Efficacy of the Second COVID-19 Vaccine Booster Dose in the Elderly
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Pan, N. Visualizing the History of Pandemics. Available online: https://www.visualcapitalist.com/history-of-pandemics-deadliest (accessed on 21 November 2022).
- Maynou, L.; Owen, R.; Konstant-Hambling, R.; Imam, T.; Arkill, S.; Bertfield, D.; Street, A.; Abrams, K.R.; Conroy, S. The association between frailty risk and COVID-19-associated all-mortality in hospitalised older people: A national cohort study. Eur. Geriatr. Med. 2022, 13, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Efficacy of COVID-19 vaccine booster doses in older people. Eur. Geriatr. Med. 2022, 13, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. Epidemia COVID-19—Aggiornamento Nazionale 18 November 2022. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati (accessed on 21 November 2021).
- Monto, A.S. The Future of SARS-CoV-2 Vaccination—Lessons from Influenza. N. Engl. J. Med. 2021, 385, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, H.A.; Khedr, A.; Koritala, T.; Bartlett, B.N.; Jain, N.K.; Khan, S.A. A review of adverse effects of COVID-19 vaccines. Infez. Med. 2022, 30, 1–10. [Google Scholar] [PubMed]
- Utami, A.M.; Rendrayani, F.; Khoiry, Q.A.; Noviyanti, D.; Suwantika, A.A.; Postma, M.J.; Zakiyah, N. Economic evaluation of COVID-19 vaccination: A systematic review. J. Glob. Health 2023, 13, 06001. [Google Scholar] [CrossRef] [PubMed]
- Dadras, O.; SeyedAlinaghi, S.; Karimi, A.; Shamsabadi, A.; Qaderi, K.; Ramezani, M.; Mirghaderi, S.P.; Mahdiabadi, S.; Vahedi, F.; Saeidi, S.; et al. COVID-19 mortality and its predictors in the elderly: A systematic review. Health Sci. Rep. 2022, 5, e657. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. COVID vaccine boosters: The most important questions. Nature 2021, 596, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, M.; Mateo-Urdiales, A.; Sacco, C.; Rota, M.C.; Petrone, D.; Bressi, M.; Del Manso, M.; Siddu, A.; Proietti, V.; Battilomo, S.; et al. Relative effectiveness of a 2nd booster dose of COVID-19 mRNA vaccine up to four months post administration in individuals aged 80 years or more in Italy: A retrospective matched cohort study. Vaccine 2023, 41, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kislaya, I.; Machado, A.; Magalhães, S.; Rodrigues, A.P.; Franco, R.; Leite, P.P.; Dias, C.M.; Nunes, B. COVID-19 mRNA vaccine effectiveness (second and first booster dose) against hospitalisation and death during Omicron BA.5 circulation: Cohort study based on electronic health records, Portugal, May to July 2022. Eurosurveillance 2022, 27, 2200697. [Google Scholar] [CrossRef] [PubMed]
- Patalon, T.; Saciuk, Y.; Peretz, A.; Perez, G.; Lurie, Y.; Maor, Y.; Gazit, S. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat. Commun. 2022, 13, 3203. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; De Nitto, S.; Gianfilippi, G.; Lippi, G. Three-month ad interim analysis of total anti-SARS-CoV-2 antibodies in healthy recipient of a single BNT162b2 vaccine booster. Clin. Chem. Lab. Med. 2022, 60, e181–e183. [Google Scholar] [CrossRef] [PubMed]
- Stieber, F.; Allen, N.; Carpenter, K.; Hu, P.; Alagna, R.; Rao, S.; Manissero, D.; Howard, J.; Nikolayevskyy, V. Durability of COVID-19 vaccine induced T-cell mediated immune responses measured using the QuantiFERON SARS-CoV-2 assay. Pulmonology 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Henry, B.M. Is cellular immunity the future key for deciphering and monitoring COVID-19 vaccines efficacy? J. Lab. Precis. Med. 2022, 7, 18. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Expected cost effectiveness of the fourth dose of COVID-19 vaccine against the omicron variant of COVID-19: A preliminary report. Int. J. Physiol. Pathophysiol. Pharmacol. 2022, 14, 272–275. [Google Scholar] [PubMed]
- Lippi, G.; Mattiuzzi, C. Bilavent vaccines against COVID-19. Biochim. Clin. 2022, 46, 279–282. [Google Scholar] [CrossRef]
- He, Q.; Sun, S.; Chen, X.; Hu, Z.; Zhang, Y.; Peng, H.; Fu, Y.X.; Yang, J.; Chen, L. The Bivalent COVID-19 Booster Immunization after Three Doses of Inactivated Vaccine Augments the Neutralizing Antibody Response against Circulating Omicron Sublineages. J. Clin. Med. 2022, 12, 146. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Weber, Z.A.; Natarajan, K.; Klein, N.P.; Kharbanda, A.B.; Stenehjem, E.; Embi, P.J.; Reese, S.E.; Naleway, A.L.; Grannis, S.J.; et al. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults—VISION Network, Nine States, September–November 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Surie, D.; De Cuir, J.; Zhu, Y.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Hospitalization Among Immunocompetent Adults Aged ≥65 Years—IVY Network, 18 States, 8 Septembe–30 November 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, T.; Onodera, T.; Takahashi, T.; Harigae, H.; Ishii, T. Acute Adverse Events at a Mass Vaccination Site after the 3rd and 4th COVID-19 Vaccinations in Japan. Tohoku J. Exp. Med. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
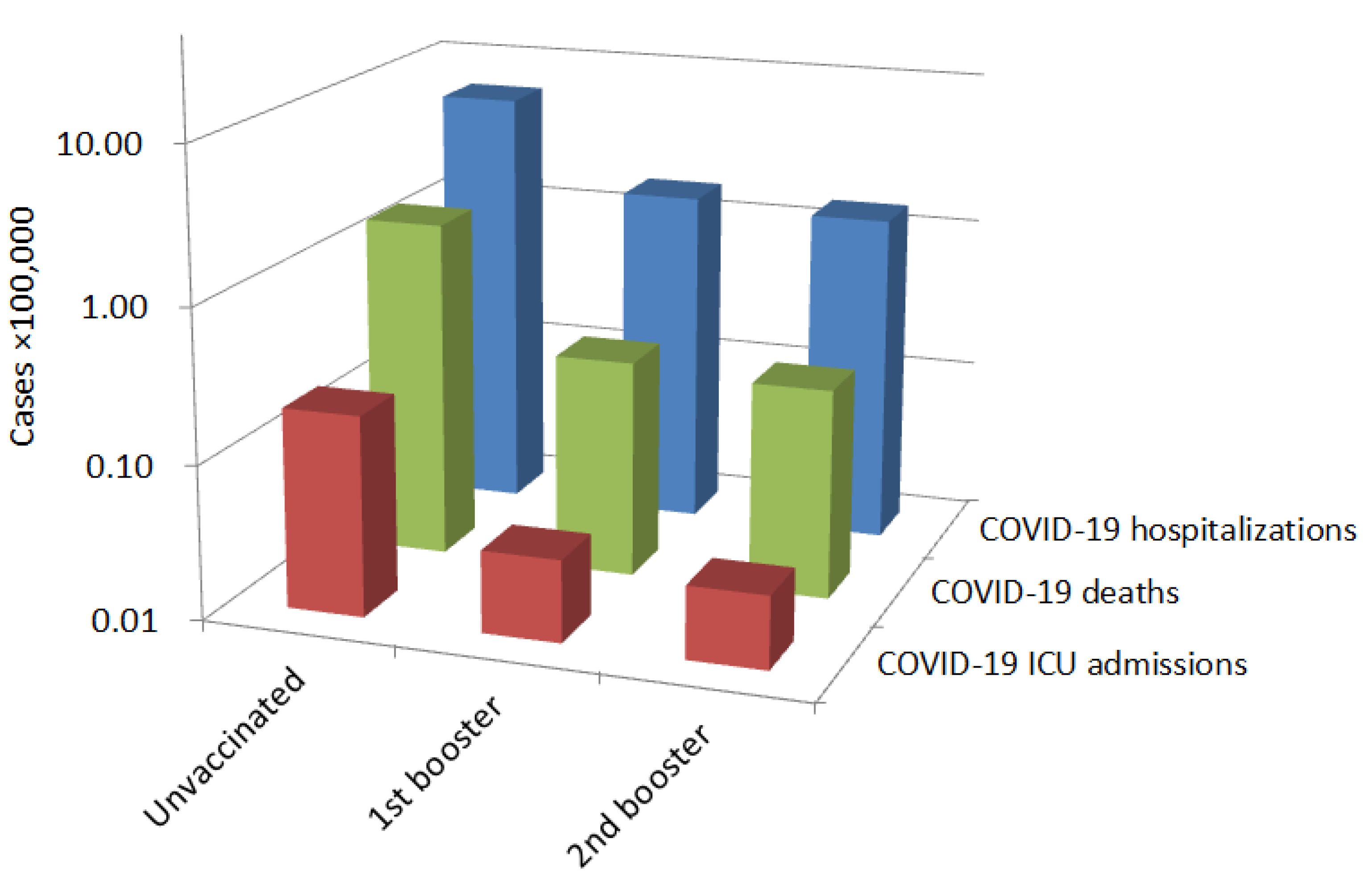
| Endpoint | Unvaccinated | 2nd Vaccine Booster |
|---|---|---|
| OR (95%CI) COVID-19-related hospitalizations | ||
| 1st vaccine booster | 0.26 (95% CI, 0.24–0.28; p < 0.001) | 0.89 (95% CI, 0.84–0.94; p < 0.001) |
| 2nd vaccine booster | 0.23 (95% CI, 0.21–0.25; p < 0.001) | - |
| OR (95%CI) COVID-19-related ICU admissions | ||
| 1st vaccine booster | 0.17 (95% CI, 0.11–0.26; p < 0.001) | 0.87 (95% CI, 0.61–1.24; p = 0.443) |
| 2nd vaccine booster | 0.15 (95% CI, 0.09–0.24; p < 0.001) | - |
| OR (95%CI) COVID-19-related deaths | ||
| 1st vaccine booster | 0.16 (95% CI, 0.14–0.19; p < 0.001) | 0.89 (95% CI, 0.78–1.01; p = 0.074) |
| 2nd vaccine booster | 0.14 (95% CI, 0.12–0.17; p < 0.001) | - |
| Endpoint | Rate (×100,000) | Odds Ratio (95% CI) |
|---|---|---|
| OR (95%CI) COVID-19-related hospitalizations | ||
| ≤120 days | 1.14 | 0.67 (95% CI, 0.61–0.73; p < 0.001) |
| >120 days | 1.71 | |
| OR (95%CI) COVID-19-related ICU admissions | ||
| ≤120 days | 0.02 | 0.53 (95% CI, 0.29–0.99; p = 0.045) |
| >120 days | 0.04 | |
| OR (95%CI) COVID-19-related deaths | ||
| ≤120 days | 0.17 | 0.56 (95% CI, 0.45–0.69; p < 0.001) |
| >120 days | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattiuzzi, C.; Lippi, G. Efficacy of the Second COVID-19 Vaccine Booster Dose in the Elderly. Vaccines 2023, 11, 213. https://doi.org/10.3390/vaccines11020213
Mattiuzzi C, Lippi G. Efficacy of the Second COVID-19 Vaccine Booster Dose in the Elderly. Vaccines. 2023; 11(2):213. https://doi.org/10.3390/vaccines11020213
Chicago/Turabian StyleMattiuzzi, Camilla, and Giuseppe Lippi. 2023. "Efficacy of the Second COVID-19 Vaccine Booster Dose in the Elderly" Vaccines 11, no. 2: 213. https://doi.org/10.3390/vaccines11020213
APA StyleMattiuzzi, C., & Lippi, G. (2023). Efficacy of the Second COVID-19 Vaccine Booster Dose in the Elderly. Vaccines, 11(2), 213. https://doi.org/10.3390/vaccines11020213







