A Cross-Sectional Study to Assess mRNA-COVID-19 Vaccine Safety among Indian Children (5–17 Years) Living in Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Study Design & Tool
2.3. Statistical Analysis
3. Results
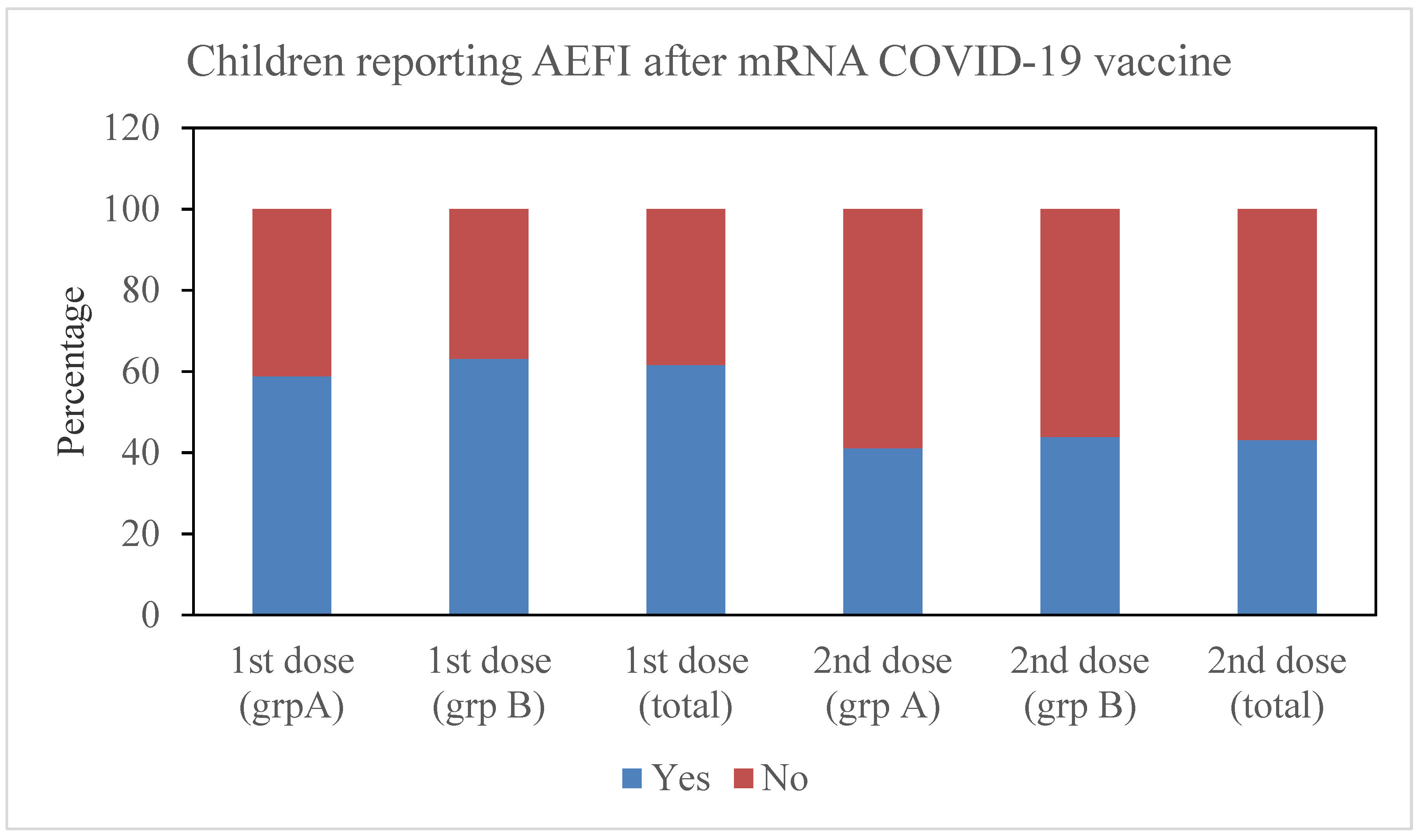
3.1. Relationship with Dose
3.2. Relationship with Gender
3.3. Relationship with Past COVID-19 Infection
3.4. Relationship with History of Allergy and AEFI with Previous Vaccines
3.5. COVID-19 Infection after Vaccination
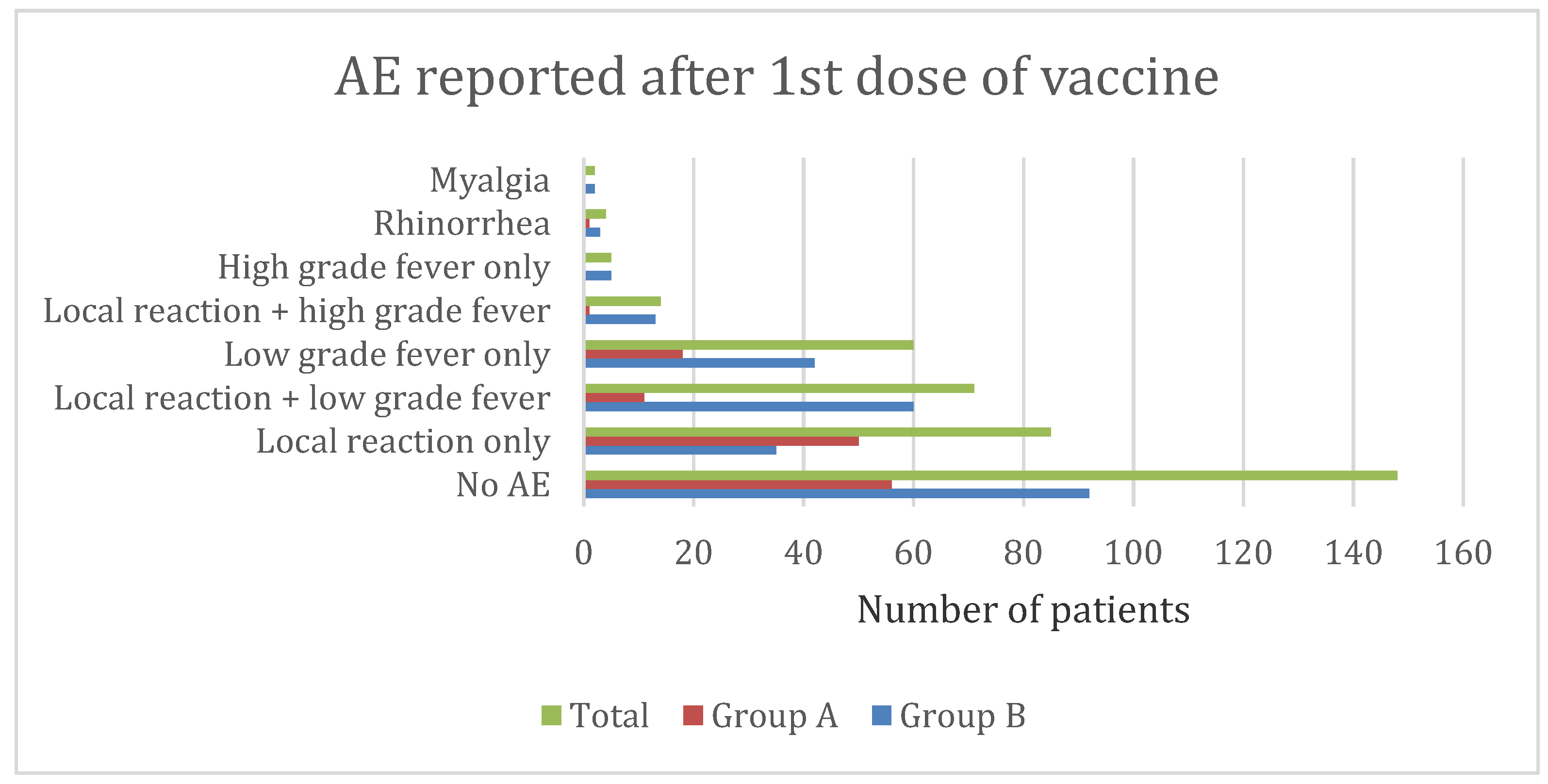
3.6. Adverse Event Characteristics in Group A (5–11 Years)
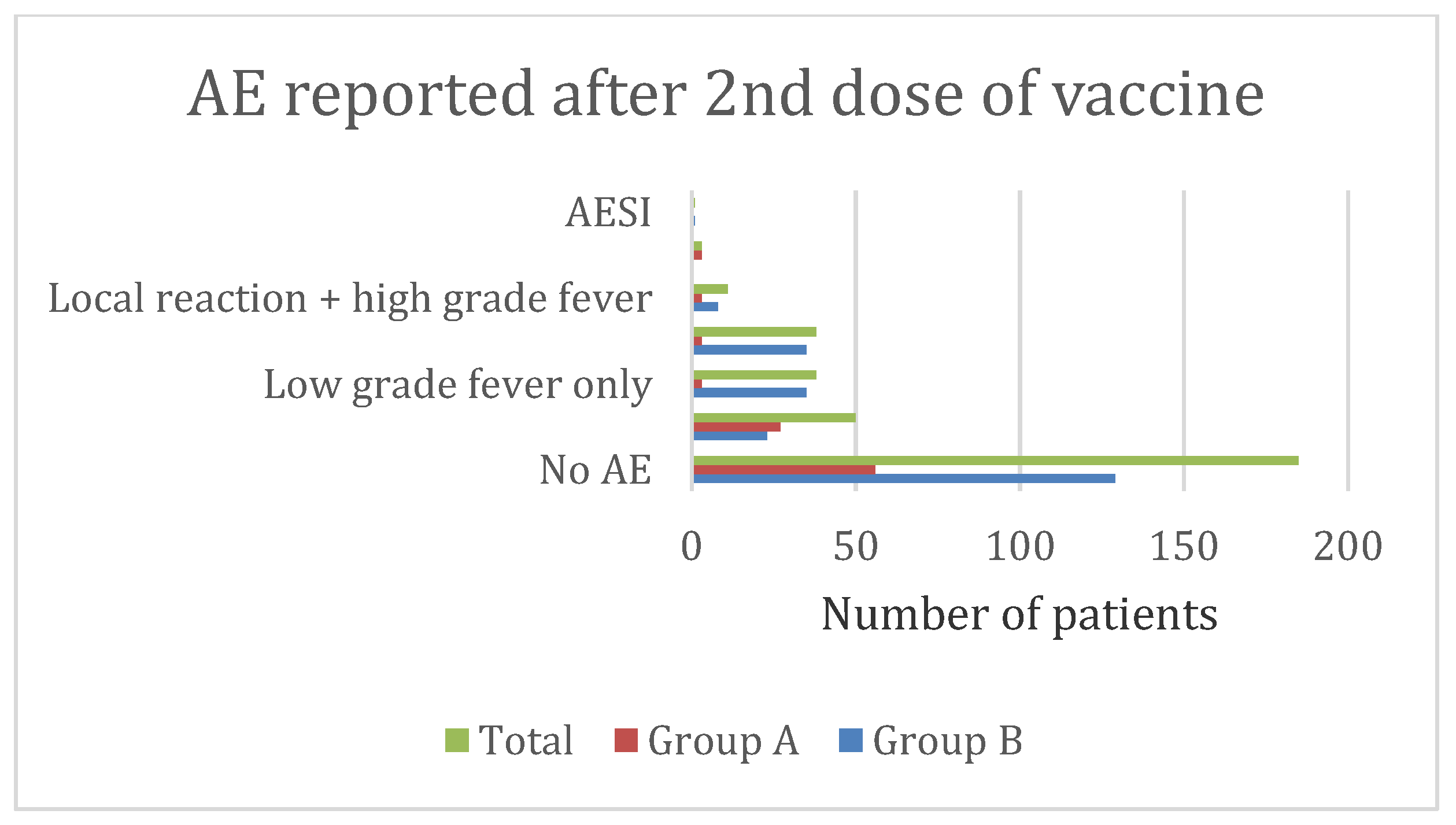
3.7. Adverse Event Characteristics in Group B (12–17 Years)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddique, F.; Abbas, R.Z.; Mansoor, M.K.; Alghamdi, E.S.; Saeed, M.; Ayaz, M.M.; Rahman, M.; Mahmood, M.S.; Iqbal, A.; Manzoor, M.; et al. An Insight Into COVID-19: A 21st Century Disaster and Its Relation to Immunocompetence and Food Antioxidants. Front. Vet. Sci. 2021, 7, 586637. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ayeh, S.K.; Chidambaram, V.; Karakousis, P.C. Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions. BMC Infect. Dis. 2021, 21, 496. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.; Tom, A.; Sen, M.S.; Sagar, R. Psychological Impact of COVID-19 on Children and Adolescents: A Systematic Review. Indian J. Psychol. Med. 2021, 43, 294–299. [Google Scholar] [CrossRef]
- Hause, A.M.; Gee, J.; Baggs, J.; Abara, W.E.; Marquez, P.; Thompson, D.; Su, J.R.; Licata, C.; Rosenblum, H.G.; Myers, T.R.; et al. COVID-19 Vaccine Safety in Adolescents Aged 12-17 Years-United States, December 14, 2020-July 16, 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Gee, J.; Su, J.R.; Zhang, B.; Thompson, D.; Shimabukuro, T.T.; Shay, D.K. COVID-19 Vaccine Safety in Children Aged 5-11 Years-United States, November 3-December 19, 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021, 36, 427–439. [Google Scholar] [CrossRef]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef]
- Suran, M. Why Parents Still Hesitate to Vaccinate Their Children Against COVID-19. JAMA 2022, 327, 23–25. [Google Scholar] [CrossRef]
- Stephenson, J. Many Parents Unlikely to Seek COVID-19 Vaccination for Newly Eligible Young Children, Survey Finds. JAMA Health Forum 2022, 3, e223206. [Google Scholar] [CrossRef]
- Jethwa, H.; Wong, R.; Abraham, S. Covid-19 vaccine trials: Ethnic diversity and immunogenicity. Vaccine 2021, 39, 3541–3543. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 8 November 2022).
- Adverse Events Following Immunization (AEFI). Available online: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi (accessed on 10 November 2022).
- Hartwig, S.C.; Siegel, J.; Schneider, P.J. Preventability and severity assessment in reporting adverse drug reactions. Am. J. Hosp. Pharm. 1992, 49, 2229–2232. [Google Scholar] [CrossRef] [PubMed]
- CIOMS. Practical Aspects of Signal Detection in Pharmacovigilance: Report of Cioms Working Group VIII; CIOMS: Geneva, Switzerland, 2010. [Google Scholar]
- Brighton Collaboration Is Working Diligently to Fight the Coronavirus Disease (COVID-19) Pandemic. Available online: https://brightoncollaboration.us/covid-19/ (accessed on 11 November 2022).
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Lam-Hine, T.; McCurdy, S.A.; Santora, L.; Duncan, L.; Corbett-Detig, R.; Kapusinszky, B.; Willis, M. Outbreak Associated with SARS-CoV-2 B.1.617.2 (Delta) Variant in an Elementary School-Marin County, California, May-June 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Reses, H.E.; Cool, A.J.; Shapiro, C.N.; Hsu, J.; Boehmer, T.K.; Cornwell, C.R.; Gray, E.B.; Henley, S.J.; Lochner, K.; et al. Trends in COVID-19 Cases, Emergency Department Visits, and Hospital Admissions Among Children and Adolescents Aged 0-17 Years-United States, August 2020–August 2021. MMWR Morb. Mortal Wkly. Rep. 2021, 70, 1249–1254. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Kant, A.; Jansen, J.; van Balveren, L.; van Hunsel, F. Description of Frequencies of Reported Adverse Events Following Immunization Among Four Different COVID-19 Vaccine Brands. Drug Saf. 2022, 45, 319–331. [Google Scholar] [CrossRef]
- Le, X.T.T.; Hoang, Q.L.; Ta, N.T.K.; Pham, Q.T.; Nguyen, T.T.; Phan, H.T.M.; Nguyen, T.V.; Le, H.T.T.; Nguyen, N.T.; Hoang, L.D.; et al. Common adverse events following immunization with the COVID-19 comirnaty vaccine (Pfizer-BioNTech) among adult population in Hanoi, Vietnam, 2021. Front. Trop. Dis. 2022, 3, 2911333. [Google Scholar] [CrossRef]
- Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines 2022, 10, 233. [Google Scholar] [CrossRef]
- Hause, A.M.; Shay, D.K.; Klein, N.P.; Abara, W.E.; Baggs, J.; Cortese, M.M.; Fireman, B.; Gee, J.; Glanz, J.M.; Goddard, K.; et al. Safety of COVID-19 Vaccination in United States Children Ages 5 to 11 Years. Pediatrics 2022, 150, e2022057313. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2021, 386, 35–46. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Ghrew, M.; Ustianowski, A.; Ahmad, S.; Borrow, R.; Papavasileiou, L.P.; Petrakis, D.; Bakerly, N.D. Self-Reported Real-World Safety and Reactogenicity of COVID-19 Vaccines: A Vaccine Recipient Survey. Life 2021, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cooper, L.T., Jr. Recovery from mRNA COVID-19 vaccine-related myocarditis. Lancet Child Adolesc. Health 2022, 6, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Husby, A.; Køber, L. COVID-19 mRNA vaccination and myocarditis or pericarditis. Lancet 2022, 399, 2168–2169. [Google Scholar] [CrossRef]
| Variables | Group A (n = 136) | Group B (n = 249) | Total (n = 385) |
|---|---|---|---|
| Region of residence | |||
| Asir region | 13 (3.3%) | 6 (2.4%) | 19 (5%) |
| Eastern region | 33 (24.3%) | 38 (15.3%) | 71 (18.4%) |
| Makkah region | 6 (4.4%) | 23 (9.2%) | 29 (7.5%) |
| Riyadh region | 56 (41.2%) | 157 (63%) | 213 (55.3%) |
| Qasim region | 18 (13.2%) | 24 (9.6%) | 42 (10.9%) |
| Others | 10 (7.4%) | 1 (0.4%) | 11 (2.9%) |
| Mean Age ± SD (in years) | 8.58 ± 2.06 | 14.26 ± 1.74 | 12.25 ± 3.29 |
| Gender | |||
| Male | 84 (61.8%) | 101 (40.6%) | 185 (48.1%) |
| Female | 52 (38.2%) | 148 (59.4%) | 200 (51.9%) |
| No: of doses of COVID-19 vaccine received | |||
| One | 41 (30.1%) | 19 (7.6%) | 60 (15.6%) |
| Two | 95 (69.9%) | 230 (92.4%) | 325 (84.4%) |
| COVID-19 infection before vaccination | |||
| Yes | 19 (14%) | 28 (11.2%) | 47 (12.2%) |
| Maybe | 7 (5.1%) | 8 (3.2%) | 15 (3.9%) |
| COVID-19 infection after vaccination | N = 95 | N = 230 | N = 325 |
| Yes | 11 (11.6%) | 34 (14.8%) | 45 (13.8%) |
| Maybe | 3 (3.2%) | 7 (3%) | 10 (3.1%) |
| History of allergy | |||
| Yes | 15 (11%) | 17 (6.8%) | 32 (8.3%) |
| Maybe | 5 (3.7%) | - | 5 (1.3%) |
| Type of allergy | |||
| Dust allergy | 17 (12.5%) | 16 (6.4%) | 33 (8.6%) |
| Pet allergy | 2 (1.5%) | - | 2 (0.5%) |
| Allergy to antibiotics | 1 (0.7%) | 1 (0.4%) | 2 (0.5%) |
| Family history of allergy | |||
| Yes | 22 (16.2%) | 17 (6.8%) | 39 (10.1%) |
| Maybe | 6 (4.4%) | 8 (3.2%) | 14 (3.6%) |
| Comorbidities | |||
| Asthma | 7 (5.14%) | 4 (1.6%) | 11 (2.9%) |
| Eczema | - | 1 (0.4%) | 1 (0.3%) |
| Adrenal insufficiency | - | 1 (0.4%) | 1 (0.3%) |
| Unwanted events during past routine immunization | |||
| Yes | 3 (2.2%) | 14 (5.6%) | 18 (4.4%) |
| Maybe | - | 7 (2.8%) | 7 (1.8%) |
| Variables | Group A | Group B | Total |
|---|---|---|---|
| Total number of events reported, n | |||
| After 1st dose | 93 | 233 | 326 |
| After 2nd dose | 45 | 144 | 189 |
| Number of patients reporting AE after 1st dose ***, n | N = 136 | N = 249 | N = 385 |
| None | 56 (41.2%) | 92 (36.9%) | 148 (38.4%) |
| One AE | 68 (50%) | 84 (33.7%) | 152 (39.4%) |
| More than one AE | 12 (8.8%) | 73 (29.3%) | 85 (22%) |
| Number of patients reporting AE after 1st dose *, n | N = 95 | N = 230 | N = 325 |
| None | 56 (58.9%) | 129 (56.1%) | 185 (56.9%) |
| One AE | 33 (24.3%) | 58 (25.2%) | 91 (28%) |
| More than one AE | 6 (4.4%) | 43 (18.7%) | 49 (15.1%) |
| Gender of patients reporting at least one AE after 1st dose, n/N # | |||
| Male | 51/84 (60.7%) | 75/101 (74.3%) ** | 126/185 (68.1%) * |
| Female | 29/52 (55.8%) | 82/148 (55.4%) | 111/200 (55.5%) |
| Gender of patients reporting at least one AE after 2nd dose, n/N # | |||
| Male | 23/62 (37.1%) | 45/90 (50%) | 68/152 (44.7%) |
| Female | 16/33 (48.5%) | 56/140 (40%) | 72/173 (41.6%) |
| Cases with history of COVID-19 infection reporting at least one AE, n/N $ | |||
| After 1st dose | 10/19 (57.9%) | 28/28 (100%) | 8/47 (80.9%) |
| After 2nd dose *** | 3/13 (23.1%) | 21/23 (91.3%) | 24/36 (66.7%) |
| Predictors of AE | Odd’s Ratio | 95% CI Upper Limit-Lower Limit | p-Value |
|---|---|---|---|
| Dose AE 1st/2nd | 2.12 | 1.57–2.86 | <0.001 |
| Age AE after 1st dose AE after 2nd dose | 0.84 0.89 | 0.55–1.28 0.55–1.44 | 0.42 0.64 |
| Gender (Male/female) AE after 1st dose AE after 2nd dose | 0.171 1.136 | 1.13–2.56 0.73–1.76 | 0.01 0.57 |
| Prior COVID-19 AE after 1st dose AE after 2nd dose | 2.95 2.98 | 1.38–6.3 1.44–6.2 | 0.05 0.003 |
| Allergy AE after 1st dose AE after 2nd dose | 0.71 0.48 | 0.36–1.41 0.22–1.08 | 0.36 0.08 |
| AEFI with routine vaccination AE after 1st dose AE after 2nd dose | 1.56 0.48 | 0.63–3.85 0.19–1.21 | 0.34 0.12 |
| AE-Related Variables in Group A | Pain, Redness, and Swelling at the Site of Injection | Fever < 102 °F | Fever > 102 °F | |||
|---|---|---|---|---|---|---|
| 1st Dose (n = 136) | 2nd Dose (n = 95) | 1st Dose (n = 136) | 2nd Dose (n = 95) | 1st Dose (n = 136) | 2nd Dose (n = 95) | |
| Number of events, N | 62 | 33 | 29 | 6 | 1 | 6 |
| Onset within, (n/N) | ||||||
| 1–3 days | 62/62 | 33/33 | 29/29 | 6/6 | 1/1 | 6/6 |
| 4–7 days | - | - | - | - | - | - |
| Duration, (n/N) | ||||||
| 1–4 days | 61/62 | 30/33 | 29/29 | 6/6 | - | - |
| 4–7 days | 1/62 | 3/33 | - | - | 1/1 | 6/6 |
| Intervention, (n/N) | ||||||
| No treatment | 45/62 | 24/33 | 14/29 | - | - | - |
| Treatment at home | 17/62 | 9/33 | 15/29 | 6/6 | 1/1 | 6/6 |
| Utilization of health resources, (n/N) | ||||||
| None | 61/62 | 30/33 | 29/29 | - | - | - |
| Medical consultation (non-urgent) | 1/62 | 3/33 | 0/29 | 6/6 | 1/1 | 6/6 |
| Outcome, % | ||||||
| Recovered | 100% | 100% | 100% | 100% | 100% | 100% |
| Unknown | - | - | - | - | - | - |
| Severity, (n/N) | ||||||
| Mild | 45/62 | 24/33 | 14/29 | - | - | - |
| Moderate | 17/62 | 9/33 | 15/29 | 6/6 | 1/1 | 6/6 |
| Seriousness | None | None | None | None | None | None |
| AE-Related Variables in Group B | Pain, Redness, and Swelling at the Site of Injection | Fever < 102 °F | Fever > 102 °F | |||
|---|---|---|---|---|---|---|
| 1st Dose (n = 249) | 2nd Dose (n = 230) | 1st Dose (n = 249) | 2nd Dose (n = 230) | 1st Dose (n = 249) | 2nd Dose (n = 230) | |
| Number of events, N | 108 | 66 | 102 | 70 | 20 | 8 |
| Onset within, n/N 1–3 days 4–7 days | 108/108 - | 66/66 - | 102/102 - | 70/70 - | 20/20 - | 8/8 - |
| Duration, n/N 1–4 days 4–7 days | 105/108 3/108 | 66/66 - | 102/102 - | 70/70 - | 17 3 | 8/8 - |
| Intervention, n/N No treatment Treatment at home | 39/108 69/108 | 37/66 29/66 | 56/102 46/102 | 21/70 49/70 | - 20/20 | - 8/8 |
| Utilization of health resources, n/N | ||||||
| None Medical consultation (non-urgent) | 91/108 17/108 | 58/66 8/66 | 96/102 6/102 | 70/70 - | 9/20 11/20 | - 8/8 |
| Outcome, % Recovered Unknown | 100% - | 100% - | 100% - | 100% - | 100% - | 100% - |
| Severity, n/N Mild Moderate | 39/108 69/108 | 37/66 29/66 | 56/102 46/102 | 21/70 49/70 | - 17/20 | - 8/8 |
| Seriousness | None | None | None | None | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, M.; Shaik, R.A.; Mallick, A.K.; Banawas, S.S.; Alruwaili, T.A.M.; Alanazi, Y.A.; Alzahrani, H.S.; Ahmad, R.K.; Ahmad, M.S.; AlAnazi, F.H.; et al. A Cross-Sectional Study to Assess mRNA-COVID-19 Vaccine Safety among Indian Children (5–17 Years) Living in Saudi Arabia. Vaccines 2023, 11, 207. https://doi.org/10.3390/vaccines11020207
Ahsan M, Shaik RA, Mallick AK, Banawas SS, Alruwaili TAM, Alanazi YA, Alzahrani HS, Ahmad RK, Ahmad MS, AlAnazi FH, et al. A Cross-Sectional Study to Assess mRNA-COVID-19 Vaccine Safety among Indian Children (5–17 Years) Living in Saudi Arabia. Vaccines. 2023; 11(2):207. https://doi.org/10.3390/vaccines11020207
Chicago/Turabian StyleAhsan, Marya, Riyaz Ahamed Shaik, Ayaz K. Mallick, Saeed S. Banawas, Thamer A. M. Alruwaili, Yousef Abud Alanazi, Hayat Saleh Alzahrani, Ritu Kumar Ahmad, Mohammad Shakil Ahmad, Faisal Holil AlAnazi, and et al. 2023. "A Cross-Sectional Study to Assess mRNA-COVID-19 Vaccine Safety among Indian Children (5–17 Years) Living in Saudi Arabia" Vaccines 11, no. 2: 207. https://doi.org/10.3390/vaccines11020207
APA StyleAhsan, M., Shaik, R. A., Mallick, A. K., Banawas, S. S., Alruwaili, T. A. M., Alanazi, Y. A., Alzahrani, H. S., Ahmad, R. K., Ahmad, M. S., AlAnazi, F. H., Alfhaid, F., Aljulifi, M. Z., Mehta, V., Almhmd, A. E., Daham, A. S. D. A., & Alruwaili, M. M. M. (2023). A Cross-Sectional Study to Assess mRNA-COVID-19 Vaccine Safety among Indian Children (5–17 Years) Living in Saudi Arabia. Vaccines, 11(2), 207. https://doi.org/10.3390/vaccines11020207










