An Insight into Current Treatment Strategies, Their Limitations, and Ongoing Developments in Vaccine Technologies against Herpes Simplex Infections
Abstract
1. Introduction
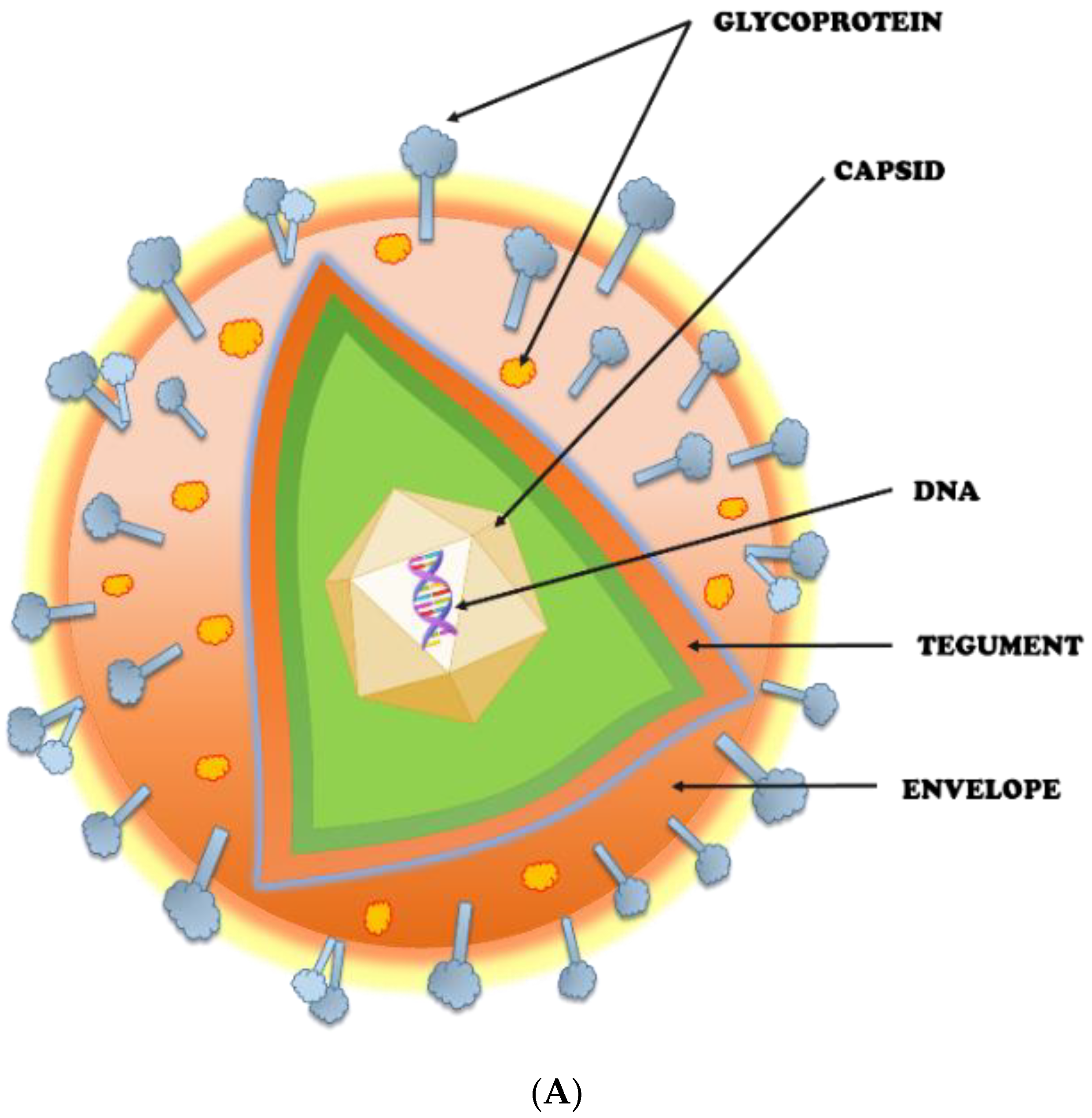
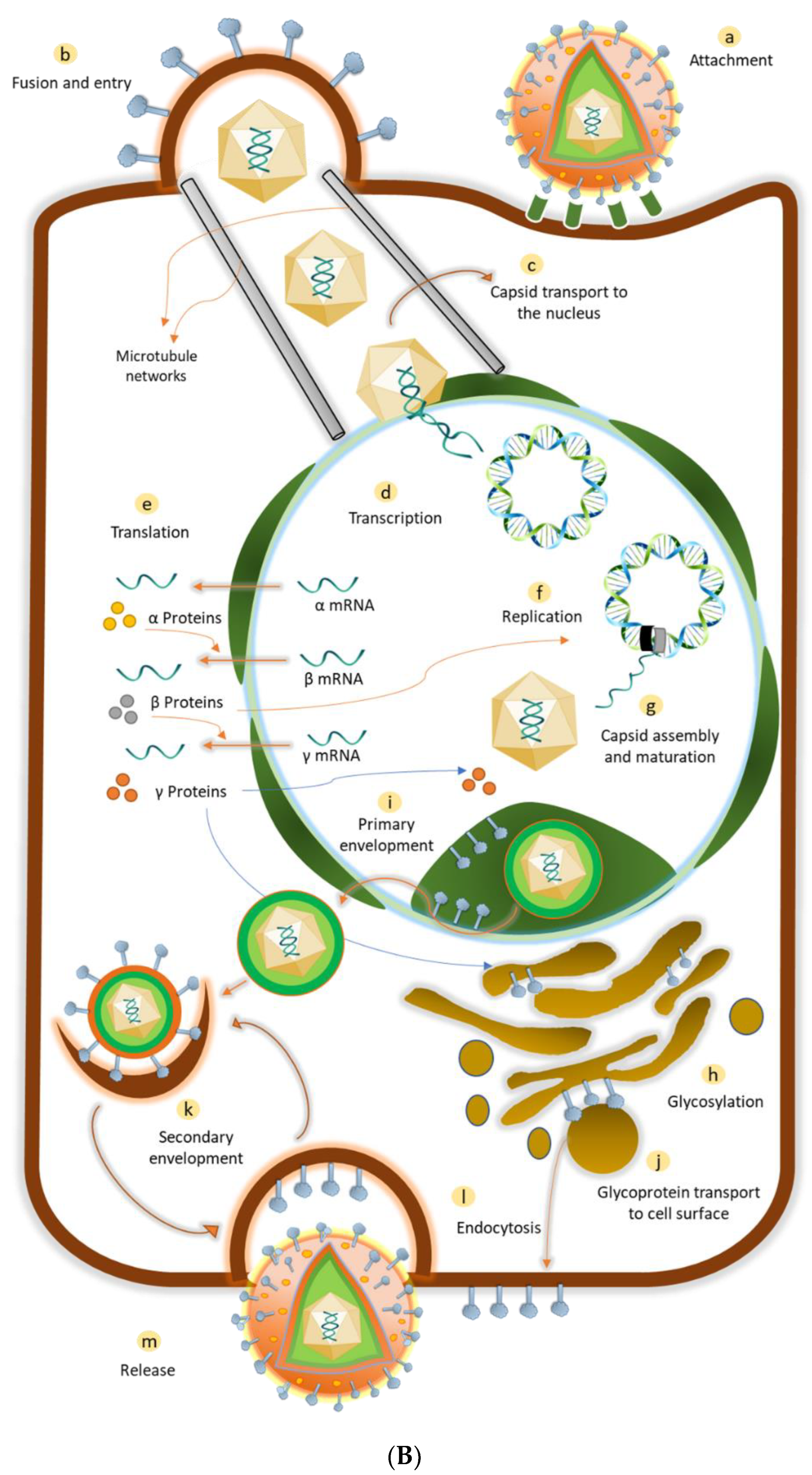
2. Herpes Simplex Virus (HSV): An Overview
2.1. Potential Targets for HSV Prevention
2.2. Interplay of HSV and Immune System
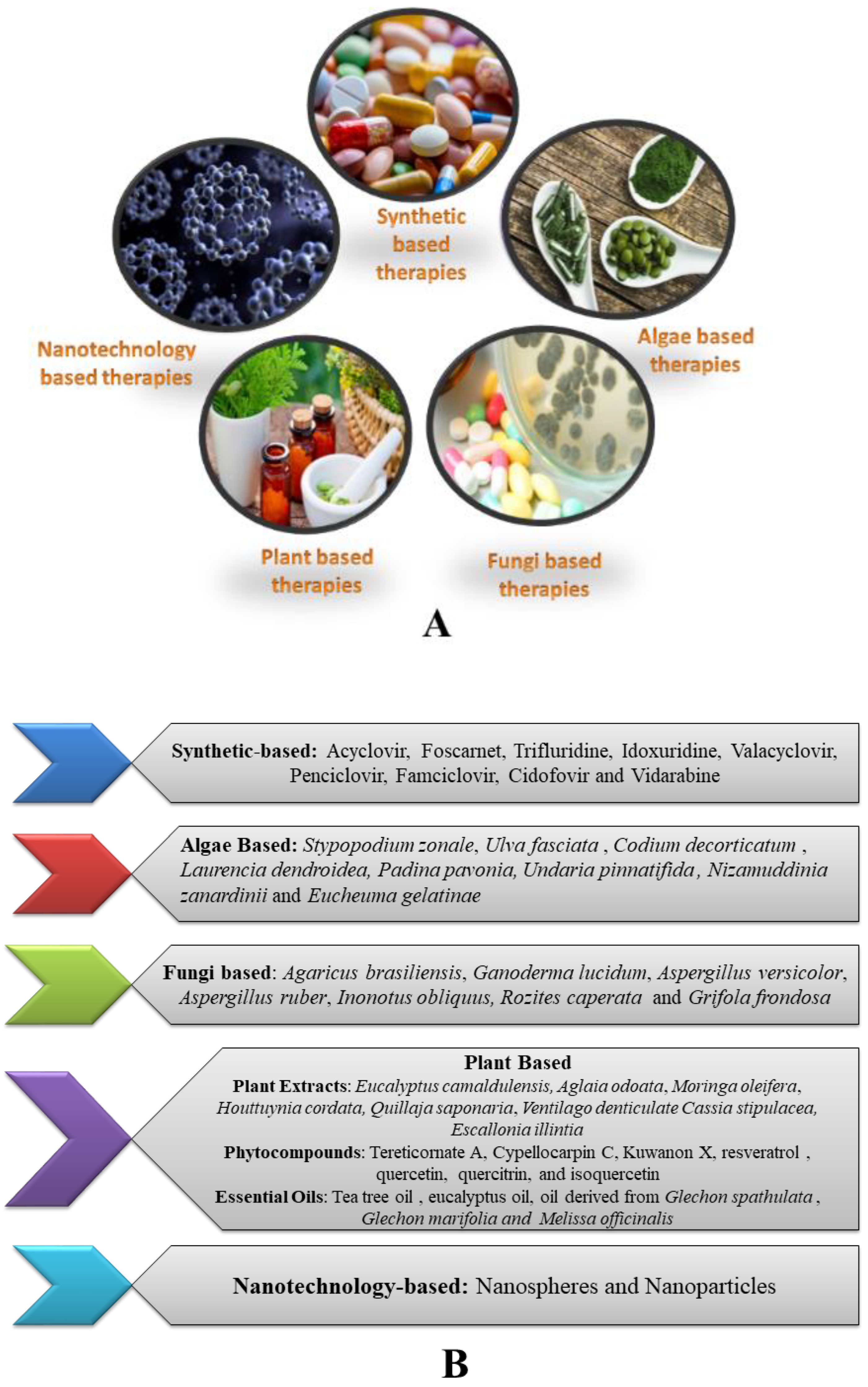
3. Therapeutic Strategies Available for Herpes Infections
3.1. Synthetic Therapeutics
3.2. Plant-Based Therapeutics
3.2.1. Plant Extracts
3.2.2. Phytocompounds
3.2.3. Essential Oils
3.3. Algae-Based Therapeutics
3.4. Fungi-Based Therapeutics
3.5. Nanotechnology-Based Therapeutics
3.6. Clinical Trial Evidence
4. Limitations of the Current Treatments and Challenges for Vaccine Development
4.1. Limitations of Current Treatments
4.2. Various Approaches Investigated for Meeting out the Limitations and Challenges
5. Preventive Methods for HSV through Vaccines
5.1. Inactivated Killed HSV Vaccines
5.2. Live-Attenuated Vaccines
5.3. Replication-Defective Virus Vaccines
5.4. Subunit HSV Vaccines
5.5. Nucleic Acid Vaccines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustafa, M.; Illzam, E.; Muniandy, R.; Sharifah, A.; Nang, M.; Ramesh, B. Herpes simplex virus infections, Pathophysiology and Management. IOSR J. Dent. Med. Sci. 2016, 15, 85–91. [Google Scholar] [CrossRef]
- Madavaraju, K.; Koganti, R.; Volety, I.; Yadavalli, T.; Shukla, D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell. Infect. Microbiol. 2021, 10, 617578. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.V.; Shukla, D. Herpes Simplex Epithelial and Stromal Keratitis: An Epidemiologic Update. Surv. Ophthalmol. 2012, 57, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.; Choudhary, A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006, 25, 355–380. [Google Scholar] [CrossRef]
- Whitley, R.; Davis, E.A.; Suppapanya, N. Incidence of Neonatal Herpes Simplex Virus Infections in a Managed-Care Population. Sex. Transm. Dis. 2007, 34, 704–708. [Google Scholar] [CrossRef]
- Mostafa, H.H.; Thompson, T.W.; Konen, A.J.; Haenchen, S.D.; Hilliard, J.G.; Macdonald, S.J.; Morrison, L.A.; Davido, D.J. Herpes Simplex Virus 1 Mutant with Point Mutations in UL39 Is Impaired for Acute Viral Replication in Mice, Establishment of Latency, and Explant-Induced Reactivation. J. Virol. 2018, 92, e01654-17. [Google Scholar] [CrossRef]
- Gonçalves, B.C.; Barbosa, M.G.L.; Olak, A.P.S.; Terezo, N.B.; Nishi, L.; Watanabe, M.A.; Marinello, P.; Rechenchoski, D.Z.; Rocha, S.P.D.; Faccin-Galhardi, L.C. Antiviral therapies: Advances and perspectives. Fundam. Clin. Pharmacol. 2020, 35, 305–320. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Majewska, A.; Malejczyk, M.; Mlynarczyk, G.; Majewski, S. Antiviral medication in sexually transmitted diseases. Part I: HSV, HPV. Mini-Rev. Med. Chem. 2013, 13, 1837–1845. [Google Scholar] [CrossRef]
- Birkmann, A.; Zimmermann, H. HSV antivirals—current and future treatment options. Curr. Opin. Virol. 2016, 18, 9–13. [Google Scholar] [CrossRef]
- Evans, T.G.; Bernstein, D.I.; Raborn, G.W.; Harmenberg, J.; Kowalski, J.; Spruance, S.L. Double-Blind, Randomized, Placebo-Controlled Study of Topical 5% Acyclovir-1% Hydrocortisone Cream (ME-609) for Treatment of UV Radiation-Induced Herpes Labialis. Antimicrob. Agents Chemother. 2002, 46, 1870–1874. [Google Scholar] [CrossRef]
- LeFlore, S.; Anderson, P.L.; Fletcher, C.V.; Fletcher, C.V.; Fletcher, C.V. A Risk-Benefit Evaluation of Aciclovir for the Treatment and Prophylaxis of Herpes Simplex Virus Infections. Drug Saf. 2000, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Arduino, P.G.; Porter, S. Herpes Simplex Virus Type 1 infection: Overview on relevant clinico-pathological features. J. Oral Pathol. Med. 2007, 37, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Javaly, K.; Wohlfeiler, M.; Kalayjian, R.; Klein, T.; Bryson, Y.; Grafford, K.; Martin-Munley, S.; Hardy, W.D. Treatment of Mucocutaneous Herpes Simplex Virus Infections Unresponsive to Acyclovir with Topical Foscarnet Cream in AIDS Patients: A Phase I/II Study. Am. J. Ther. 1999, 21, 301–306. [Google Scholar] [CrossRef]
- Chen, F.; Xu, H.; Liu, J.; Cui, Y.; Luo, X.; Zhou, Y.; Chen, Q.; Jiang, L. Efficacy and safety of nucleoside antiviral drugs for treatment of recurrent herpes labialis: A systematic review and meta-analysis. J. Oral Pathol. Med. 2017, 46, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.; Gottlieb, S.L.; Wald, A. Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine 2016, 34, 2948–2952. [Google Scholar] [CrossRef]
- Akhrameyeva, N.V.; Zhang, P.; Sugiyama, N.; Behar, S.M.; Yao, F. Development of a Glycoprotein D-Expressing Dominant-Negative and Replication-Defective Herpes Simplex Virus 2 (HSV-2) Recombinant Viral Vaccine against HSV-2 Infection in Mice. J. Virol. 2011, 85, 5036–5047. [Google Scholar] [CrossRef]
- Khan, A.A.; Srivastava, R.; Lopes, P.P.; Wang, C.; Pham, T.T.; Cochrane, J.; Thai, N.T.U.; Gutierrez, L.; BenMohamed, L. Asymptomatic memory CD8+T cells. Hum. Vaccines Immunother. 2014, 10, 945–963. [Google Scholar] [CrossRef]
- Cliffe, A.; Chang, L.; Colgrove, R.; Knipe, D.M. Herpes Simplex Virus. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Taylor, T.J.; Brockman, M.A.; McNamee, E.E.; Knipe, D.M. Herpes simplex virus. Front. Biosci.-Landmark 2002, 7, 752–764. [Google Scholar] [CrossRef]
- Krishnan, R.; Stuart, P.M. Developments in Vaccination for Herpes Simplex Virus. Front. Microbiol. 2021, 12, 798927. [Google Scholar] [CrossRef]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Kortekangas-Savolainen, O.; Orhanen, E.; Puodinketo, T.; Vuorinen, T. Epidemiology of Genital Herpes Simplex Virus Type 1 and 2 Infections in Southwestern Finland During a 10-Year Period (2003–2012). Sex. Transm. Dis. 2014, 41, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Akkus, S.E.a.A. Genital Herpes. In Fundamentals of Sexually Transmitted Infections; IntechOpen: London, UK, 2017. [Google Scholar]
- Benedetti, J.; Corey, L.; Ashley, R. Recurrence Rates in Genital Herpes after Symptomatic First-Episode Infection. Ann. Intern. Med. 1994, 121, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Roizman, B.; Zhou, G. The 3 facets of regulation of herpes simplex virus gene expression: A critical inquiry. Virology 2015, 479–480, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Kulkarni, S.; Mukherjee, A. Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home. Front. Microbiol. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Hu, K.; He, S.; Wang, P.; Zhang, M.; Huang, X.; Du, T.; Zheng, C.; Liu, Y.; Hu, Q. Contribution of N-linked glycans on HSV-2 gB to cell–cell fusion and viral entry. Virology 2015, 483, 72–82. [Google Scholar] [CrossRef]
- Akhtar, J.; Shukla, D. Viral entry mechanisms: Cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009, 276, 7228–7236. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, S.; Zhu, D.; Tang, H.; Wang, N.; Chen, W.; Gao, Q.; Li, Y.; Wang, J.; Liu, H.; et al. Structure of the herpes simplex virus type 2 C-capsid with capsid-vertex-specific component. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Mahiet, C.; Ergani, A.; Huot, N.; Alende, N.; Azough, A.; Salvaire, F.; Bensimon, A.; Conseiller, E.; Wain-Hobson, S.; Labetoulle, M.; et al. Structural Variability of the Herpes Simplex Virus 1 Genome In Vitro and In Vivo. J. Virol. 2012, 86, 8592–8601. [Google Scholar] [CrossRef]
- Slobedman, B.; Zhang, X.; Simmons, A. Herpes Simplex Virus Genome Isomerization: Origins of Adjacent Long Segments in Concatemeric Viral DNA. J. Virol. 1999, 73, 810–813. [Google Scholar] [CrossRef]
- Dolan, A.; Jamieson, F.E.; Cunningham, C.; Barnett, B.C.; McGeoch, D.J. The Genome Sequence of Herpes Simplex Virus Type 2. J. Virol. 1998, 72, 2010–2021. [Google Scholar] [CrossRef]
- Baines, J.D.; Pellett, P.E. Genetic comparison of human alphaherpesvirus genomes. In Human Herpesviruses: Biology, Therapy, Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Bowman, B.R.; Baker, M.L.; Rixon, F.J.; Chiu, W.; Quiocho, F.A. Structure of the herpesvirus major capsid protein. EMBO J. 2003, 22, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Shim, J.; Kim, S.J. Stunning symmetries involved in the self-assembly of the HSV-1 capsid. J. Korean Phys. Soc. 2021, 78, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.J.; Fraefel, C.; Cunningham, A.L.; Diefenbach, R.J. Functional roles of the tegument proteins of herpes simplex virus type 1. Virus Res. 2009, 145, 173–186. [Google Scholar] [CrossRef]
- Diefenbach, R.; Miranda-Saksena, M.; Douglas, M.W.; Cunningham, A.L. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 2007, 18, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.J.; Dong, L.; Vilalta, A.; Byrd, B.; Wilhelm, K.M.; McClurkan, C.L.; Margalith, M.; Liu, C.; Kaslow, D.; Sidney, J.; et al. Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J. Gen. Virol. 2009, 90, 1153–1163. [Google Scholar] [CrossRef]
- Jaggi, U.; Wang, S.; Tormanen, K.; Matundan, H.; Ljubimov, A.V.; Ghiasi, H. Role of Herpes Simplex Virus Type 1 (HSV-1) Glycoprotein K (gK) Pathogenic CD8+ T Cells in Exacerbation of Eye Disease. Front. Immunol. 2018, 9, 2895. [Google Scholar] [CrossRef]
- Saleh, D.; Yarrarapu, S.N.S.; Sharma, S. Herpes Simplex Type 1; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mathew, J.J.; Sapra, A. Herpes Simplex Type 2; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cook, M.L.; Stevens, J.G. Pathogenesis of Herpetic Neuritis and Ganglionitis in Mice: Evidence for Intra-Axonal Transport of Infection. Infect. Immun. 1973, 7, 272–288. [Google Scholar] [CrossRef]
- Rødahl, E.; Stevens, J.G. Differential accumulation of herpes simplex virus type 1 latency-associated transcripts in sensory and autonomic ganglia. Virology 1992, 189, 385–388. [Google Scholar] [CrossRef]
- Speck, P.G.; Simmons, A. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J. Virol. 1991, 65, 4001–4005. [Google Scholar] [CrossRef]
- Speck, P.G.; Simmons, A. Synchronous appearance of antigen-positive and latently infected neurons in spinal ganglia of mice infected with a virulent strain of herpes simplex virus. J. Gen. Virol. 1992, 73, 1281–1285. [Google Scholar] [CrossRef]
- Simmons, A.; Tscharke, D. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: Implications for the fate of virally infected neurons. J. Exp. Med. 1992, 175, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.A.; Tscharke, D.C. Lytic Promoters Express Protein during Herpes Simplex Virus Latency. PLoS Pathog. 2016, 12, e1005729. [Google Scholar] [CrossRef] [PubMed]
- Sawtell, N.M.; Thompson, R.L. Herpes simplex virus and the lexicon of latency and reactivation: A call for defining terms and building an integrated collective framework. F1000Research 2016, 5, 2038. [Google Scholar] [CrossRef] [PubMed]
- Rohde, W.; Mikelens, P.; Jackson, J.; Blackman, J.; Whitcher, J.; Levinson, W. Hydroxyquinolines Inhibit Ribonucleic Acid-Dependent Deoxyribonucleic Acid Polymerase and Inactivate Rous Sarcoma Virus and Herpes Simplex Virus. Antimicrob. Agents Chemother. 1976, 10, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, D.R.; Oien, N.L.; Hopkins, T.A.; Knechtel, M.L.; Brideau, R.J.; Wathen, M.W.; Homa, F.L. Amino Acid Changes within Conserved Region III of the Herpes Simplex Virus and Human Cytomegalovirus DNA Polymerases Confer Resistance to 4-Oxo-Dihydroquinolines, a Novel Class of Herpesvirus Antiviral Agents. J. Virol. 2003, 77, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Schnute, M.E.; Cudahy, M.M.; Brideau, R.J.; Homa, F.L.; Hopkins, T.A.; Knechtel, M.L.; Oien, N.L.; Pitts, T.W.; Poorman, R.A.; Wathen, M.W.; et al. 4-Oxo-4,7-dihydrothieno[2,3-b]pyridines as Non-Nucleoside Inhibitors of Human Cytomegalovirus and Related Herpesvirus Polymerases. J. Med. Chem. 2005, 48, 5794–5804. [Google Scholar] [CrossRef] [PubMed]
- Brideau, R.J.; Knechtel, M.L.; Huang, A.; Vaillancourt, V.A.; Vera, E.E.; Oien, N.L.; Hopkins, T.A.; Wieber, J.L.; Wilkinson, K.F.; Rush, B.D.; et al. Broad-spectrum antiviral activity of PNU-183792, a 4-oxo-dihydroquinoline, against human and animal herpesviruses. Antivir. Res. 2001, 54, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Oien, N.L.; Brideau, R.J.; Hopkins, T.A.; Wieber, J.L.; Knechtel, M.L.; Shelly, J.A.; Anstadt, R.A.; Wells, P.A.; Poorman, R.A.; Huang, A.; et al. Broad-Spectrum Antiherpes Activities of 4-Hydroxyquinoline Carboxamides, a Novel Class of Herpesvirus Polymerase Inhibitors. Antimicrob. Agents Chemother. 2002, 46, 724–730. [Google Scholar] [CrossRef]
- Spear, P.G.; Eisenbergbc, R.J.; H.Cohenbd, G. Three Classes of Cell Surface Receptors for Alphaherpesvirus Entry. Virology 2000, 275, 1–8. [Google Scholar] [CrossRef]
- Gopinath, S.C.B.; Hayashi, K.; Kumar, P.K.R. Aptamer That Binds to the gD Protein of Herpes Simplex Virus 1 and Efficiently Inhibits Viral Entry. J. Virol. 2012, 86, 6732–6744. [Google Scholar] [CrossRef]
- Yadavalli, T.; Agelidis, A.; Jaishankar, D.; Mangano, K.; Thakkar, N.; Penmetcha, K.; Shukla, D. Targeting Herpes Simplex Virus-1 gD by a DNA Aptamer Can Be an Effective New Strategy to Curb Viral Infection. Mol. Ther.-Nucleic Acids 2017, 9, 365–378. [Google Scholar] [CrossRef]
- Betz, U.A.K.; Fischer, R.; Kleymann, G.; Hendrix, M.; Rübsamen-Waigmann, H. Potent In Vivo Antiviral Activity of the Herpes Simplex Virus Primase-Helicase Inhibitor BAY 57-1293. Antimicrob. Agents Chemother. 2002, 46, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Dutia, B.M.; Frame, M.C.; Subak-Sharpe, J.H.; Clark, W.N.; Marsden, H.S. Specific inhibition of herpesvirus ribonucleotide reductase by synthetic peptides. Nature 1986, 321, 439–441. [Google Scholar] [CrossRef]
- McClements, W.; Yamanaka, G.; Garsky, V.; Perry, H.; Bacchetti, S.; Colonno, R.; Stein, R. Oligopeptides inhibit the ribonucleotide reductase of herpes simplex virus by causing subunit separation. Virology 1988, 162, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Roehm, P.C.; Shekarabi, M.; Wollebo, H.S.; Bellizzi, A.; He, L.; Salkind, J.; Khalili, K. Inhibition of HSV-1 Replication by Gene Editing Strategy. Sci. Rep. 2016, 6, 23146. [Google Scholar] [CrossRef]
- Hamilton, S.T.; Milbradt, J.; Marschall, M.; Rawlinson, W.D. Human Cytomegalovirus Replication Is Strictly Inhibited by siRNAs Targeting UL54, UL97 or UL122/123 Gene Transcripts. PLoS ONE 2014, 9, e97231. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Bausch, N.; Couté, Y.; Diaz, J.J. Characterization by two-dimensional gel electrophoresis of host proteins whose synthesis is sustained or stimulated during the course of herpes simplex virus type 1 infection. Electrophoresis 2000, 21, 2522–2530. [Google Scholar] [CrossRef]
- Francke, B. Cell-free synthesis of herpes simplex virus DNA: The influence of polyamines. Biochemistry 1978, 17, 5494–5499. [Google Scholar] [CrossRef]
- Greco, A.; Callé, A.; Morfin, F.; Thouvenot, D.; Cayre, M.; Kindbeiter, K.; Martin, L.; Levillain, O.; Diaz, J. S-adenosyl methionine decarboxylase activity is required for the outcome of herpes simplex virus type 1 infection and represents a new potential therapeutic target. FASEB J. 2005, 19, 1128–1130. [Google Scholar] [CrossRef]
- Schang, L.M.; Bantly, A.; Schaffer, P.A. Explant-Induced Reactivation of Herpes Simplex Virus Occurs in Neurons Expressing Nuclear cdk2 and cdk4. J. Virol. 2002, 76, 7724–7735. [Google Scholar] [CrossRef]
- Mostafa, H.H.; Thompson, T.W.; Davido, D.J. N-Terminal Phosphorylation Sites of Herpes Simplex Virus 1 ICP0 Differentially Regulate Its Activities and Enhance Viral Replication. J. Virol. 2013, 87, 2109–2119. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Seidi, K.; Majidinia, M.; Karimian, A.; Yousefi, B.; Nabavi, S.M.; Astani, A.; Berindan-Neagoe, I.; Gulei, D.; Fallarino, F.; et al. Toll-like receptors as novel therapeutic targets for herpes simplex virus infection. Rev. Med. Virol. 2019, 29, e2048. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhan, G.; Zheng, C. Evasion of host antiviral innate immunity by HSV-1, an update. Virol. J. 2016, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Peri, P.; Mattila, R.K.; Kantola, H.; Broberg, E.; Karttunen, H.S.; Waris, M.; Vuorinen, T.; Hukkanen, V. Herpes Simplex Virus Type 1 Us3 Gene Deletion Influences Toll-like Receptor Responses in Cultured Monocytic Cells. Virol. J. 2008, 5, 140. [Google Scholar] [CrossRef]
- van Lint, A.L.; Murawski, M.R.; Goodbody, R.E.; Severa, M.; Fitzgerald, K.A.; Finberg, R.W.; Knipe, D.M.; Kurt-Jones, E.A. Herpes Simplex Virus Immediate-Early ICP0 Protein Inhibits Toll-Like Receptor 2-Dependent Inflammatory Responses and NF-κB Signaling. J. Virol. 2010, 84, 10802–10811. [Google Scholar] [CrossRef]
- Gu, H. What role does cytoplasmic ICP0 play in HSV-1 infection? Futur. Virol. 2018, 13, 375–378. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Wang, K.; Zheng, C. Herpes simplex virus 1 DNA polymerase processivity factor UL42 inhibits TNF-α-induced NF-κB activation by interacting with p65/RelA and p50/NF-κB1. Med. Microbiol. Immunol. 2013, 202, 313–325. [Google Scholar] [CrossRef]
- Jin, H.; Ma, Y.; Yan, Z.; Prabhakar, B.S.; He, B. Activation of NF-κB in CD8 + Dendritic Cells Ex Vivo by the γ 1 34.5 Null Mutant Correlates with Immunity against Herpes Simplex Virus 1. J. Virol. 2012, 86, 1059–1068. [Google Scholar] [CrossRef]
- Cotter, C.R.; Kim, W.-K.; Nguyen, M.L.; Yount, J.S.; López, C.B.; Blaho, J.A.; Moran, T.M. The Virion Host Shutoff Protein of Herpes Simplex Virus 1 Blocks the Replication-Independent Activation of NF-κB in Dendritic Cells in the Absence of Type I Interferon Signaling. J. Virol. 2011, 85, 12662–12672. [Google Scholar] [CrossRef]
- Horng, T.; Barton, G.M.; Flavell, R.A.; Medzhitov, R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 2002, 420, 329–333. [Google Scholar] [CrossRef]
- Finberg, R.W.; Knipe, D.M.; Kurt-Jones, E.A. Herpes Simplex Virus and Toll-Like Receptors. Viral Immunol. 2005, 18, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Masopust, D. Tissue-Resident Memory T Cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Pauken, K.E.; Vezys, V.; Masopust, D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 2014, 346, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.G. Regulatory T cells versus Th17: Differentiation of Th17 versus treg, are they mutually exclusive? Am. J. Clin. Exp. Immunol. 2012, 2, 91–107. [Google Scholar] [CrossRef]
- Manickan, E.; Rouse, R.J.; Yu, Z.; Wire, W.S.; Rouse, B.T. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J. Immunol. 1995, 155, 259–265. [Google Scholar] [CrossRef]
- Suvas, S.; Kumaraguru, U.; Pack, C.D.; Lee, S.; Rouse, B.T. CD4+CD25+ T Cells Regulate Virus-specific Primary and Memory CD8+ T Cell Responses. J. Exp. Med. 2003, 198, 889–901. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Bin Wei, B. Immune response of T cells during herpes simplex virus type 1 (HSV-1) infection. J. Zhejiang Univ. B 2017, 18, 277–288. [Google Scholar] [CrossRef]
- Rubio, R.M.; Mohr, I. Inhibition of ULK1 and Beclin1 by an α-herpesvirus Akt-like Ser/Thr kinase limits autophagy to stimulate virus replication. Proc. Natl. Acad. Sci. USA 2019, 116, 26941–26950. [Google Scholar] [CrossRef]
- Waisner, H.; Kalamvoki, M. The ICP0 Protein of Herpes Simplex Virus 1 (HSV-1) Downregulates Major Autophagy Adaptor Proteins Sequestosome 1 and Optineurin during the Early Stages of HSV-1 Infection. J. Virol. 2019, 93, e01258-19. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Dybas, J.M.; Hughes, J.; Weitzman, M.D.; Boutell, C. The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection. Virus Res. 2020, 285, 198015. [Google Scholar] [CrossRef]
- Xu, X.; He, Y.; Fan, S.; Feng, M.; Jiang, G.; Wang, L.; Zhang, Y.; Liao, Y.; Li, Q. Reducing Viral Inhibition of Host Cellular Apoptosis Strengthens the Immunogenicity and Protective Efficacy of an Attenuated HSV-1 Strain. Virol. Sin. 2019, 34, 673–687. [Google Scholar] [CrossRef]
- DuRaine, G.; Wisner, T.W.; Howard, P.; Johnson, D.C. Kinesin-1 Proteins KIF5A, -5B, and -5C Promote Anterograde Transport of Herpes Simplex Virus Enveloped Virions in Axons. J. Virol. 2018, 92, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Liu, X.; Ma, Y.; Cao, Y.; He, B. Herpes Simplex Virus 1 γ 1 34.5 Protein Inhibits STING Activation That Restricts Viral Replication. J. Virol. 2018, 92, e01015-18. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Su, C.; Zheng, C. Herpes Simplex Virus 1 Tegument Protein UL41 Counteracts IFIT3 Antiviral Innate Immunity. J. Virol. 2016, 90, 11056–11061. [Google Scholar] [CrossRef]
- Rao, P.; Wen, X.; Lo, J.H.; Kim, S.; Li, X.; Chen, S.; Feng, X.; Akbari, O.; Yuan, W. Herpes Simplex Virus 1 Specifically Targets Human CD1d Antigen Presentation to Enhance Its Pathogenicity. J. Virol. 2018, 92, e01490-18. [Google Scholar] [CrossRef]
- Zheng, C.; Su, C. Herpes simplex virus 1 infection dampens the immediate early antiviral innate immunity signaling from peroxisomes by tegument protein VP16. Virol. J. 2017, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.S.; Fakioglu, E.; Herold, B.C. Novel approaches in fighting herpes simplex virus infections. Expert Rev. Anti-Infect. Ther. 2009, 7, 559–568. [Google Scholar] [CrossRef]
- Kukhanova, M.K.; Korovina, A.N.; Kochetkov, S.N. Human herpes simplex virus: Life cycle and development of inhibitors. Biochem. Pharmacol. 2014, 79, 1635–1652. [Google Scholar] [CrossRef]
- Hammer, K.D.; Dietz, J.; Lo, T.S.; Johnson, E.M. A Systematic Review on the Efficacy of Topical Acyclovir, Penciclovir, and Docosanol for the Treatment of Herpes Simplex Labialis. Dermatology 2018, 6, 118–123. [Google Scholar] [CrossRef]
- De Clercq, E. Selective Anti-Herpesvirus Agents. Antivir. Chem. Chemother. 2013, 23, 93–101. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Whitley, R.J. Antiviral Therapy of HSV-1 and -2, in Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Emmert, D.H. Treatment of common cutaneous herpes simplex virus infection. Am. Fam. Physician 2000, 61, 1697–1704. [Google Scholar] [PubMed]
- Soul-Lawton, J.; Seaber, E.; On, N.; Wootton, R.; Rolan, P.; Posner, J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob. Agents Chemother. 1995, 39, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Luber, A.D.; Flaherty, J.F. Famciclovir for Treatment of Herpesvirus Infections. Ann. Pharmacother. 1996, 30, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-C.; Wang, S.-H.; Delamere, F.M.; Wojnarowska, F.; Peters, M.C.; Kanjirath, P.P. Interventions for prevention of herpes simplex labialis (cold sores on the lips). Cochrane Database Syst. Rev. 2015, 2016, CD010095. [Google Scholar] [CrossRef]
- Ormrod, D.; Scott, L.J.; Perry, C.M. Valaciclovir: A review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs 2000, 59, 839–863. [Google Scholar] [CrossRef]
- Simpson, D.; Lyseng-Williamson, K.A. Famciclovir: A review of its use in herpes zoster and genital and orolabial herpes. Drugs 2006, 66, 2397–2416. [Google Scholar] [CrossRef]
- Wilhelmus, K.R. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst. Rev. 2015, 1, CD002898. [Google Scholar] [CrossRef]
- Roozbahani, M.; Hammersmith, K.M. Management of herpes simplex virus epithelial keratitis. Curr. Opin. Ophthalmol. 2018, 29, 360–364. [Google Scholar] [CrossRef]
- Chou, T.Y.; Hong, B.Y. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: Background, effectiveness, tolerability, safety, and future applications. Ther. Clin. Risk Manag. 2014, 10, 665–681. [Google Scholar] [CrossRef]
- Poole, C.L.; James, S.H. Antiviral Therapies for Herpesviruses: Current Agents and New Directions. Clin. Ther. 2018, 40, 1282–1298. [Google Scholar] [CrossRef]
- Crumpacker, C.S. Mechanism of action of foscarnet against viral polymerases. Am. J. Med. 1992, 92, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Chilukuri, S.; Rosen, T. Management of acyclovir-resistant herpes simplex virus. Dermatol. Clin. 2003, 21, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wyles, D.L.; Patel, A.; Madinger, N.; Bessesen, M.; Krause, P.R.; Weinberg, A. Development of Herpes Simplex Virus Disease in Patients Who Are Receiving Cidofovir. Clin. Infect. Dis. 2005, 41, 676–680. [Google Scholar] [CrossRef]
- Dvorak, C.C.; Weintrub, P.S.; Cowan, M.J.; Horn, B. Development of Herpes Simplex Virus Stomatitis during Receipt of Cidofovir Therapy. Clin. Infect. Dis. 2009, 49, e92–e95. [Google Scholar] [CrossRef]
- Reusser, P. Herpesvirus resistance to antiviral drugs: A review of the mechanisms, clinical importance and therapeutic options. J. Hosp. Infect. 1996, 33, 235–248. [Google Scholar] [CrossRef]
- Blot, N.; Schneider, P.; Young, P.; Janvresse, C.; Dehesdin, D.; Tron, P.; Vannier, J. Treatment of an acyclovir and foscarnet-resistant herpes simplex virus infection with cidofovir in a child after an unrelated bone marrow transplant. Bone Marrow Transplant. 2000, 26, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P.; Sasadeusz, J.; Carapetis, J.; Waters, K.; Curtis, N. Successful treatment of foscarnet-resistant herpes simplex stomatitis with intravenous cidofovir in a child. Pediatr. Infect. Dis. J. 2001, 20, 1083–1086. [Google Scholar] [CrossRef]
- Strand, A.; Böttiger, D.; Gever, L.N.; Wheeler, W. Safety and Tolerability of Combination Acyclovir 5% and Hydrocortisone 1% Cream in Adolescents with Recurrent Herpes Simplex Labialis. Pediatr. Dermatol. 2011, 29, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-B.; Challacombe, S. Management of recurrent oral herpes simplex infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, S12.e1–S12.e18. [Google Scholar] [CrossRef] [PubMed]
- Bélec, L.; Tevi-Benissan, C.; Bianchi, A.; Cotigny, S.; Beumont-Mauviel, M.; Si-Mohamed, A.; Malkin, J.-E. In vitro inactivation of Chlamydia trachomatis and of a panel of DNA (HSV-2, CMV, adenovirus, BK virus) and RNA (RSV, enterovirus) viruses by the spermicide benzalkonium chloride. J. Antimicrob. Chemother. 2000, 46, 685–693. [Google Scholar] [CrossRef]
- Godfrey, H.R.; Godfrey, N.J.; Godfrey, J.C.; Riley, D. A randomized clinical trial on the treatment of oral herpes with topical zinc oxide/glycine. Altern. Ther. Health Med. 2001, 7, 49–56. [Google Scholar] [PubMed]
- Sacks, S.L.; Thisted, R.A.; Jones, T.M.; Barbarash, R.A.; Mikolich, D.J.; Ruoff, G.E.; Jorizzo, J.L.; Gunnilla, L.B.; Katz, D.H.; Khalil, M.; et al. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 2001, 45, 222–230. [Google Scholar] [CrossRef] [PubMed]
- E Pope, L.; Marcelletti, J.F.; Katz, L.R.; Lin, J.Y.; Katz, D.H.; Parish, M.L.; Spear, P.G. The anti-herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antivir. Res. 1998, 40, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Safety and Effectiveness of Health Care Antiseptics. Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Final rule. Fed. Regist. 2017, 82, 60474–60503. [Google Scholar]
- Crimi, S.; Fiorillo, L.; Bianchi, A.; D’Amico, C.; Amoroso, G.; Gorassini, F.; Mastroieni, R.; Marino, S.; Scoglio, C.; Catalano, F.; et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses 2019, 11, 463. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Masarčíková, R.; Berchová-Bímová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Le Cleach, L.; Trinquart, L.; Do, G.; Maruani, A.; Lebrun-Vignes, B.; Ravaud, P.; Chosidow, O. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database Syst. Rev. 2014, 8, Cd009036. [Google Scholar] [CrossRef]
- Heslop, R.; Roberts, H.; Flower, D.; Jordan, V. Interventions for men and women with their first episode of genital herpes. Cochrane Database Syst. Rev. 2016, 2016, CD010684. [Google Scholar] [CrossRef]
- Garber, A.; Barnard, L.; Pickrell, C. Review of Whole Plant Extracts with Activity Against Herpes Simplex Viruses In Vitro and In Vivo. J. Evid.-Based Integr. Med. 2021, 26, 2515690x20978394. [Google Scholar] [CrossRef]
- Mahmoud, H.; Abu-Jafar, A.; Huleihel, M. Antiviral activity of Eucalyptus camaldulensis leaves ethanolic extract on herpes viruses infection. Int. J. Clin. Virol. 2017, 1, 001–009. [Google Scholar] [CrossRef]
- Lipipun, V.; Kurokawa, M.; Suttisri, R.; Taweechotipatr, P.; Pramyothin, P.; Hattori, M.; Shiraki, K. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antivir. Res. 2003, 60, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Yang, Z.; Wang, J.; Xu, Y.; Tan, R.-X.; Li, E. Houttuynia cordata blocks HSV infection through inhibition of NF-κB activation. Antivir. Res. 2011, 92, 341–345. [Google Scholar] [CrossRef]
- Pacheco, P.; Sierra, J.; Schmeda-Hirschmann, G.; Potter, C.W.; Jones, B.M.; Moshref, M. Antiviral activity of chilean medicinal plant extracts. Phytother. Res. 1993, 7, 415–418. [Google Scholar] [CrossRef]
- Roner, M.R.; Sprayberry, J.; Spinks, M.; Dhanji, S. Antiviral activity obtained from aqueous extracts of the Chilean soapbark tree (Quillaja saponaria Molina). J. Gen. Virol. 2007, 88, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Okba, M.M.; El Gedaily, R.A.; Ashour, R.M. UPLC–PDA–ESI–qTOF-MS profiling and potent anti-HSV-II activity of Eucalyptus sideroxylon leaves. J. Chromatogr. B 2017, 1068–1069, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Brezáni, V.; Leláková, V.; Hassan, S.T.S.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef]
- Ma, F.; Shen, W.; Zhang, X.; Li, M.; Wang, Y.; Zou, Y.; Li, Y.; Wang, H. Anti-HSV Activity of Kuwanon X from Mulberry Leaves with Genes Expression Inhibitory and HSV-1 Induced NF-κB Deactivated Properties. Biol. Pharm. Bull. 2016, 39, 1667–1674. [Google Scholar] [CrossRef]
- Chen, X.; Qiao, H.; Liu, T.; Yang, Z.; Xu, L.; Xu, Y.; Ge, H.M.; Tan, R.-X.; Li, E. Inhibition of herpes simplex virus infection by oligomeric stilbenoids through ROS generation. Antivir. Res. 2012, 95, 30–36. [Google Scholar] [CrossRef]
- Faith, S.A.; Sweet, T.J.; Bailey, E.; Booth, T.; Docherty, J.J. Resveratrol suppresses nuclear factor-κB in herpes simplex virus infected cells. Antivir. Res. 2006, 72, 242–251. [Google Scholar] [CrossRef]
- Leyton, L.; Hott, M.; Acuña, F.; Caroca, J.; Nuñez, M.; Martin, C.; Zambrano, A.; Concha, M.I.; Otth, C. Nutraceutical activators of AMPK/Sirt1 axis inhibit viral production and protect neurons from neurodegenerative events triggered during HSV-1 infection. Virus Res. 2015, 205, 63–72. [Google Scholar] [CrossRef]
- Rattanathongkom, A.; Lee, J.-B.; Hayashi, K.; Sripanidkulchai, B.-O.; Kanchanapoom, T.; Hayashi, T. Evaluation of Chikusetsusaponin IV a Isolated from Alternanthera philoxeroides for Its Potency Against Viral Replication. Planta Med. 2009, 75, 829–835. [Google Scholar] [CrossRef]
- Barquero, A.A.; Alché, L.E.; Coto, C.E. Antiviral activity of meliacine on the replication of a thymidine kinase-deficient mutant of Herpes simplex virus type 1 alone and in combination with acyclovir. Int. J. Antimicrob. Agents 1997, 9, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Petrera, E.; Coto, C.E. Therapeutic effect of meliacine, an antiviral derived from Melia azedarach L., in mice genital herpetic infection. Phytother. Res. 2009, 23, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Brand, Y.M.; Roa-Linares, V.C.; Betancur-Galvis, L.A.; Durán-García, D.C.; Stashenko, E. Antiviral activity of Colombian Labiatae and Verbenaceae family essential oils and monoterpenes on Human Herpes viruses. J. Essent. Oil Res. 2015, 28, 130–137. [Google Scholar] [CrossRef]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.L.; Roehe, P.M.; Fuentefria, A.M.; Henriques, A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulate and Glechon marifolia. Pharm. Biol. 2014, 53, 682–688. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schön, K.; Reichling, J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Die Pharm. 2001, 56, 682–688. [Google Scholar]
- Mazzanti, G.; Battinelli, L.; Pompeo, C.; Serrilli, A.; Rossi, R.; Sauzullo, I.; Mengoni, F.; Vullo, V. Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Nat. Prod. Res. 2008, 22, 1433–1440. [Google Scholar] [CrossRef]
- Allahverdiyev, A.; Duran, N.; Ozguven, M.; Koltas, S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine 2004, 11, 657–661. [Google Scholar] [CrossRef]
- Buck, C.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef]
- Lee, C.-K.; Kim, H.S.; Nam, J.R.; Lee, M.-J.; Yim, J.-H.; Lee, H.K.; De Clercq, E. Anti-Picornavirus Activity and Other Antiviral Activity of Sulfated Exopolysaccharide from the Marine Microalga Gyrodinium impudicum Strain KG03. Antivir. Res. 2009, 82, A40. [Google Scholar] [CrossRef]
- Lee, J.-B.; Hayashi, K.; Hayashi, T.; Sankawa, U.; Maeda, M. Antiviral Activities against HSV-1, HCMV, and HIV-1 of Rhamnan Sulfate from Monostroma latissimum. Planta Med. 1999, 65, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.; Dutcher, J.D.; Adams, E.V.; Sherman, J.H. Protective Effect of Seaweed Extracts for Chicken Embryos Infected with Influenza B or Mumps Virus. Exp. Biol. Med. 1958, 99, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.A.; Ray, S.; Ray, B.; Damonte, E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Faral-Tello, P.; Mirazo, S.; Dutra, C.; Pérez, A.; Geis-Asteggiante, L.; Frabasile, S.; Koncke, E.; Davyt, D.; Cavallaro, L.; Heinzen, H.; et al. Cytotoxic, Virucidal, and Antiviral Activity of South American Plant and Algae Extracts. Sci. World J. 2012, 2012, 174837. [Google Scholar] [CrossRef]
- Soares, A.R.; Robaina, M.C.S.; Mendes, G.S.; Silva, T.S.L.; Gestinari, L.M.S.; Pamplona, O.S.; Yoneshigue-Valentin, Y.; Kaiser, C.R.; Romanos, M.T.V. Antiviral activity of extracts from Brazilian seaweeds against herpes simplex virus. Rev. Bras. De Farm. 2012, 22, 714–723. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2018, 124, 131–137. [Google Scholar] [CrossRef]
- Jin, F.; Zhuo, C.; He, Z.; Wang, H.; Liu, W.; Zhang, R.; Wang, Y. Anti-herpes simplex virus activity of polysaccharides from Eucheuma gelatinae. World J. Microbiol. Biotechnol. 2015, 31, 453–460. [Google Scholar] [CrossRef]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef]
- Sun, Q.-L.; Li, Y.; Ni, L.-Q.; Li, Y.-X.; Cui, Y.-S.; Jiang, S.-L.; Xie, E.-Y.; Du, J.; Deng, F.; Dong, C.-X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2019, 229, 115487. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.M.L.; Abrantes, J.L.; Epifanio, R.D.A.; Fontes, C.F.L.; Frugulhetti, I.C.P.P. The Alkaloid 4-Methylaaptamine Isolated from the Sponge Aaptos aaptos Impairs Herpes simplex Virus Type 1 Penetration and Immediate-Early Protein Synthesis. Planta Med. 2007, 73, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.D.S.; Bravin, I.C.; Yoneshigue-Valentin, Y.; Yokoya, N.S.; Romanos, M.T.V. Anti-HSV activity of Hypnea musciformis cultured with different phytohormones. Rev. Bras. De Farm. 2012, 22, 789–794. [Google Scholar] [CrossRef]
- Santoyo, S.; Jaime, L.; Plaza, M.; Herrero, M.; Rodriguez-Meizoso, I.; Ibañez, E.; Reglero, G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2012, 24, 731–741. [Google Scholar] [CrossRef]
- Keivan, Z.; Moradali, F.; Parisa, P.; Kohzad, S. Evaluation of in vitro antiviral activity of a brown alga (Cystoseira myrica) from the Persian Gulf against herpes simplex virus type 1. Afr. J. Biotechnol. 2007, 6, 2511–2514. [Google Scholar] [CrossRef]
- Cardozo, F.T.G.D.S.; Camelini, C.M.; Leal, P.C.; Kratz, J.M.; Nunes, R.J.; de Mendonça, M.M.; Simões, C.M.O. Antiherpetic Mechanism of a Sulfated Derivative of Agaricus brasiliensis Fruiting Bodies Polysaccharide. Intervirology 2014, 57, 375–383. [Google Scholar] [CrossRef]
- Eo, S.-K.; Kim, Y.-S.; Lee, C.-K.; Han, S.-S. Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. J. Ethnopharmacol. 2000, 72, 475–481. [Google Scholar] [CrossRef]
- Huang, Z.; Nong, X.; Ren, Z.; Wang, J.; Zhang, X.; Qi, S. Anti-HSV-1, antioxidant and antifouling phenolic compounds from the deep-sea-derived fungus Aspergillus versicolor SCSIO 41502. Bioorganic Med. Chem. Lett. 2017, 27, 787–791. [Google Scholar] [CrossRef]
- Liang, T.-M.; Fang, Y.-W.; Zheng, J.-Y.; Shao, C.-L. Secondary Metabolites Isolated from the Gorgonian-Derived Fungus Aspergillus ruber and Their Antiviral Activity. Chem. Nat. Compd. 2018, 54, 559–561. [Google Scholar] [CrossRef]
- Pradeep, P.; Manju, V.; Ahsan, M.F. Antiviral Potency of Mushroom Constituents. In Medicinal Mushrooms: Recent Progress in Research and Development; Agrawal, D., Dhanasekaran, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 275–297. [Google Scholar]
- Gu, C.-Q.; Li, J.-W.; Chao, F.; Jin, M.; Wang, X.-W.; Shen, Z.-Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antivir. Res. 2007, 75, 250–257. [Google Scholar] [CrossRef]
- Mamo, T.; Moseman, E.A.; Kolishetti, N.; Salvador-Morales, C.; Shi, J.; Kuritzkes, D.R.; Langer, R.; von Andrian, U.; Farokhzad, O.C. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine 2010, 5, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Barras, A.; Khanal, M.; Pagneux, Q.; Boukherroub, R. Nanostructures for the Inhibition of Viral Infections. Molecules 2015, 20, 14051–14081. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Law, W.-C.; Aalinkeel, R.; Reynolds, J.L.; Nair, B.B.; E Sykes, D.; Yong, K.-T.; Roy, I.; Prasad, P.N.; A Schwartz, S. Anti-HIV-1 nanotherapeutics: Promises and challenges for the future. Int. J. Nanomed. 2012, 7, 5301–5314. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhubiab, B.E.; Nair, A.B.; Kumria, R.; Attimarad, M.; Harsha, S. Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids Surf. B Biointerfaces 2015, 136, 878–884. [Google Scholar] [CrossRef]
- Rai, M.; Shegokar, R. Metal Nanoparticles in Pharma; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Ramadan, M.A.; Shawkey, A.E.; Rabeh, M.A.; Abdellatif, A.O. Promising antimicrobial activities of oil and silver nanoparticles obtained from Melaleuca alternifolia leaves against selected skin-infecting pathogens. J. Herb. Med. 2019, 20, 100289. [Google Scholar] [CrossRef]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 387. [Google Scholar] [CrossRef]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020, 32, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Dhanasezhian, A.; Srivani, S.; Govindaraju, K.; Parija, P.; Sasikala, S.; Kumar, M. Anti-Herpes Simplex Virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J. Mar. Sci. 2019, 48. [Google Scholar]
- I, R.-I.; Mj, S.; R, G.; Fj, D.L.M.; Mj, B.; Ma, M.-F. Gold Nanoparticles Crossing Blood-Brain Barrier Prevent HSV-1 Infection and Reduce Herpes Associated Amyloid-βsecretion. J. Clin. Med. 2020, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, E.; Studzińska, M.; Jabłońska, A.; Lozovski, V.; Rusinchuk, N.; Mukha, I.; Vitiuk, N.; Leśnikowski, Z.J. Antiviral Effect of Nonfunctionalized Gold Nanoparticles against Herpes Simplex Virus Type-1 (HSV-1) and Possible Contribution of Near-Field Interaction Mechanism. Molecules 2021, 26, 5960. [Google Scholar] [CrossRef] [PubMed]
- Krzyzowska, M.; Chodkowski, M.; Janicka, M.; Dmowska, D.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Bednarczyk, K.; Celichowski, G.; Grobelny, J. Lactoferrin-Functionalized Noble Metal Nanoparticles as New Antivirals for HSV-2 Infection. Microorganisms 2022, 10, 110. [Google Scholar] [CrossRef]
- Antoine, T.E.; Mishra, Y.K.; Trigilio, J.; Tiwari, V.; Adelung, R.; Shukla, D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antivir. Res. 2012, 96, 363–375. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R.; Röhl, C.; Shukla, D.; Spors, F.; Tiwari, V. Virostatic potential of micro–nano filopodia-like ZnO structures against herpes simplex virus-1. Antivir. Res. 2011, 92, 305–312. [Google Scholar] [CrossRef]
- Shen, M.-X.; Ma, N.; Li, M.-K.; Liu, Y.-Y.; Chen, T.; Wei, F.; Liu, D.-Y.; Hou, W.; Xiong, H.-R.; Yang, Z.-Q. Antiviral Properties of R. tanguticum Nanoparticles on Herpes Simplex Virus Type I In Vitro and In Vivo. Front. Pharmacol. 2019, 10, 959. [Google Scholar] [CrossRef]
- Tarallo, R.; Carberry, T.P.; Falanga, A.; Vitiello, M.; Galdiero, S.; Galdiero, M.; Weck, M. Dendrimers functionalized with membrane-interacting peptides for viral inhibition. Int. J. Nanomed. 2013, 8, 521–534. [Google Scholar] [CrossRef]
- Chono, K.; Katsumata, K.; Kontani, T.; Kobayashi, M.; Sudo, K.; Yokota, T.; Konno, K.; Shimizu, Y.; Suzuki, H. ASP2151, a novel helicase-primase inhibitor, possesses antiviral activity against varicella-zoster virus and herpes simplex virus types 1 and 2. J. Antimicrob. Chemother. 2010, 65, 1733–1741. [Google Scholar] [CrossRef]
- Kalu, N.N.; Desai, P.J.; Shirley, C.M.; Gibson, W.; Dennis, P.A.; Ambinder, R.F. Nelfinavir Inhibits Maturation and Export of Herpes Simplex Virus 1. J. Virol. 2014, 88, 5455–5461. [Google Scholar] [CrossRef]
- Quenelle, D.C.; Lampert, B.; Collins, D.J.; Rice, T.L.; Painter, G.R.; Kern, E.R. Efficacy of CMX001 against Herpes Simplex Virus Infections in Mice and Correlations with Drug Distribution Studies. J. Infect. Dis. 2010, 202, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Kern, E.R.; Hartline, C.B.; Lanier, E.R.; Quenelle, D.C. CMX001 Potentiates the Efficacy of Acyclovir in Herpes Simplex Virus Infections. Antimicrob. Agents Chemother. 2011, 55, 4728–4734. [Google Scholar] [CrossRef]
- Wald, A.; Corey, L.; Timmler, B.; Magaret, A.; Warren, T.; Tyring, S.; Johnston, C.; Kriesel, J.; Fife, K.; Galitz, L.; et al. Helicase–Primase Inhibitor Pritelivir for HSV-2 Infection. New Engl. J. Med. 2014, 370, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, K.Y. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: Current state of the art. Antivir. Res. 2009, 82, A84–A98. [Google Scholar] [CrossRef]
- Kleymann, G.; Fischer, R.; Betz, U.A.; Hendrix, M.; Bender, W.; Schneider, U.; Handke, G.; Eckenberg, P.; Hewlett, G.; Pevzner, V.; et al. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nat. Med. 2002, 8, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Patick, A.K.; Mo, H.; Markowitz, M.; Appelt, K.; Wu, B.; Musick, L.; Kalish, V.; Kaldor, S.; Reich, S.; Ho, D.; et al. Antiviral and resistance studies of AG1343, an orally bioavailable inhibitor of human immunodeficiency virus protease. Antimicrob. Agents Chemother. 1996, 40, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, M.; Conant, M.; Hurley, A.; Schluger, R.; Duran, M.; Peterkin, J.; Chapman, S.; Patick, A.; Hendricks, A.; Yuen, G.J.; et al. A preliminary evaluation of nelfinavir mesylate, an inhibitor of human immunodeficiency virus (HIV)-1 protease, to treat HIV infection. J. Infect. Dis. 1998, 177, 1533–1540. [Google Scholar] [CrossRef]
- Semprini, A.; Singer, J.; Braithwaite, I.; Shortt, N.; Thayabaran, D.; McConnell, M.; Weatherall, M.; Beasley, R. Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: A randomised controlled trial. BMJ Open 2019, 9, e026201. [Google Scholar] [CrossRef]
- Gupta, R.; Wald, A.; Krantz, E.; Selke, S.; Warren, T.; Vargas-Cortes, M.; Miller, G.; Corey, L. Valacyclovir and Acyclovir for Suppression of Shedding of Herpes Simplex Virus in the Genital Tract. J. Infect. Dis. 2004, 190, 1374–1381. [Google Scholar] [CrossRef]
- Moomaw, M.D.; Cornea, P.; Rathbun, R.C.; A Wendel, K. Review of antiviral therapy for herpes labialis, genital herpes and herpes zoster. Expert Rev. Anti-Infect. Ther. 2003, 1, 283–295. [Google Scholar] [CrossRef]
- Boyd, M.R.; Safrin, S.; Kern, E.R. Penciclovir: A review of its spectrum of activity, selectivity, and cross-resistance pattern. Antivir. Chem. Chemother. 1993, 4, 3–11. [Google Scholar] [CrossRef]
- Harmenberg, J.; Öberg, B.; Spruance, S. Prevention of Ulcerative Lesions by Episodic Treatment of Recurrent Herpes Labialis: A Literature Review. Acta Derm. -Venereol. 2010, 90, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Paintsil, E.; Cheng, Y.-C. Antiviral Agents. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 223–257. [Google Scholar]
- Sadjadi, S.-A.; Regmi, S.; Chau, T. Acyclovir Neurotoxicity in a Peritoneal Dialysis Patient: Report of a Case and Review of the Pharmacokinetics of Acyclovir. Am. J. Case Rep. 2018, 19, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Frobert, E.; Ooka, T.; Cortay, J.C.; Lina, B.; Thouvenot, D.; Morfin, F. Herpes Simplex Virus Thymidine Kinase Mutations Associated with Resistance to Acyclovir: A Site-Directed Mutagenesis Study. Antimicrob. Agents Chemother. 2005, 49, 1055–1059. [Google Scholar] [CrossRef]
- Sarisky, R.T.; Bacon, T.; Boon, R.; Locke, L.; Nguyen, T.T.; Leary, J.; Esser, K.; Saltzman, R. Penciclovir Susceptibilities of Herpes Simplex Virus Isolates from Patients Using Penciclovir Cream for Treatment of Recurrent Herpes Labialis. Antimicrob. Agents Chemother. 2002, 46, 2848–2853. [Google Scholar] [CrossRef]
- Sarisky, R.T.; Bacon, T.H.; Boon, R.J.; Duffy, K.E.; Esser, K.M.; Leary, J.; Locke, L.A.; Nguyen, T.T.; Quail, M.R.; Saltzman, R. Profiling penciclovir susceptibility and prevalence of resistance of herpes simplex virus isolates across eleven clinical trials. Arch. Virol. 2003, 148, 1757–1769. [Google Scholar] [CrossRef]
- Bacon, T.H.; Levin, M.J.; Leary, J.J.; Sarisky, R.T.; Sutton, D. Herpes Simplex Virus Resistance to Acyclovir and Penciclovir after Two Decades of Antiviral Therapy. Clin. Microbiol. Rev. 2003, 16, 114–128. [Google Scholar] [CrossRef]
- Spruance, S.L. Prophylactic chemotherapy with acyclovir for recurrent herpes simplex labialis. J. Med. Virol. 1993, 41, 27–32. [Google Scholar] [CrossRef]
- Mindel, A. Is it meaningful to treat patients with recurrent herpetic infections? Scand. J. Infect. Dis. Suppl. 1991, 80, 27–32. [Google Scholar]
- Spruance, S.L.; Stewart, J.C.B.; Rowe, N.H.; McKeough, M.B.; Wenerstrom, G.; Freeman, D.J. Treatment of Recurrent Herpes Simplex Labialis with Oral Acyclovir. J. Infect. Dis. 1990, 161, 185–190. [Google Scholar] [CrossRef]
- Sauerbrei, A. Optimal management of genital herpes: Current perspectives. Infect. Drug Resist. 2016, 9, 129–141. [Google Scholar] [CrossRef]
- Sela, M.; Hilleman, M.R. Therapeutic vaccines: Realities of today and hopes for tomorrow. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. S2), 14559. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.R.; Smith, J.B.; Sandgren, K.J.; Cunningham, A.L. Mechanisms of Immune Control of Mucosal HSV Infection: A Guide to Rational Vaccine Design. Front. Immunol. 2019, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.F. Protection from Experimental Ocular Herpetic Keratitis by a Heat-Killed Virus Vaccine. Arch. Ophthalmol. 1980, 98, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Rajcani, J.; Kutinová, L.; Vonka, V. Restriction of latent herpes virus infection in rabbits immunized with subviral herpes simplex virus vaccine. Acta. Virologica. 1980, 24, 183–193. [Google Scholar]
- Hoshino, Y.; Pesnicak, L.; Dowdell, K.C.; Lacayo, J.; Dudek, T.; Knipe, D.M.; Straus, S.E.; Cohen, J.I. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine 2008, 26, 4034–4040. [Google Scholar] [CrossRef]
- Liu, X.; Broberg, E.; Watanabe, D.; Dudek, T.; DeLuca, N.; Knipe, D.M. Genetic engineering of a modified herpes simplex virus 1 vaccine vector. Vaccine 2009, 27, 2760–2767. [Google Scholar] [CrossRef]
- Peng, B.; Wang, L.R.; Gómez-Román, V.R.; Davis-Warren, A.; Montefiori, D.C.; Kalyanaraman, V.S.; Venzon, D.; Zhao, J.; Kan, E.; Rowell, T.J.; et al. Replicating Rather than Nonreplicating Adenovirus-Human Immunodeficiency Virus Recombinant Vaccines Are Better at Eliciting Potent Cellular Immunity and Priming High-Titer Antibodies. J. Virol. 2005, 79, 10200–10209. [Google Scholar] [CrossRef]
- Halford, W.P.; Weisend, C.; Grace, J.; Soboleski, M.; Carr, D.J.J.; Balliet, J.W.; Imai, Y.; Margolis, T.P.; Gebhardt, B.M. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: Implications for the regulation of viral latency. Virol. J. 2006, 3, 44. [Google Scholar] [CrossRef]
- Huang, X.; Lu, B.; Yu, W.; Fang, Q.; Liu, L.; Zhuang, K.; Shen, T.; Wang, H.; Tian, P.; Zhang, L.; et al. A Novel Replication-Competent Vaccinia Vector MVTT Is Superior to MVA for Inducing High Levels of Neutralizing Antibody via Mucosal Vaccination. PLoS ONE 2009, 4, e4180. [Google Scholar] [CrossRef]
- Liu, H.; Yu, W.; Tang, X.; Wang, H.; Ouyang, W.; Zhou, J.; Chen, Z. The route of inoculation determines the tissue tropism of modified vaccinia tiantan expressing the spike glycoprotein of SARS-CoV in mice. J. Med. Virol. 2010, 82, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.; Gregory, S.; Nakashima, H.; Viapiano, M.S.; Knipe, D.M. Intramuscular delivery of replication-defective herpes simplex virus gives antigen expression in muscle syncytia and improved protection against pathogenic HSV-2 strains. Virology 2017, 513, 129–135. [Google Scholar] [CrossRef]
- Petro, C.; A González, P.; Cheshenko, N.; Jandl, T.; Khajoueinejad, N.; Bénard, A.; Sengupta, M.; Herold, B.C.; Jacobs, W.R. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife 2015, 4, e06054. [Google Scholar] [CrossRef] [PubMed]
- Petro, C.D.; Weinrick, B.; Khajoueinejad, N.; Burn, C.; Sellers, R.; Jacobs, W.R.; Herold, B.C. HSV-2 ΔgD elicits FcγR-effector antibodies that protect against clinical isolates. J. Clin. Investig. 2016, 1, e88529. [Google Scholar] [CrossRef]
- Prichard, M.N.; Kaiwar, R.; Jackman, W.T.; Quenelle, D.C.; Collins, D.J.; Kern, E.R.; Kemble, G.M.; Spaete, R.R. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine 2005, 23, 5424–5431. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Cardin, R.D.; Smith, G.A.; Pickard, G.E.; Sollars, P.J.; Dixon, D.A.; Pasula, R.; Bravo, F.J. The R2 non-neuroinvasive HSV-1 vaccine affords protection from genital HSV-2 infections in a guinea pig model. NPJ Vaccines 2020, 5, 104. [Google Scholar] [CrossRef]
- Odegard, J.M.; Flynn, P.; Campbell, D.J.; Robbins, S.H.; Dong, L.; Wang, K.; ter Meulen, J.; Cohen, J.I.; Koelle, D. A novel HSV-2 subunit vaccine induces GLA-dependent CD4 and CD8 T cell responses and protective immunity in mice and guinea pigs. Vaccine 2015, 34, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.K.; Swaims-Kohlmeier, A.; Herbst-Kralovetz, M.M. IL-36γ Is a Key Regulator of Neutrophil Infiltration in the Vaginal Microenvironment and Limits Neuroinvasion in Genital HSV-2 Infection. J. Immunol. 2019, 203, 2655–2664. [Google Scholar] [CrossRef]
- Aschner, C.B.; Knipe, D.M.; Herold, B.C. Model of vaccine efficacy against HSV-2 superinfection of HSV-1 seropositive mice demonstrates protection by antibodies mediating cellular cytotoxicity. Vaccines 2020, 5, 35. [Google Scholar] [CrossRef]
- McLean, C.; Níchallanáin, D.; Duncan, I.; Boursnell, M.; Jennings, R.; Inglis, S. Induction of a protective immune response by mucosal vaccination with a DISC HSV-1 vaccine. Vaccine 1996, 14, 987–992. [Google Scholar] [CrossRef]
- Boursnell, M.; Entwisle, C.; Blakeley, D.; Roberts, C.; Duncan, I.A.; Chisholm, S.E.; Martin, G.M.; Jennings, R.; Ni Challanain, D.; Sobek, I.; et al. A Genetically Inactivated Herpes Simplex Virus Type 2 (HSV-2) Vaccine Provides Effective Protection against Primary and Recurrent HSV-2 Disease. J. Infect. Dis. 1997, 175, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Dudek, T.; Mathews, L.C.; Knipe, D.M. Disruption of the UL41 gene in the herpes simplex virus 2 dl5-29 mutant increases its immunogenicity and protective capacity in a murine model of genital herpes. Virology 2008, 372, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Dropulic, L.K.; Oestreich, M.C.; Pietz, H.L.; Laing, K.J.; Hunsberger, S.; Lumbard, K.; Garabedian, D.; Turk, S.P.; Chen, A.; Hornung, R.L.; et al. A Randomized, Double-Blinded, Placebo-Controlled, Phase 1 Study of a Replication-Defective Herpes Simplex Virus (HSV) Type 2 Vaccine, HSV529, in Adults with or Without HSV Infection. J. Infect. Dis. 2019, 220, 990–1000. [Google Scholar] [CrossRef]
- Wang, K.; Dropulic, L.; Bozekowski, J.; Pietz, H.L.; Jegaskanda, S.; Dowdell, K.; Vogel, J.S.; Garabedian, D.; Oestreich, M.; Nguyen, H.; et al. Serum and Cervicovaginal Fluid Antibody Profiling in Herpes Simplex Virus–Seronegative Recipients of the HSV529 Vaccine. J. Infect. Dis. 2021, 224, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Reszka, N.J.; Dudek, T.; Knipe, D.M. Construction and properties of a herpes simplex virus 2 dl5-29 vaccine candidate strain encoding an HSV-1 virion host shutoff protein. Vaccine 2010, 28, 2754–2762. [Google Scholar] [CrossRef]
- Zhang, P.; Xie, L.; Balliet, J.W.; Casimiro, D.R.; Yao, F. A Herpes Simplex Virus 2 (HSV-2) Glycoprotein D-expressing Nonreplicating Dominant-Negative HSV-2 Virus Vaccine Is Superior to a gD2 Subunit Vaccine against HSV-2 Genital Infection in Guinea Pigs. PLoS ONE 2014, 9, e101373. [Google Scholar] [CrossRef]
- Geiss, B.J.; Smith, T.J.; Leib, D.A.; Morrison, L.A. Disruption of Virion Host Shutoff Activity Improves the Immunogenicity and Protective Capacity of a Replication-Incompetent Herpes Simplex Virus Type 1 Vaccine Strain. J. Virol. 2000, 74, 11137–11144. [Google Scholar] [CrossRef]
- Keadle, T.; Morrison, L.; Morris, J.; Pepose, J.; Stuart, P. Therapeutic immunization with a virion host shutoff (vhs) defective, replication-incompetent HSV-1 strain limits recurrent herpetic ocular infection. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3854. [Google Scholar]
- Bernard, M.-C.; Barban, V.; Pradezynski, F.; De Montfort, A.; Ryall, R.; Caillet, C.; Londoño-Hayes, P. Immunogenicity, Protective Efficacy, and Non-Replicative Status of the HSV-2 Vaccine Candidate HSV529 in Mice and Guinea Pigs. PLoS ONE 2015, 10, e0121518. [Google Scholar] [CrossRef]
- Ann Arbor, M. BlueWillow Biologics Awarded Patent for Intranasal Genital Herpes Vaccine. BlueWillow News, 23 July 2019.
- Richards, A.L.; Sollars, P.J.; Pitts, J.D.; Stults, A.M.; Heldwein, E.E.; Pickard, G.E.; Smith, G.A. The pUL37 tegument protein guides alpha-herpesvirus retrograde axonal transport to promote neuroinvasion. PLoS Pathog. 2017, 13, e1006741. [Google Scholar] [CrossRef]
- Stanfield, B.A.; Pahar, B.; Chouljenko, V.N.; Veazey, R.; Kousoulas, K.G. Vaccination of rhesus macaques with the live-attenuated HSV-1 vaccine VC2 stimulates the proliferation of mucosal T cells and germinal center responses resulting in sustained production of highly neutralizing antibodies. Vaccine 2017, 35, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, B.A.; Rider, P.J.; Caskey, J.; Del Piero, F.; Kousoulas, K.G. Intramuscular vaccination of guinea pigs with the live-attenuated human herpes simplex vaccine VC2 stimulates a transcriptional profile of vaginal Th17 and regulatory Tr1 responses. Vaccine 2018, 36, 2842–2849. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, B.A.; Stahl, J.; Chouljenko, V.N.; Subramanian, R.; Charles, A.-S.; Saied, A.A.; Walker, J.D.; Kousoulas, K.G. A Single Intramuscular Vaccination of Mice with the HSV-1 VC2 Virus with Mutations in the Glycoprotein K and the Membrane Protein UL20 Confers Full Protection against Lethal Intravaginal Challenge with Virulent HSV-1 and HSV-2 Strains. PLoS ONE 2014, 9, e109890. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Püschel, R.; Gershburg, E.; Wilber, A.; Gershburg, S.; Rakowski, B. A Live-Attenuated HSV-2 ICP0− Virus Elicits 10 to 100 Times Greater Protection against Genital Herpes than a Glycoprotein D Subunit Vaccine. PLoS ONE 2011, 6, e17748. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Püschel, R.; Rakowski, B. Herpes Simplex Virus 2 ICP0− Mutant Viruses Are Avirulent and Immunogenic: Implications for a Genital Herpes Vaccine. PLoS ONE 2010, 5, e12251. [Google Scholar] [CrossRef]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.A.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef] [PubMed]
- HSV-040 Study Group. Safety and immunogenicity of a glycoprotein D genital herpes vaccine in healthy girls 10–17 years of age: Results from a randomised, controlled, double-blind trial. Vaccine 2013, 31, 6136–6143. [Google Scholar] [CrossRef]
- Mo, A.; Musselli, C.; Chen, H.; Pappas, J.; LeClair, K.; Liu, A.; Chicz, R.M.; Truneh, A.; Monks, S.; Levey, D.L.; et al. A heat shock protein based polyvalent vaccine targeting HSV-2: CD4+ and CD8+ cellular immunity and protective efficacy. Vaccine 2011, 29, 8530–8541. [Google Scholar] [CrossRef]
- Wald, A.; Koelle, D.M.; Fife, K.; Warren, T.; LeClair, K.; Chicz, R.M.; Monks, S.; Levey, D.L.; Musselli, C.; Srivastava, P.K. Safety and immunogenicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. Vaccine 2011, 29, 8520–8529. [Google Scholar] [CrossRef]
- Egan, K.; Hook, L.M.; Naughton, A.; Friedman, H.M.; Awasthi, S. Herpes simplex virus type 2 trivalent protein vaccine containing glycoproteins C, D and E protects guinea pigs against HSV-1 genital infection. Hum. Vaccines Immunother. 2020, 16, 2109–2113. [Google Scholar] [CrossRef]
- Awasthi, S.; Lubinski, J.M.; Shaw, C.E.; Barrett, S.M.; Cai, M.; Wang, F.; Betts, M.; Kingsley, S.; DiStefano, D.J.; Balliet, J.W.; et al. Immunization with a Vaccine Combining Herpes Simplex Virus 2 (HSV-2) Glycoprotein C (gC) and gD Subunits Improves the Protection of Dorsal Root Ganglia in Mice and Reduces the Frequency of Recurrent Vaginal Shedding of HSV-2 DNA in Guinea Pigs Compared to Immunization with gD Alone. J. Virol. 2011, 85, 10472–10486. [Google Scholar] [CrossRef]
- Awasthi, S.; Hook, L.M.; Pardi, N.; Wang, F.; Myles, A.; Cancro, M.P.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci. Immunol. 2019, 4, eaaw7083. [Google Scholar] [CrossRef]
- Awasthi, S.; Hook, L.M.; Shaw, C.E.; Pahar, B.; Stagray, J.A.; Liu, D.; Veazey, R.S.; Friedman, H.M. An HSV-2 Trivalent Vaccine Is Immunogenic in Rhesus Macaques and Highly Efficacious in Guinea Pigs. PLoS Pathog. 2017, 13, e1006141. [Google Scholar] [CrossRef]
- Hook, L.M.; Awasthi, S.; Dubin, J.; Flechtner, J.; Long, D.; Friedman, H.M. A trivalent gC2/gD2/gE2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine 2018, 37, 664–669. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Vannucci, L.; De Luca, A.; Lai, M.; Matteoli, B.; Freer, G.; Manservigi, R.; Ceccherini-Nelli, L.; Maggi, F.; Bendinelli, M.; et al. A Lentiviral Vector-Based, Herpes Simplex Virus 1 (HSV-1) Glycoprotein B Vaccine Affords Cross-Protection against HSV-1 and HSV-2 Genital Infections. J. Virol. 2012, 86, 6563–6574. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Flechtner, J.B.; McNeil, L.K.; Heineman, T.; Oliphant, T.; Tasker, S.; Wald, A.; Hetherington, S. Therapeutic HSV-2 vaccine decreases recurrent virus shedding and recurrent genital herpes disease. Vaccine 2019, 37, 3443–3450. [Google Scholar] [CrossRef]
- Flechtner, J.B.; Long, D.; Larson, S.; Clemens, V.; Baccari, A.; Kien, L.; Chan, J.; Skoberne, M.; Brudner, M.; Hetherington, S. Immune responses elicited by the GEN-003 candidate HSV-2 therapeutic vaccine in a randomized controlled dose-ranging phase 1/2a trial. Vaccine 2016, 34, 5314–5320. [Google Scholar] [CrossRef]
- Van Wagoner, N.; Fife, K.; A Leone, P.; I Bernstein, D.; Warren, T.; Panther, L.; Novak, R.M.; Beigi, R.; Kriesel, J.; Tyring, S.; et al. Effects of Different Doses of GEN-003, a Therapeutic Vaccine for Genital Herpes Simplex Virus-2, on Viral Shedding and Lesions: Results of a Randomized Placebo-Controlled Trial. J. Infect. Dis. 2018, 218, 1890–1899. [Google Scholar] [CrossRef]
- Skoberne, M.; Cardin, R.; Lee, A.; Kazimirova, A.; Zielinski, V.; Garvie, D.; Lundberg, A.; Larson, S.; Bravo, F.J.; Bernstein, D.I.; et al. An Adjuvanted Herpes Simplex Virus 2 Subunit Vaccine Elicits a T Cell Response in Mice and Is an Effective Therapeutic Vaccine in Guinea Pigs. J. Virol. 2013, 87, 3930–3942. [Google Scholar] [CrossRef]
- Chandra, J.; Woo, Y.; Dutton, J.L.; Xu, Y.; Li, B.; Kinrade, S.; Druce, J.; Finlayson, N.; Griffin, P.; Laing, K.J.; et al. Immune responses to a HSV-2 polynucleotide immunotherapy COR-1 in HSV-2 positive subjects: A randomized double blinded phase I/IIa trial. PLoS ONE 2019, 14, e0226320. [Google Scholar] [CrossRef]
- Dutton, J.L.; Li, B.; Woo, Y.; Marshak, J.O.; Xu, Y.; Huang, M.-L.; Dong, L.; Frazer, I.H.; Koelle, D.M. A Novel DNA Vaccine Technology Conveying Protection against a Lethal Herpes Simplex Viral Challenge in Mice. PLoS ONE 2013, 8, e76407. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Dou, J.; Yu, F.; He, X.; Yuan, X.; Wang, Y.; Liu, C.; Gu, N. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine 2011, 29, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Veselenak, R.L.; Shlapobersky, M.; Pyles, R.B.; Wei, Q.; Sullivan, S.M.; Bourne, N. A Vaxfectin®-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine 2012, 30, 7046–7051. [Google Scholar] [CrossRef]
- Riedmann, E.M. Vical initiates vaccine trials against HSV-2 and CMV. Hum. Vaccines Immunother. 2014, 10, 255. [Google Scholar]
- Shlapobersky, M.; Marshak, J.O.; Dong, L.; Huang, M.-L.; Wei, Q.; Chu, A.; Rolland, A.; Sullivan, S.; Koelle, D.M. Vaxfectin-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J. Gen. Virol. 2012, 93, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Ravani, L.; Rinaldi, F.; Marconi, P.; Drechsler, M.; Manservigi, M.; Argnani, R.; Menegatti, E.; Esposito, E.; Manservigi, R. Intranasal immunization in mice with non-ionic surfactants vesicles containing HSV immunogens: A preliminary study as possible vaccine against genital herpes. Int. J. Pharm. 2013, 440, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Straus, S.; Savarese, B.; Krause, P.; Kost, R.; Meier, J.; Corey, L.; Barnum, G.; Burke, R.; Sekulovich, R.; Adair, S.; et al. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 1994, 343, 1460–1463. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Zhang, X.; Dervillez, X.; Chentoufi, A.A.; Badakhshan, T.; Bettahi, I.; BenMohamed, L. Targeting the Genital Tract Mucosa with a Lipopeptide/Recombinant Adenovirus Prime/Boost Vaccine Induces Potent and Long-Lasting CD8+ T Cell Immunity against Herpes: Importance of MyD88. J. Immunol. 2012, 189, 4496–4509. [Google Scholar] [CrossRef]
- Khanna, K.M.; Bonneau, R.H.; Kinchington, P.R.; Hendricks, R.L. Herpes Simplex Virus-Specific Memory CD8+ T Cells Are Selectively Activated and Retained in Latently Infected Sensory Ganglia. Immunity 2003, 18, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Posavad, C.M.; Huang, M.L.; Barcy, S.; Koelle, D.M.; Corey, L. Long Term Persistence of Herpes Simplex Virus-Specific CD8+ CTL in Persons with Frequently Recurring Genital Herpes. J. Immunol. 2000, 165, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Merecz-Sadowska, A.; Kowalczyk, T.; Wieczfinska, J.; Zajdel, R.; Śliwiński, T. Potential Synergistic Action of Bioactive Compounds from Plant Extracts against Skin Infecting Microorganisms. Int. J. Mol. Sci. 2020, 21, 5105. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, F.J.; Farías, M.A.; Gonzalez-Troncoso, M.P.; Corrales, N.; Duarte, L.F.; Retamal-Díaz, A.; Gonzalez, P.A. Experimental Dissection of the Lytic Replication Cycles of Herpes Simplex Viruses in vitro. Front. Microbiol. 2018, 9, 2406. [Google Scholar] [CrossRef]
- Tachjian, A.; Maria, V.; Jahangir, A. Use of Herbal Products and Potential Interactions in Patients with Cardiovascular Diseases. J. Am. Coll. Cardiol. 2010, 55, 515–525. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jun, S.A.; Hong, S.S.; Ahn, Y.C.; Lee, D.S.; Son, C.G. Systematic Review of Adverse Effects from Herbal Drugs Reported in Randomized Controlled Trials. Phytother. Res. 2016, 30, 1412–1419. [Google Scholar] [CrossRef]
- Borse, S.P.; Singh, D.P.; Nivsarkar, M. Understanding the relevance of herb–drug interaction studies with special focus on interplays: A prerequisite for integrative medicine. Porto Biomed. J. 2019, 4, e15. [Google Scholar] [CrossRef]
| Mechanism of Action | Potential Target | Drugs | References |
|---|---|---|---|
| Suppresses herpes virus genome replication at the protein level | DNA polymerase | 4-hydroxy-quinoline-3-carboxamide molecule and 8-hydroxyquinoline. | [52,53] |
| Blocks attachments and invasion by targeting viral proteins | Glycoproteins | PRO 2000, polymethylene hydroquinone sulfonate, and cellulose sulphate | [55,56] |
| Inhibits HSV DNA replication | Helicase primase complex | 2-amino-thiazole and thiazolylsulfonamide | [57] |
| Ribonucleotide reductase inhibitors | Ribonucleotide reductase | Peptidomimetic inhibitors | [58,59] |
| Inhibits polyamine biosynthetic pathway to block replication of HSV | Polyamine pathway | SAMDC inhibitors | [63,64] |
| CDK-2 inhibitors | CDK-2 | Roscovitine | [65,66] |
| Blocks attachment and penetration | Entry receptors (HVEM, glycosaminoglycans) | Anti-HVEM antibodies | [54] |
| TLR inhibitors | TLR | G-ODN, CpG oligonucleotide | [67] |
| Sr. No. | Type of Vaccine | Candidate Name | Modification in Vaccine | Host | Phase | Results | Origin | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | Inactivated vaccine | HSV-2 dl5-29 | deletions of UL5 and UL29 | mice and guinea pigs | Pre-clinical | enhance immune responses, protection against reactivates HSV infection | Harvard Medical School, Boston | [234] |
| 2 | Inactivated vaccine | SC16ΔgH | deletion of HSV-1 gH deletion | mice | Clinical | has no impact on viral shedding and inability to demonstrate immunity against reactivated genital herpes infections | Boston, Massachusetts | [16] |
| 3 | Replication-Defective Viral Vaccines | CJ2-gD2 | Dominant negative HSV-2 with CJ2-gD2 | guinea pigs | Pre-Clinical | provide protection against primary infection and reactivates HSV-2 genital infections | - | [235] |
| 4 | Replication-Defective Viral Vaccines | HSV-1 vhs-/ICP8- | deletions of vhs in an ICP8 | mice | Pre-Clinical | significantly enhances protective efficacy | - | [236] |
| Δ41Δ29 | deletions in the gene for virion host shutoff (vhs) protein | BALB/c mice | Pre-Clinical | decreased the frequency of UV-B-induced recurrent viral shedding in mice with latent infection | Washington University School of Medicine | [237] | ||
| 5 | Replication-Defective Viral Vaccines | HSV529 | Deletion of UL5, UL29 in HSV-2 dl5-29 mice | mice and HSV-1 seropositive guinea pigs | immunogenicity reported | Sanofi Pasteur | [238] | |
| HSV529 | - | human participants | Phase I | induced moderate CD4+ T-cell responses and neutralising antibodies in HSV-seronegative vaccine recipients | Sanofi Pasteur | [232,233] | ||
| 6 | Live-attenuated vaccine | HSV-2 ΔgD2 | Deletion of gD-2 | C57BL/6 or BALB/c mice | Pre-clinical | protection by non-neutralising Fc-mediated humoral responses | Albert Einstein College of Medicine | [222] |
| C57BL/6 mice | Pre-clinical | infected mice were protected by gD-2 | Albert Einstein College of Medicine | [223] | ||||
| 7 | Live-attenuated vaccine | AD472 | Deletion of γ134.5 gene, UL55-56, UL43.5 and the US10-12 region | guinea pigs | Pre-clinical | reduce lesion development and infection severity in guinea pigs | Albert Einstein College of Medicine | [224] |
| 8 | Live-attenuated vaccine | NE-HSV-2 | Antigens such as gB2 and gD2 were used to make nanoemulsion | C57BL/6 mice | Pre-Clinical | reduction of reactivated lesions and viral shedding by more than 50% | BlueWillow Biologics | [239] |
| 9 | Live-attenuated vaccine | R2 | Mutated pUL37 gene in the R2 region | guinea pigs | Pre-Clinical | reduction of reactivated virus shedding | Thyreos LLC | [225,240] |
| 10 | Live-attenuated vaccine | HSV-1 VC2 | Mutations in glycoprotein K (gK) and UL20 | rhesus macaques | Pre-Clinical | induction of immune response | Louisiana State University | [241] |
| - | guinea pigs | Pre-Clinical | diminishes HSV-2 replication | Louisiana State University | [242] | |||
| Mutations in glycoprotein K (gK) and UL20 | mice | Pre-Clinical | protect mice against lethal intravaginal infection | Louisiana State University | [243] | |||
| 11 | Live-attenuated vaccine | HSV-2 0ΔNLS | Deletion of ICP0− | mice | Phase II | represent avirulence and immunogenicity | Southern Illinois University | [244] |
| Pre-Clinical | protected against lethal challenge with wild-type HSV-2 | Southern Illinois University | [245] | |||||
| 12 | Subunit Vaccine | gD2 subunit vaccine | gD2 adjuvant with AS04 | HSV-1– and HSV-2–seronegative women | Phase 3 | efficacy against HSV-1 and HSV-1 genital disease was 58% and 35% respectively | GlaxoSmithKline | [246] |
| girls aged 10–17 years | Phase 3 | vaccine was tolerated and immunogenic | GlaxoSmithKline | [247] | ||||
| 13 | Subunit Vaccine | HerpV | 32 synthetic 35mer HSV-2 peptides complexed with Hsc70 protein + QS21 (saponin adjuvant) | HLA-A2 transgenic mice | Pre-clinical | immunogenic, CD4(+) and CD8(+) T cell activators | Agenus | [248] |
| HerpV+ QS-21 | human participant | Phase II (DC) | significant CD4+ T and CD8(+) T cell response | Agenus | [249] | |||
| 14 | Subunit Vaccine | HSV-2 trivalent vaccine | Combination of glycoproteins C, D, and E (gC2, gD2, gE2) | guinea pig | Pre-clinical | trivalent protein vaccine provides protection to prevent herpes infection | University of Pennsylvania | [250,251,252] |
| rhesus macaques and guinea pig | Pre-clinical | viral shedding was reduced | University of Pennsylvania | [253] | ||||
| guinea pigs | Pre-clinical | immune evasion domains on HSV-2 glycoproteins | University of Pennsylvania | [254] | ||||
| 15 | Subunit Vaccine | HSV1 gB lentiviral vector | HSV-1 glycoprotein B (gB1) with feline immunodeficiency virus (FIV) vector | C57BL/6 mice | Pre-Clinical | significant reduction in viral infectivity | University of Pisa, Italy | [255] |
| 16 | Subunit Vaccine | GEN-003 | gD2ΔTMR + M2 | seropositive participants | Completed Phase II (DC) | reduction in shedding and lesion rate was reported | University of Cincinnati, | [256] |
| gD2 (truncated)+ICP4 fragment | healthy participants | Phase 2 | reduction in shedding rate and lesion rate | Genocea | [257,258] | |||
| GEN-003/MM-2 | guinea pigs | Pre-clinical | elicits humoral immune responses, CD4+ and CD8+ T cells | Genocea | [259] | |||
| 17 | Subunit Vaccine | G103 | HSV-2 gD, deletions in UL19 and UL25 | Mice and guinea pigs | Pre-clinical | reductions of no. of lesions and lesion area | Immune Design | [226] |
| 18 | DNA Vaccines | COR-1 | gD2 ubiquitin tag with COR-1 DNA or placebo | HSV-2 seropositive subjects | Phase II | reduction in viral shedding | Admedus | [260] |
| BALB/c mice | Pre-clinical | protects against lethal viral challenge and reduces ganglionic latency | University of Washington | [261] | ||||
| 19 | DNA Vaccines | pRSC-gD-IL-2123 | HSV-1 gD combined with IL-21 | mice | Pre-clinical | inhibition of HSK in HSV-1-infected mice | Southeast University, China | [262] |
| 20 | DNA Vaccines | Vaxfectin®-gD2/UL46/UL47 | HSV-2 glycoprotein D and UL46 and UL47 genes/Vaxfectin | mice | Pre-clinical | reduction of frequency of reactivated disease and viral shedding | Vical | [263,264] |
| Vaxfectin-gD2 pDNA | pDNA + FL gD2 Vaxfectin | guinea pig | Pre-clinical | reduction in HSV-2 DNA copy number | University of Washington | [265] | ||
| 21 | DNA Vaccines | gB1s-NISV | NISV+HSV-1 gB+CpG | mice | Pre-Clinical | provides protection against a heterologous lethal vaginal challenge with HSV-2 | - | [266] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.; Sharma, S.; Akojwar, N.; Dondulkar, A.; Yenorkar, N.; Pandita, D.; Prasad, S.K.; Dhobi, M. An Insight into Current Treatment Strategies, Their Limitations, and Ongoing Developments in Vaccine Technologies against Herpes Simplex Infections. Vaccines 2023, 11, 206. https://doi.org/10.3390/vaccines11020206
Sharma D, Sharma S, Akojwar N, Dondulkar A, Yenorkar N, Pandita D, Prasad SK, Dhobi M. An Insight into Current Treatment Strategies, Their Limitations, and Ongoing Developments in Vaccine Technologies against Herpes Simplex Infections. Vaccines. 2023; 11(2):206. https://doi.org/10.3390/vaccines11020206
Chicago/Turabian StyleSharma, Divya, Supriya Sharma, Natasha Akojwar, Ayusha Dondulkar, Nikhil Yenorkar, Deepti Pandita, Satyendra K. Prasad, and Mahaveer Dhobi. 2023. "An Insight into Current Treatment Strategies, Their Limitations, and Ongoing Developments in Vaccine Technologies against Herpes Simplex Infections" Vaccines 11, no. 2: 206. https://doi.org/10.3390/vaccines11020206
APA StyleSharma, D., Sharma, S., Akojwar, N., Dondulkar, A., Yenorkar, N., Pandita, D., Prasad, S. K., & Dhobi, M. (2023). An Insight into Current Treatment Strategies, Their Limitations, and Ongoing Developments in Vaccine Technologies against Herpes Simplex Infections. Vaccines, 11(2), 206. https://doi.org/10.3390/vaccines11020206








