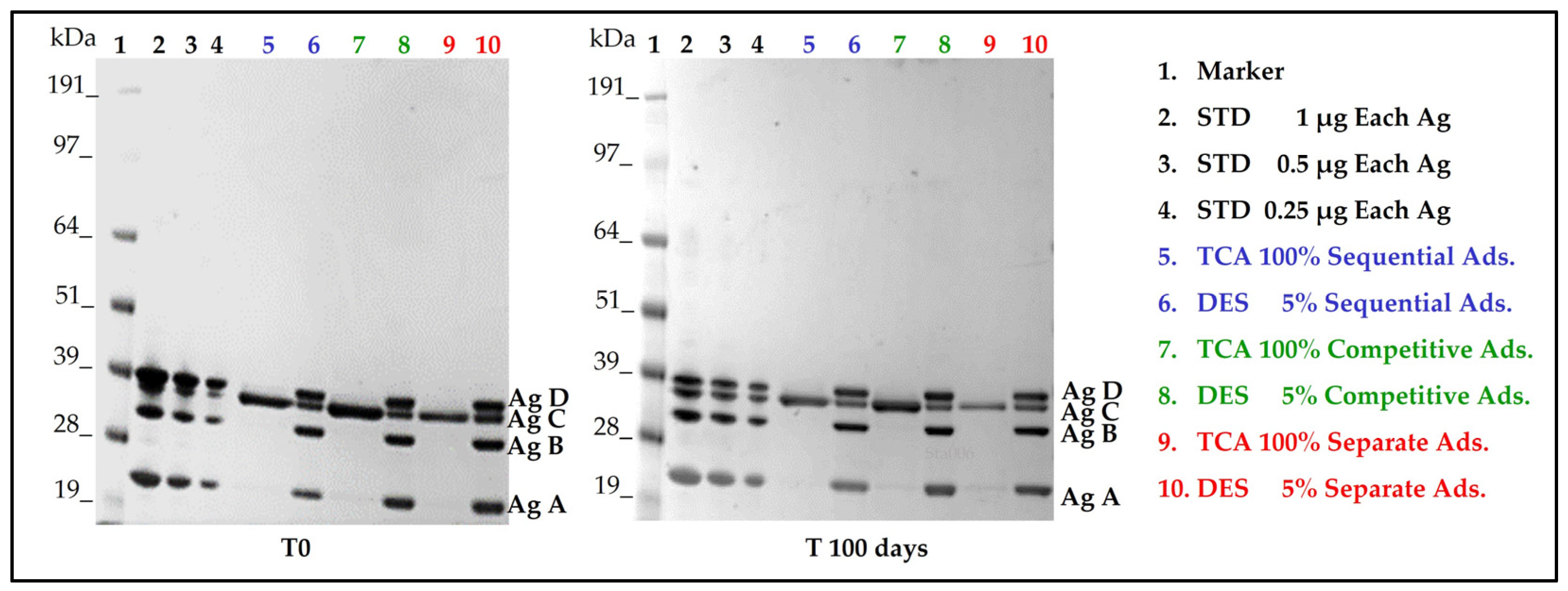
3.2. Antigen Integrity and Adsorption onto AlumOH
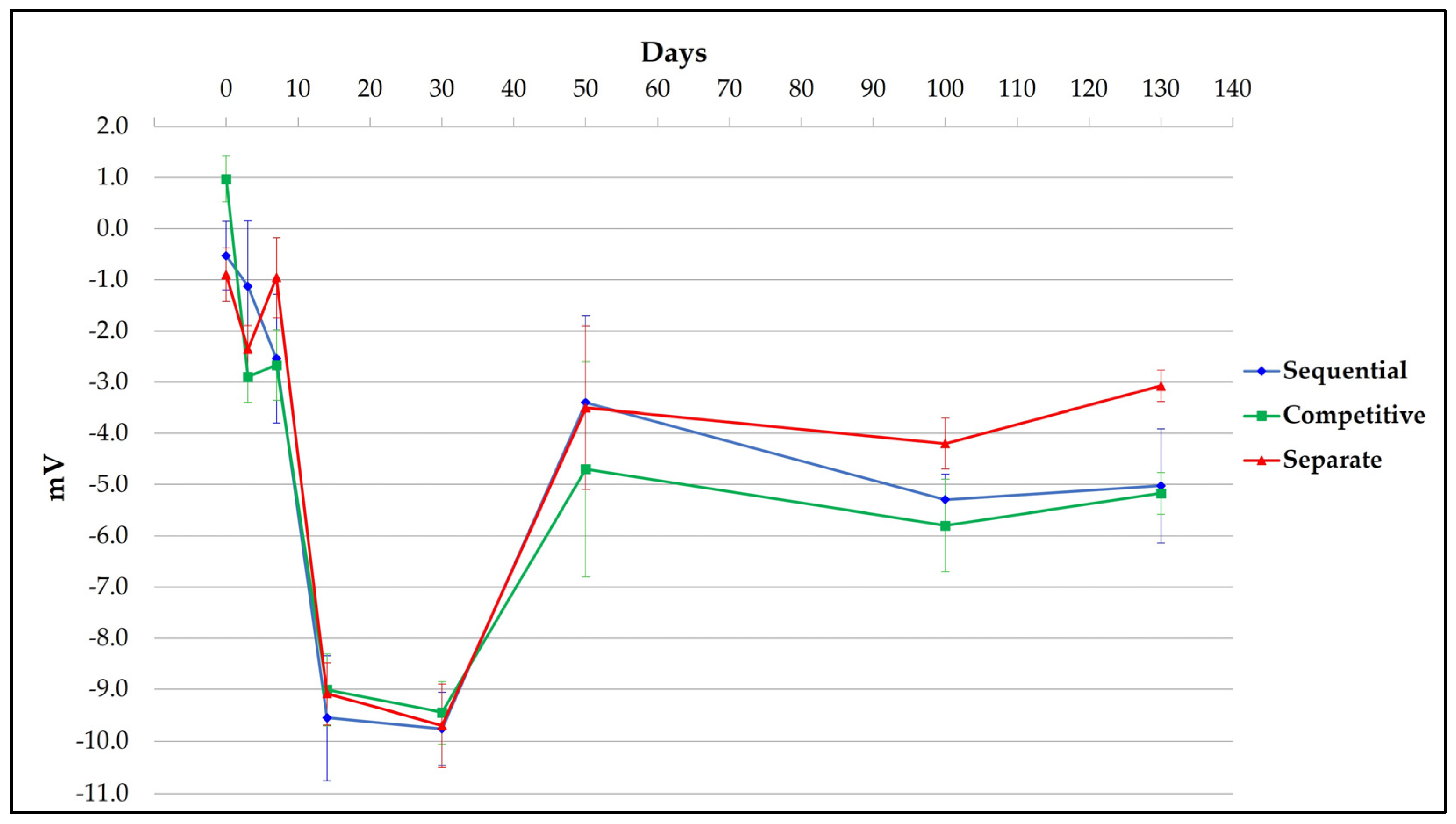
3.3. Surface Charge of Different Formulation Samples
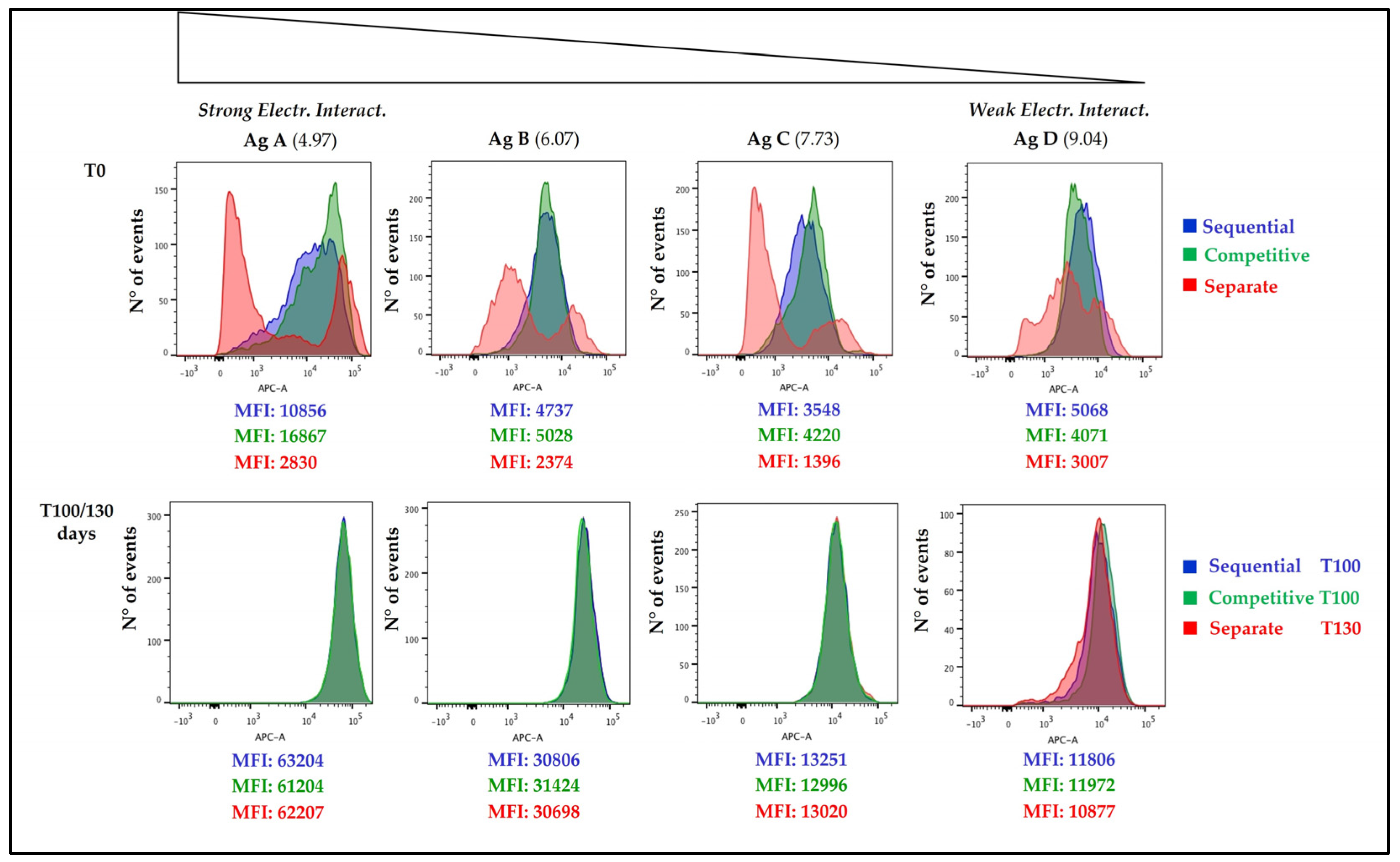
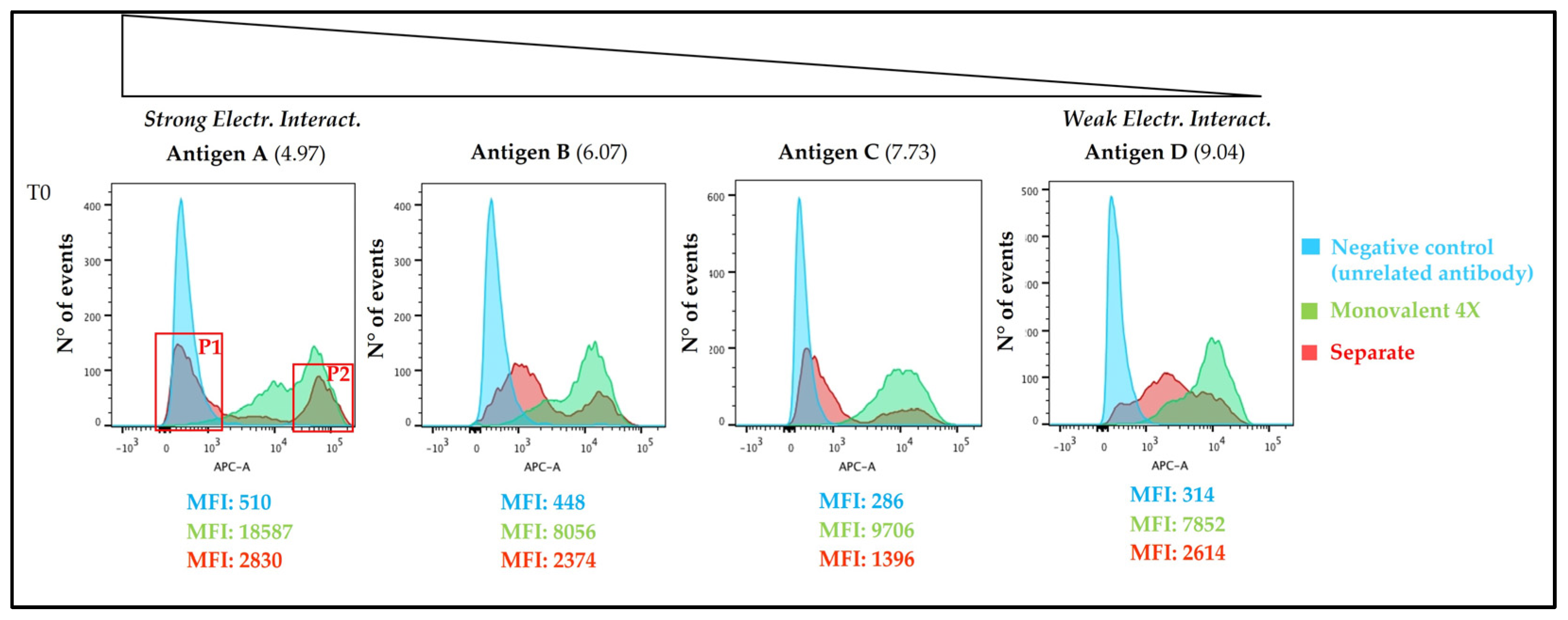
3.4. Antigens Distribution over AlumOH Particles through FC Analysis
3.4.1. Time Point 0 and 100/130 Days
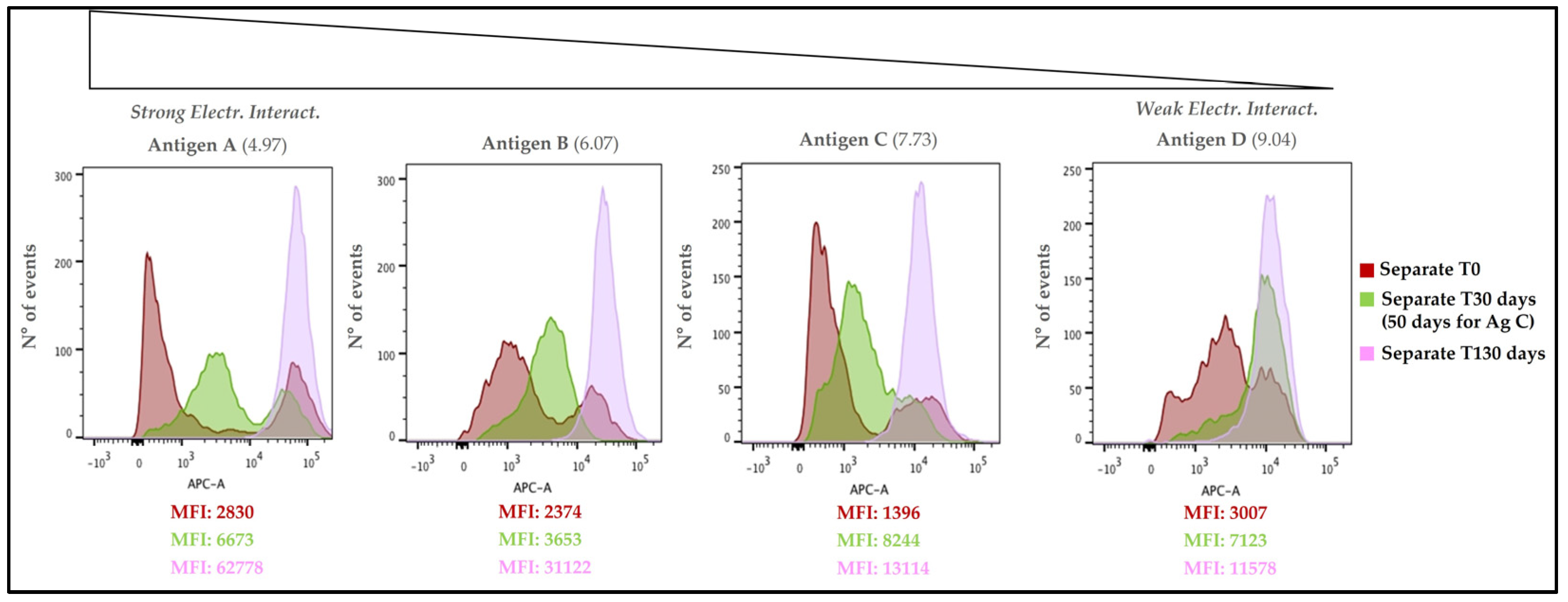
3.4.2. Overlay of Different Time Points for Separate Adsorption Approach Sample
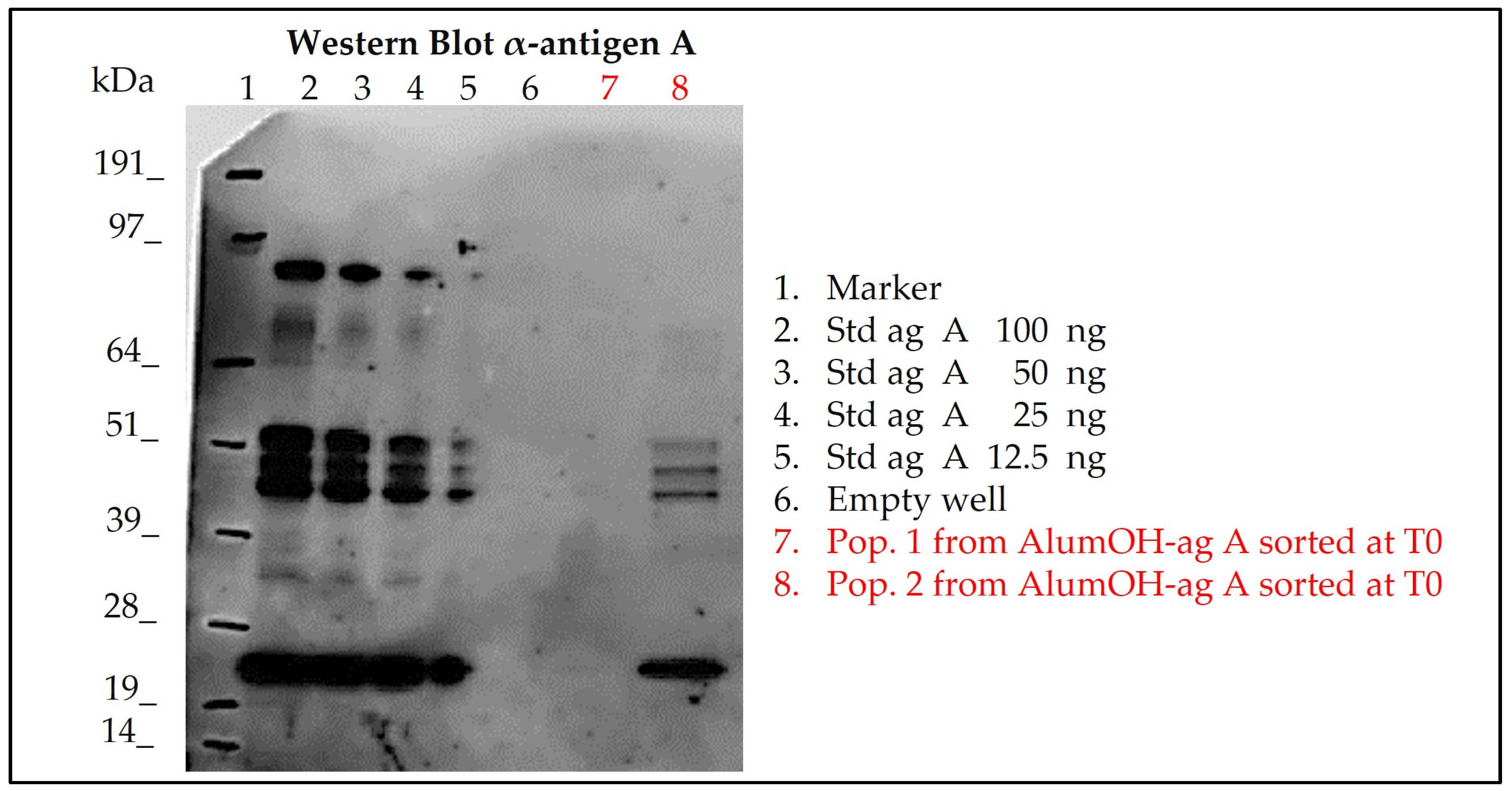
3.4.3. Sorting of Separate Adsorption Sample and Western Blot Analysis for Antigen A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Panhuis, W.G.; Grefenstette, J.; Jung, S.Y.; Chok, N.S.; Cross, A.; Eng, H.; Lee, B.Y.; Zadorozhny, V.; Brown, S.; Cummings, D.; et al. Contagious diseases in the United States from 1888 to the present. N. Engl. J. Med. 2013, 369, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Serruto, D.; Rappuoli, R. Post-genomic vaccine development. FEBS Lett. 2006, 580, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; O’Hagan, D. Advances in vaccine adjuvants. Nat. Biotechnol. 1999, 17, 1075–1081. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; De Gregorio, E. The path to a successful vaccine adjuvant—‘The long and winding road’. Drug Discov. Today 2009, 14, 541–551. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Friedland, L.R.; Hanon, E.; Didierlaurent, A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 2004, 82, 497–505. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccin. Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Hem, S.L.; Hogenesch, H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert. Rev. Vaccines 2007, 6, 685–698. [Google Scholar] [CrossRef]
- Romero Mendez, I.Z.; Shi, Y.; HogenEsch, H.; Hem, S.L. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine 2007, 25, 825–833. [Google Scholar] [CrossRef]
- Noe, S.M.; Green, M.A.; HogenEsch, H.; Hem, S.L. Mechanism of immunopotentiation by aluminum-containing adjuvants elucidated by the relationship between antigen retention at the inoculation site and the immune response. Vaccine 2010, 28, 3588–3594. [Google Scholar] [CrossRef]
- Hutchison, S.; Benson, R.A.; Gibson, V.B.; Pollock, A.H.; Garside, P.; Brewer, J.M. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012, 26, 1272–1279. [Google Scholar] [CrossRef]
- Clapp, T.; Siebert, P.; Chen, D.; Jones Braun, L. Vaccines with aluminum-containing adjuvants: Optimizing vaccine efficacy and thermal stability. J. Pharm. Sci. 2011, 100, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Lipsitch, M.; Almond, J.W. Vaccine production, distribution, access, and uptake. Lancet 2011, 378, 428–438. [Google Scholar] [CrossRef]
- Ugozzoli, M.; Laera, D.; Nuti, S.; Skibinski, D.A.; Bufali, S.; Sammicheli, C.; Tavarini, S.; Singh, M.; O’Hagan, D.T. Flow cytometry: An alternative method for direct quantification of antigens adsorbed to aluminum hydroxide adjuvant. Anal. Biochem. 2011, 418, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Morefield, G.L.; HogenEsch, H.; Robinson, J.P.; Hem, S.L. Distribution of adsorbed antigen in mono-valent and combination vaccines. Vaccine 2004, 22, 1973–1984. [Google Scholar] [CrossRef]
- Agnolon, V.; Bruno, C.; Galletti, B.; Mori, E.; Ugozzoli, M.; Pergola, C.; O’Hagan, D.T.; Baudner, B.C. Multiplex immunoassay for in vitro characterization of acellular pertussis antigens in combination vaccines. Vaccine 2016, 34, 1040–1046. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, T.; Cao, L.; Wang, X.; Cao, J.; Huang, X.; Cai, Y.; Lin, Z.; Pan, H.; Yuan, Q.; et al. Simultaneous in situ visualization and quantitation of dual antigens adsorbed on adjuvants using high content analysis. Nanomedicine 2019, 14, 2535–2548. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, S.; Brummelman, J.; van Els, C.; Marino, F.; Heck, A.J.R.; van Riet, E.; Metz, B.; Kersten, G.F.A.; Pennings, J.L.A.; Meiring, H.D. Vaccine antigens modulate the innate response of monocytes to Al(OH)3. PLoS ONE 2018, 13, e0197885. [Google Scholar] [CrossRef]
- Ostergaard, E.; Frandsen, P.L.; Sandberg, E. Determination of freeze damage on HPV vaccines by use of flow cytometry. Biologicals 2015, 43, 266–273. [Google Scholar] [CrossRef]
- He, L.; Su, J.; Ming, M.; Bernardo, L.; Chen, T.; Gisonni-Lex, L.; Gajewska, B. Flow cytometry: An efficient method for antigenicity measurement and particle characterization on an adjuvanted vaccine candidate H4-IC31 for tuberculosis. J. Immunol. Methods 2018, 452, 39–45. [Google Scholar] [CrossRef]
- Rinella, J.V.; White, J.L.; Hem, S.L. Effect of pH on the Elution of Model Antigens from Aluminum-Containing Adjuvants. J. Colloid. Interface Sci. 1998, 205, 161–165. [Google Scholar] [CrossRef]
- Hem, S.L.; White, J.L. Structure and properties of aluminum-containing adjuvants. Pharm. Biotechnol. 1995, 6, 249–276. [Google Scholar] [CrossRef] [PubMed]
- Callahan, P.M.; Shorter, A.L.; Hem, S.L. The importance of surface charge in the optimization of antigen-adjuvant interactions. Pharm. Res. 1991, 8, 851–858. [Google Scholar] [CrossRef]
- al-Shakhshir, R.; Regnier, F.; White, J.L.; Hem, S.L. Effect of protein adsorption on the surface charge characteristics of aluminium-containing adjuvants. Vaccine 1994, 12, 472–474. [Google Scholar] [CrossRef] [PubMed]
- al-Shakhshir, R.H.; Regnier, F.E.; White, J.L.; Hem, S.L. Contribution of electrostatic and hydrophobic interactions to the adsorption of proteins by aluminium-containing adjuvants. Vaccine 1995, 13, 41–44. [Google Scholar] [CrossRef]
- Huang, M.; Wang, W. Factors affecting alum-protein interactions. Int. J. Pharm. 2014, 466, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Matheis, W.; Zott, A.; Schwanig, M. The role of the adsorption process for production and control combined adsorbed vaccines. Vaccine 2001, 20, 67–73. [Google Scholar] [CrossRef]
- Chang, M.F.; White, J.L.; Nail, S.L.; Hem, S.L. Role of the electrostatic attractive force in the adsorption of proteins by aluminum hydroxide adjuvant. PDA J. Pharm. Sci. Technol. 1997, 51, 25–29. [Google Scholar]
- Langford, A.; Horwitz, T.; Adu-Gyamfi, E.; Wiley, C.; Holding, E.; Zimmermann, D.; Ignatius, A.A.; Ohtake, S. Impact of Formulation and Suspension Properties on Redispersion of Aluminum-Adjuvanted Vaccines. J. Pharm. Sci. 2020, 109, 1460–1466. [Google Scholar] [CrossRef]
- Jerajani, K.; Wan, Y.; Hickey, J.M.; Kumru, O.S.; Sharma, N.; Pullagurla, S.R.; Ogun, O.; Mapari, S.; Whitaker, N.; Brendle, S.; et al. Analytical and preformulation characterization studies of human papillomavirus virus-like particles to enable quadrivalent multi-dose vaccine formulation development. J. Pharm. Sci. 2022, 111, 2983–2997. [Google Scholar] [CrossRef]
- Iyer, V.; Hu, L.; Liyanage, M.R.; Esfandiary, R.; Reinisch, C.; Meinke, A.; Maisonneuve, J.; Volkin, D.B.; Joshi, S.B.; Middaugh, C.R. Preformulation characterization of an aluminum salt-adjuvanted trivalent recombinant protein-based vaccine candidate against Streptococcus pneumoniae. J. Pharm. Sci. 2012, 101, 3078–3090. [Google Scholar] [CrossRef] [PubMed]
- Ahl, P.L.; Mensch, C.; Hu, B.; Pixley, H.; Zhang, L.; Dieter, L.; Russell, R.; Smith, W.J.; Przysiecki, C.; Kosinski, M.; et al. Accelerating Vaccine Formulation Development Using Design of Experiment Stability Studies. J. Pharm. Sci. 2016, 105, 3046–3056. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.M.; Archer, M.C.; Orr, M.T.; Dubois Cauwelaert, N.; Beebe, E.A.; Huang, P.D.; Dowling, Q.M.; Schwartz, A.M.; Fedor, D.M.; Vedvick, T.S.; et al. Development of a thermostable nanoemulsion adjuvanted vaccine against tuberculosis using a design-of-experiments approach. Int. J. Nanomed. 2018, 13, 3689–3711. [Google Scholar] [CrossRef]
- Sesardic, D.; Rijpkema, S.; Patel, B.P. New adjuvants: EU regulatory developments. Expert Rev. Vaccines 2007, 6, 849–861. [Google Scholar] [CrossRef]
- Verch, T.; Trausch, J.J.; Shank-Retzlaff, M. Principles of vaccine potency assays. Bioanalysis 2018, 10, 163–180. [Google Scholar] [CrossRef]
- Rappuoli, R. Towards animal free and science based measures of critical quality attributes for vaccine quality control and release. Vaccine 2019, 37, 3745–3746. [Google Scholar] [CrossRef] [PubMed]
- Westdijk, J.; Metz, B.; Spruit, N.; Tilstra, W.; van der Gun, J.; Hendriksen, C.; Kersten, G. Antigenic fingerprinting of diphtheria toxoid adsorbed to aluminium phosphate. Biologicals 2017, 47, 69–75. [Google Scholar] [CrossRef]
- Wittayanukulluk, A.; Jiang, D.; Regnier, F.E.; Hem, S.L. Effect of microenvironment pH of aluminum hydroxide adjuvant on the chemical stability of adsorbed antigen. Vaccine 2004, 22, 1172–1176. [Google Scholar] [CrossRef]
- Otto, R.B.; Burkin, K.; Amir, S.E.; Crane, D.T.; Bolgiano, B. Patterns of binding of aluminum-containing adjuvants to Haemophilus influenzae type b and meningococcal group C conjugate vaccines and components. Biologicals 2015, 43, 355–362. [Google Scholar] [CrossRef]
- Latour, R.A. Fundamental Principles of the Thermodynamics and Kinetics of Protein Adsorption to Material Surfaces. Colloids Surf. Biointerfaces 2020, 191, 110992. [Google Scholar] [CrossRef]
- Jiang, D.; Morefield, G.L.; HogenEsch, H.; Hem, S.L. Relationship of adsorption mechanism of antigens by aluminum-containing adjuvants to in vitro elution in interstitial fluid. Vaccine 2006, 24, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Colaprico, A.; Senesi, S.; Ferlicca, F.; Brunelli, B.; Ugozzoli, M.; Pallaoro, M.; O’Hagan, D.T. Adsorption onto aluminum hydroxide adjuvant protects antigens from degradation. Vaccine 2020, 38, 3600–3609. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Hickey, J.M.; McAdams, D.; White, J.A.; Sitrin, R.; Khandke, L.; Cryz, S.; Joshi, S.B.; Volkin, D.B. Effect of Aluminum Adjuvant and Preservatives on Structural Integrity and Physicochemical Stability Profiles of Three Recombinant Subunit Rotavirus Vaccine Antigens. J. Pharm. Sci. 2020, 109, 476–487. [Google Scholar] [CrossRef] [PubMed]

| Antigen Id. | Molecular Weight (Da) | Isoelectric Point (IEP) | Supposed Adsorption Strength |
|---|---|---|---|
| A | 22,813 | 4.97 | Strong |
| B | 27,301 | 6.07 | Intermediate |
| C | 33,525 | 7.73 | Intermediate |
| D | 32,425 | 9.04 | Weak |
| Attribute | Method | Expected Result |
|---|---|---|
| pH | pH-metry | 6.5 ± 0.5 (Histidine buffer) |
| AlumOH aggregation profile | Static Light Scattering | 1–20 μm |
| Antigen integrity and adsorption | SDS-PAGE | No degradation and almost complete adsorption |
| AlumOH surface charge | Zeta Potential | ≤0 (mV) |
| Antigen distribution | Flow Cytometry | Variation of Mean Fluorescence Intensity (MFI) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laera, D.; Scarpellini, C.; Tavarini, S.; Baudner, B.; Marcelli, A.; Pergola, C.; Meppen, M.; O’Hagan, D.T. Maturation of Aluminium Adsorbed Antigens Contributes to the Creation of Homogeneous Vaccine Formulations. Vaccines 2023, 11, 155. https://doi.org/10.3390/vaccines11010155
Laera D, Scarpellini C, Tavarini S, Baudner B, Marcelli A, Pergola C, Meppen M, O’Hagan DT. Maturation of Aluminium Adsorbed Antigens Contributes to the Creation of Homogeneous Vaccine Formulations. Vaccines. 2023; 11(1):155. https://doi.org/10.3390/vaccines11010155
Chicago/Turabian StyleLaera, Donatello, Camilla Scarpellini, Simona Tavarini, Barbara Baudner, Agnese Marcelli, Carlo Pergola, Malte Meppen, and Derek T. O’Hagan. 2023. "Maturation of Aluminium Adsorbed Antigens Contributes to the Creation of Homogeneous Vaccine Formulations" Vaccines 11, no. 1: 155. https://doi.org/10.3390/vaccines11010155
APA StyleLaera, D., Scarpellini, C., Tavarini, S., Baudner, B., Marcelli, A., Pergola, C., Meppen, M., & O’Hagan, D. T. (2023). Maturation of Aluminium Adsorbed Antigens Contributes to the Creation of Homogeneous Vaccine Formulations. Vaccines, 11(1), 155. https://doi.org/10.3390/vaccines11010155







