Toll-like Receptor 2 Mediated Immune Regulation in Simian Immunodeficiency Virus-Infected Rhesus Macaques
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement, SIV Infection, and Sample Collection
2.2. Plasma Isolation
2.3. Hematology
2.4. Quantitative SIV Plasma Virus Load (PVL)
2.5. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.6. Isolation of Jejunal Lamina Propria Leukocytes (LPL)
2.7. Isolation of Cells from Lymph Nodes (LNs)
2.8. In Vitro TLR2 Stimulation
2.9. Quantification of Cytokines and Chemokines in Plasma and Cell Culture Supernatant
2.10. Quantification of Microbial Translocation by Measuring Intestinal Fatty Acid Binding Protein (I-FABP)
2.11. Soluble CD14 (sCD14) Marker Quantification
2.12. Quantification of Plasma REG3A
2.13. Quantification of Soluble TLR2 (sTLR2) in Plasma
2.14. Flow Cytometry Analysis of Membrane-Bound TLR2 (mb-TLR2) Expression
2.15. Statistical Analyses
3. Results
3.1. Dynamics of Plasma Viral Load and Absolute Peripheral CD4 Count
3.2. An Increased Level of sTLR2 Was Detected in the Plasma of SIV-Infected RM
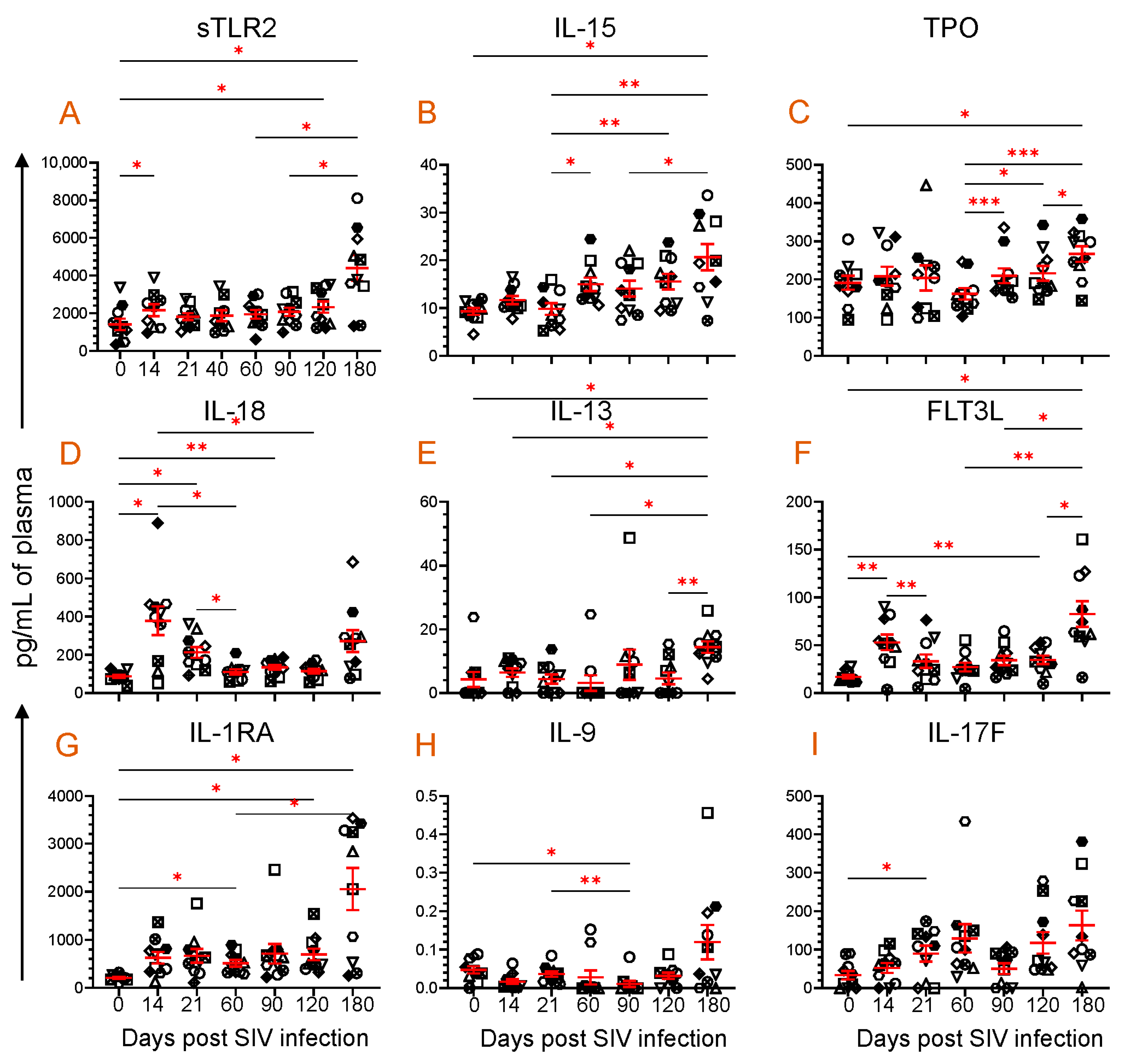
3.3. Dynamics of Cytokine Expression during SIV Infection
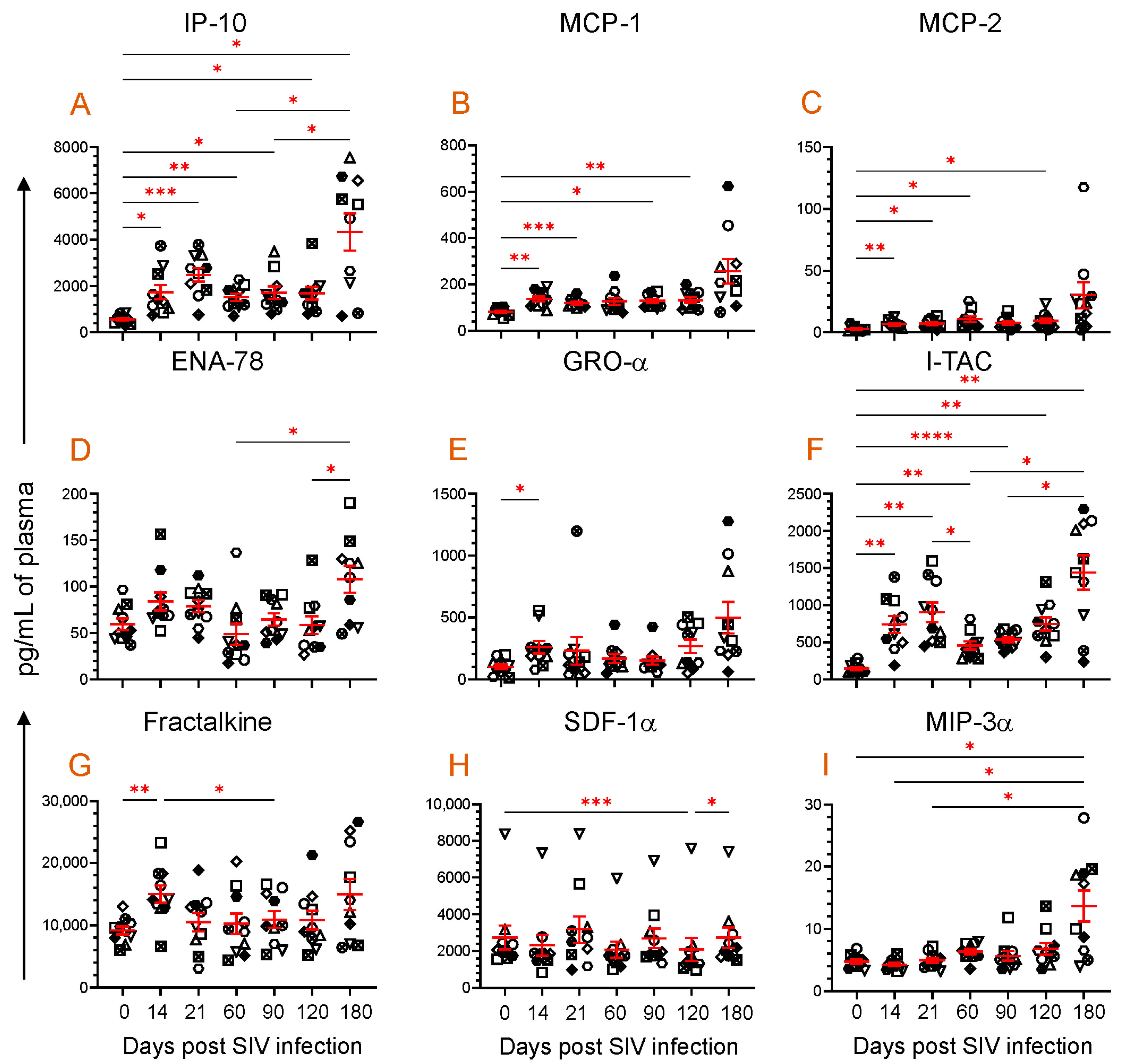
3.4. Dynamics of Chemokine Expression during SIV Infection
3.5. Correlation of Plasma Viral Load with Plasma sTLR2, IL-18, and FLT3L Concentration
3.6. Evidence of a Positive Correlation between Plasma sTLR2 and Different Cytokines/Chemokines
3.7. Absence of Correlation between Plasma sTLR2 and Mucosal Inflammatory Markers
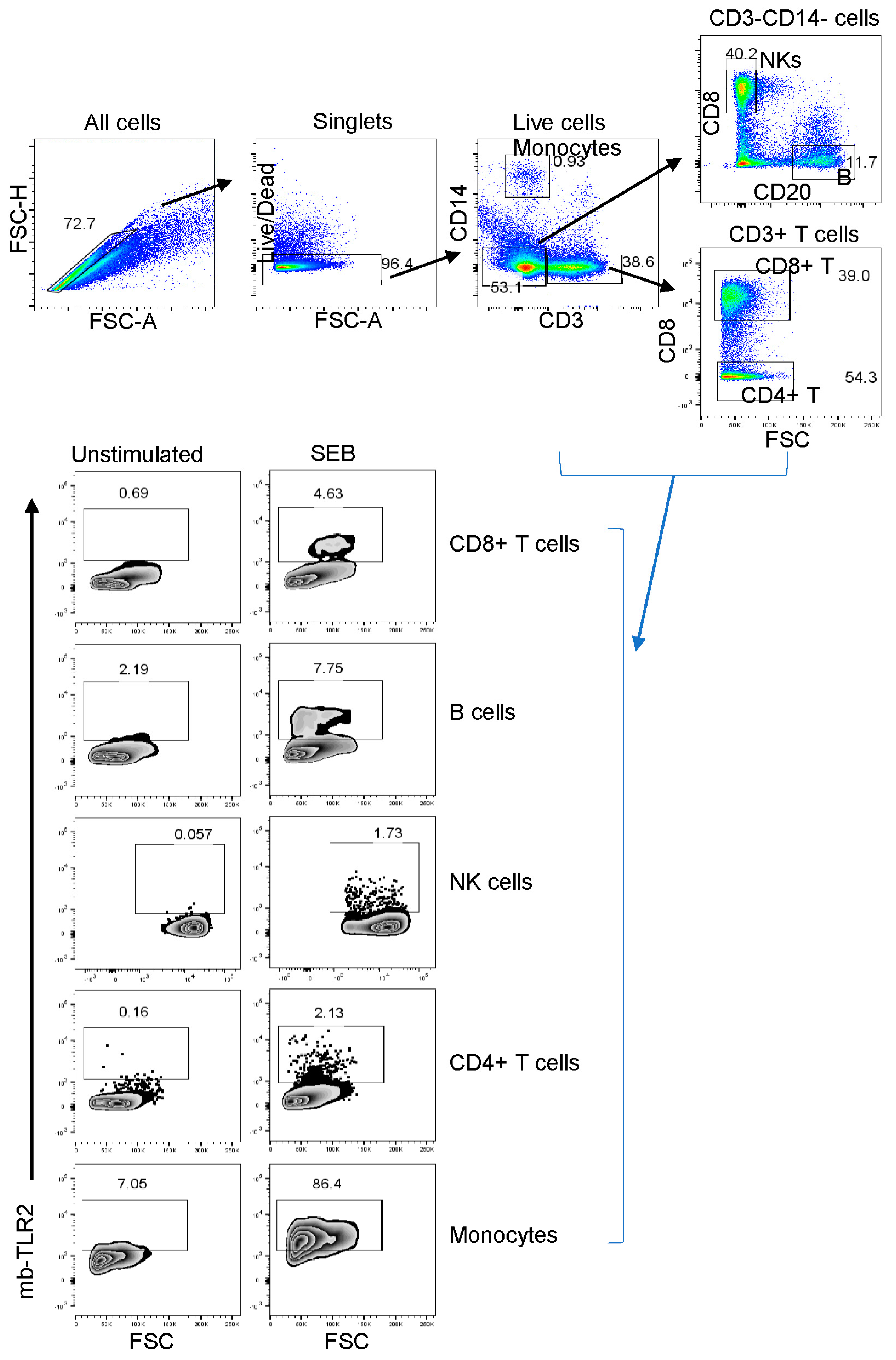
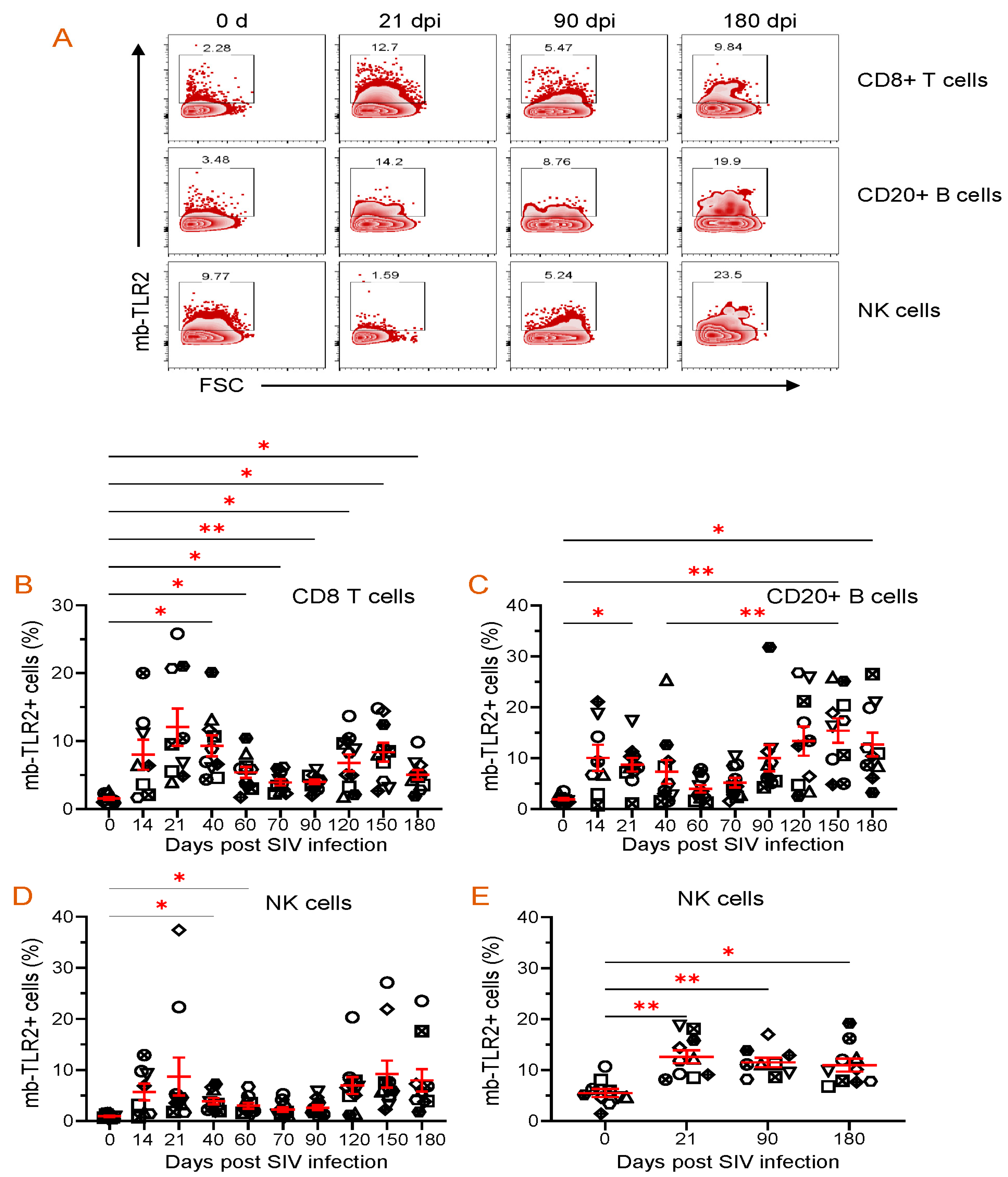
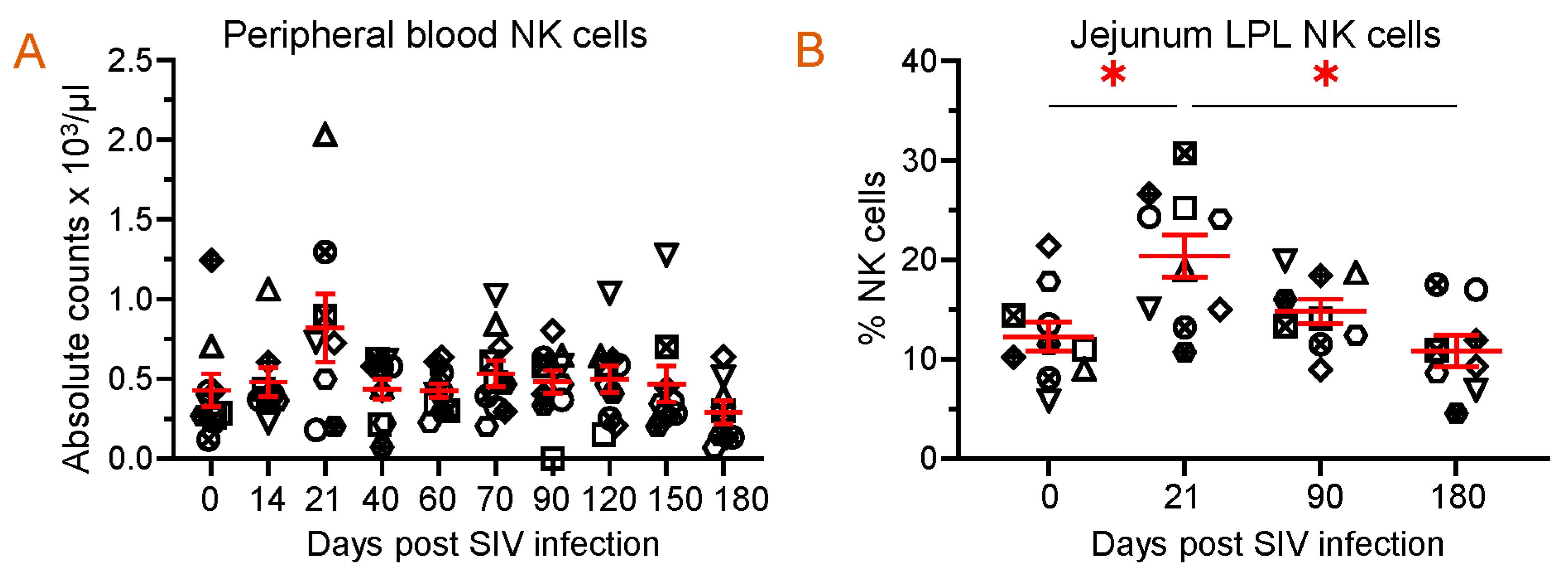
3.8. PBMC or Jejunum LPL NK, B, and CD8+ T Cells mb-TLR2 Expression Was Significantly Upregulated after Infection
3.9. TLR2 Agonist Modulates Cytokine/Chemokine Production In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chege, D.; Sheth, P.M.; Kain, T.; Kim, C.J.; Kovacs, C.; Loutfy, M.; Halpenny, R.; Kandel, G.; Chun, T.-W.; Ostrowski, M.; et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS 2011, 25, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; George, M.D.; Reay, E.; Guadalupe, M.; Flamm, J.; Prindiville, T.; Dandekar, S. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J. Virol. 2008, 82, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Boby, N.; Cao, X.; Ransom, A.; Pace, B.T.; Mabee, C.; Shroyer, M.N.; Das, A.; Didier, P.J.; Srivastav, S.K.; Porter, E.; et al. Identification, Characterization, and Transcriptional Reprogramming of Epithelial Stem Cells and Intestinal Enteroids in Simian Immunodeficiency Virus Infected Rhesus Macaques. Front. Immunol. 2021, 12, 769990. [Google Scholar] [CrossRef]
- Heise, C.; Vogel, P.; Miller, C.J.; Halsted, C.H.; Dandekar, S. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am. J. Pathol. 1993, 142, 1759–1771. [Google Scholar] [PubMed]
- Rajasuriar, R.; Wright, E.; Lewin, S.R. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr. Opin. HIV AIDS 2015, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Kelleher, A.D. Immune activation and immune aging in HIV infection. Curr. Opin. HIV AIDS 2016, 11, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Doitsh, G.; Greene, W.C. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe 2016, 19, 280–291. [Google Scholar] [CrossRef]
- Boby, N.; Ransom, A.; Pace, B.T.; Williams, K.M.; Mabee, C.; Das, A.; Srivastav, S.K.; Porter, E.; Pahar, B. Enhanced Intestinal TGF-Beta/SMAD-Dependent Signaling in Simian Immunodeficiency Virus Infected Rhesus Macaques. Cells 2021, 10, 806. [Google Scholar] [CrossRef]
- Pahar, B.; Lackner, A.A.; Piatak, M., Jr.; Lifson, J.D.; Wang, X.; Das, A.; Ling, B.; Montefiori, D.C.; Veazey, R.S. Control of viremia and maintenance of intestinal CD4(+) memory T cells in SHIV(162P3) infected macaques after pathogenic SIV(MAC251) challenge. Virology 2009, 387, 273–284. [Google Scholar] [CrossRef]
- Kenway-Lynch, C.S.; Das, A.; Pan, D.; Lackner, A.A.; Pahar, B. Dynamics of cytokine/chemokine responses in intestinal CD4+ and CD8+ T Cells during Acute Simian Immunodeficiency Virus Infection. J. Virol. 2013, 87, 11916–11923. [Google Scholar] [CrossRef]
- Liew, F.Y.; Xu, D.; Brint, E.K.; O’Neill, L.A. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, G.; Hayden, M.S.; Greenblatt, M.B.; Bussey, C.; Flavell, R.A.; Ghosh, S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 2004, 303, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, A. Use of single-cell technology to improve our understanding of the role of TLR2 in macrophage-Mycobacterium tuberculosis interaction. mSystems 2023, 8, e0073023. [Google Scholar] [CrossRef]
- Bieback, K.; Lien, E.; Klagge, I.M.; Avota, E.; Schneider-Schaulies, J.; Duprex, W.P.; Wagner, H.; Kirschning, C.J.; Ter Meulen, V.; Schneider-Schaulies, S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 2002, 76, 8729–8736. [Google Scholar] [CrossRef]
- Duesberg, U.; von dem Bussche, A.; Kirschning, C.; Miyake, K.; Sauerbruch, T.; Spengler, U. Cell activation by synthetic lipopeptides of the hepatitis C virus (HCV)–core protein is mediated by toll like receptors (TLRs) 2 and 4. Immunol. Lett. 2002, 84, 89–95. [Google Scholar] [CrossRef]
- Sun, P.P.; Li, D.; Su, M.; Ren, Q.; Guo, W.P.; Wang, J.L.; Du, L.Y.; Xie, G.C. Cell membrane-bound toll-like receptor-1/2/4/6 monomers and -2 heterodimer inhibit enterovirus 71 replication by activating the antiviral innate response. Front. Immunol. 2023, 14, 1187035. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Silva, C.S.; Marinho, R.L.; Cabral, J.G.; Gurrao, E.; Dos Santos, P.A.S.; Casseb, S.M.M.; Lima, K.V.B.; Lima, L. Analysis of Epidemiological Factors and SNP rs3804100 of TLR2 for COVID-19 in a Cohort of Professionals Who Worked in the First Pandemic Wave in Belem-PA, Brazil. Genes 2023, 14, 1907. [Google Scholar] [CrossRef]
- Meier, A.; Kirschning, C.J.; Nikolaus, T.; Wagner, H.; Heesemann, J.; Ebel, F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003, 5, 561–570. [Google Scholar] [CrossRef]
- Campos, M.A.; Almeida, I.C.; Takeuchi, O.; Akira, S.; Valente, E.P.; Procopio, D.O.; Travassos, L.R.; Smith, J.A.; Golenbock, D.T.; Gazzinelli, R.T. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 2001, 167, 416–423. [Google Scholar] [CrossRef]
- Vijay Kumar, J.E.B. Toll-Like Receptors (TLRs) in Health and Disease: An Overview. In Handbook of Experimental Pharmacology; Michel, M.C., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. Toll-like Receptor Response to Human Immunodeficiency Virus Type 1 or Co-Infection with Hepatitis B or C Virus: An Overview. Int. J. Mol. Sci. 2023, 24, 9624. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.C.; Stevenson, M.; Latz, E.; Urcuqui-Inchima, S. HIV type 1 infection up-regulates TLR2 and TLR4 expression and function in vivo and in vitro. AIDS Res. Hum. Retroviruses 2012, 28, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wan, X.; Li, X.; Pan, K.; Maimaitiaili, W.; Zhang, Y. Expression of TLR4 gene is downregulated in acquired immune deficiency syndrome-associated Kaposi’s sarcoma. Exp. Ther. Med. 2019, 17, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Reuven, E.M.; Ali, M.; Rotem, E.; Schwarzer, R.; Gramatica, A.; Futerman, A.H.; Shai, Y. The HIV-1 envelope transmembrane domain binds TLR2 through a distinct dimerization motif and inhibits TLR2-mediated responses. PLoS Pathog. 2014, 10, e1004248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nazli, A.; Kafka, J.K.; Ferreira, V.H.; Anipindi, V.; Mueller, K.; Osborne, B.J.; Dizzell, S.; Chauvin, S.; Mian, M.F.; Ouellet, M.; et al. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J. Immunol. 2013, 191, 4246–4258. [Google Scholar] [CrossRef] [PubMed]
- Heggelund, L.; Muller, F.; Lien, E.; Yndestad, A.; Ueland, T.; Kristiansen, K.I.; Espevik, T.; Aukrust, P.; Froland, S.S. Increased expression of toll-like receptor 2 on monocytes in HIV infection: Possible roles in inflammation and viral replication. Clin. Infect. Dis. 2004, 39, 264–269. [Google Scholar] [CrossRef][Green Version]
- Victoria, S.; Temerozo, J.R.; Gobbo, L.; Pimenta-Inada, H.K.; Bou-Habib, D.C. Activation of Toll-like receptor 2 increases macrophage resistance to HIV-1 infection. Immunobiology 2013, 218, 1529–1536. [Google Scholar] [CrossRef]
- Thibault, S.; Fromentin, R.; Tardif, M.R.; Tremblay, M.J. TLR2 and TLR4 triggering exerts contrasting effects with regard to HIV-1 infection of human dendritic cells and subsequent virus transfer to CD4+ T cells. Retrovirology 2009, 6, 42. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Boby, N.; Srivastav, A.; Srivastav, S.K.; Pahar, B. Divergent Cytokine and Chemokine Responses at Early Acute Simian Immunodeficiency Virus Infection Correlated with Virus Replication and CD4 T Cell Loss in a Rhesus Macaque Model. Vaccines 2023, 11, 264. [Google Scholar] [CrossRef]
- Boby, N.; Cao, X.; Williams, K.; Gadila, S.K.G.; Shroyer, M.N.; Didier, P.J.; Srivastav, S.K.; Das, A.; Baker, K.; Sha, Q.; et al. Simian Immunodeficiency Virus Infection Mediated Changes in Jejunum and Peripheral SARS-CoV-2 Receptor ACE2 and Associated Proteins or Genes in Rhesus Macaques. Front. Immunol. 2022, 13, 835686. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Kenway-Lynch, C.S.; Lala, W.; Veazey, R.S.; Lackner, A.A.; Das, A.; Pahar, B. Lack of interleukin-10-mediated anti-inflammatory signals and upregulated interferon gamma production are linked to increased intestinal epithelial cell apoptosis in pathogenic simian immunodeficiency virus infection. J. Virol. 2014, 88, 13015–13028. [Google Scholar] [CrossRef] [PubMed]
- Pahar, B.; Gray, W.; Fahlberg, M.; Grasperge, B.; Hunter, M.; Das, A.; Mabee, C.; Aye, P.P.; Schiro, F.; Hensley, K.; et al. Recombinant Simian Varicella Virus-Simian Immunodeficiency Virus Vaccine Induces T and B Cell Functions and Provides Partial Protection against Repeated Mucosal SIV Challenges in Rhesus Macaques. Viruses 2022, 14, 2819. [Google Scholar] [CrossRef] [PubMed]
- Kenway-Lynch, C.S.; Das, A.; Lackner, A.A.; Pahar, B. Cytokine/Chemokine responses in activated CD4+ and CD8+ T cells isolated from peripheral blood, bone marrow, and axillary lymph nodes during acute simian immunodeficiency virus infection. J. Virol. 2014, 88, 9442–9457. [Google Scholar] [CrossRef]
- Pahar, B.; Lackner, A.A.; Veazey, R.S. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur. J. Immunol. 2006, 36, 583–592. [Google Scholar] [CrossRef]
- Kamohara, A.; Hirata, H.; Xu, X.; Shiraki, M.; Yamada, S.; Zhang, J.Q.; Kukita, T.; Toyonaga, K.; Hara, H.; Urano, Y.; et al. IgG immune complexes with Staphylococcus aureus protein A enhance osteoclast differentiation and bone resorption by stimulating Fc receptors and TLR2. Int. Immunol. 2020, 32, 89–104. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I.; Inamura, S.; Giese, T.; Moll, H.; Endres, S.; Sing, A.; Zahringer, U.; Hartmann, G. Staphylococcus aureus protein A triggers T cell-independent B cell proliferation by sensitizing B cells for TLR2 ligands. J. Immunol. 2007, 178, 2803–2812. [Google Scholar] [CrossRef]
- de Graaf, C.A.; Metcalf, D. Thrombopoietin and hematopoietic stem cells. Cell Cycle 2011, 10, 1582–1589. [Google Scholar] [CrossRef]
- Stylianou, E.; Bjerkeli, V.; Yndestad, A.; Heggelund, L.; Waehre, T.; Damas, J.K.; Aukrust, P.; Froland, S.S. Raised serum levels of interleukin-18 is associated with disease progression and may contribute to virological treatment failure in HIV-1-infected patients. Clin. Exp. Immunol. 2003, 132, 462–466. [Google Scholar] [CrossRef]
- Zurawski, G.; de Vries, J.E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today 1994, 15, 19–26. [Google Scholar] [CrossRef]
- Horiuchi, K.; Morioka, H.; Takaishi, H.; Akiyama, H.; Blobel, C.P.; Toyama, Y. Ectodomain shedding of FLT3 ligand is mediated by TNF-alpha converting enzyme. J. Immunol. 2009, 182, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Nouhin, J.; Pean, P.; Madec, Y.; Chevalier, M.F.; Didier, C.; Borand, L.; Blanc, F.X.; Scott-Algara, D.; Laureillard, D.; Weiss, L. Interleukin-1 receptor antagonist, a biomarker of response to anti-TB treatment in HIV/TB co-infected patients. J. Infect. 2017, 74, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Dripps, D.J.; Brandhuber, B.J.; Thompson, R.C.; Eisenberg, S.P. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J. Biol. Chem. 1991, 266, 10331–10336. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Huang, M.; Yao, Y.M. Biology of Interleukin-17 and Its Pathophysiological Significance in Sepsis. Front. Immunol. 2020, 11, 1558. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor. Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Walz, A.; Schmutz, P.; Mueller, C.; Schnyder-Candrian, S. Regulation and function of the CXC chemokine ENA-78 in monocytes and its role in disease. J. Leukoc. Biol. 1997, 62, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Maruszewska, A.; Bosiacki, M.; Chlubek, D.; Baranowska-Bosiacka, I. The Potential Importance of CXCL1 in the Physiological State and in Noncancer Diseases of the Cardiovascular System, Respiratory System and Skin. Int. J. Mol. Sci. 2022, 24, 205. [Google Scholar] [CrossRef]
- White, G.E.; Greaves, D.R. Fractalkine: A survivor’s guide: Chemokines as antiapoptotic mediators. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 589–594. [Google Scholar] [CrossRef]
- Niebuhr, M.; Schorling, K.; Heratizadeh, A.; Werfel, T. Staphylococcal alpha-toxin induces a functional upregulation of TLR-2 on human peripheral blood monocytes. Exp. Dermatol. 2015, 24, 381–383. [Google Scholar] [CrossRef]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.G.; Lee, J.O. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef]
- Lester, R.T.; Yao, X.D.; Ball, T.B.; McKinnon, L.R.; Kaul, R.; Wachihi, C.; Jaoko, W.; Plummer, F.A.; Rosenthal, K.L. Toll-like receptor expression and responsiveness are increased in viraemic HIV-1 infection. AIDS 2008, 22, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Yao, X.D.; Rosenthal, K.L.; INFANT Study Team. HIV-1 Structural Proteins Serve as PAMPs for TLR2 Heterodimers Significantly Increasing Infection and Innate Immune Activation. Front. Immunol. 2015, 6, 426. [Google Scholar] [CrossRef]
- Lin, W.; Li, L.; Guo, P.; He, Y.; He, H.; Li, H.; Zhong, H.; Liu, C.; Du, P.; Cai, W.; et al. Early on-treatment plasma interleukin-18 as a promising indicator for long-term virological response in patients with HIV-1 infection. Front. Med. 2023, 10, 1170208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Spector, S.A. CCL2 increases X4-tropic HIV-1 entry into resting CD4+ T cells. J. Biol. Chem. 2008, 283, 30745–30753. [Google Scholar] [CrossRef] [PubMed]
- Mothapo, K.M.; Ten Oever, J.; Koopmans, P.; Stelma, F.F.; Burm, S.; Bajramovic, J.; Verbeek, M.M.; Rikkert, M.G.O.; Netea, M.G.; Koopman, G.; et al. Soluble TLR2 and 4 concentrations in cerebrospinal fluid in HIV/SIV-related neuropathological conditions. J. Neurovirology 2017, 23, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Pu, A.; Zheng, H.; Liu, M.; Chen, W.; Wang, W.; Xiao, W.; Yang, H. TLR2-Dependent Signaling for IL-15 Production Is Essential for the Homeostasis of Intestinal Intraepithelial Lymphocytes. Mediat. Inflamm. 2016, 2016, 4281865. [Google Scholar] [CrossRef]
- Henrick, B.M.; Yao, X.D.; Drannik, A.G.; Abimiku, A.; Rosenthal, K.L.; INFANT Study Team. Soluble toll-like receptor 2 is significantly elevated in HIV-1 infected breast milk and inhibits HIV-1 induced cellular activation, inflammation and infection. AIDS 2014, 28, 2023–2032. [Google Scholar] [CrossRef]
- Hernandez, J.C.; Arteaga, J.; Paul, S.; Kumar, A.; Latz, E.; Urcuqui-Inchima, S. Up-regulation of TLR2 and TLR4 in dendritic cells in response to HIV type 1 and coinfection with opportunistic pathogens. AIDS Res. Hum. Retroviruses 2011, 27, 1099–1109. [Google Scholar] [CrossRef]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Wang, J.; Guo, J. Role of Regenerating Islet-Derived Protein 3A in Gastrointestinal Cancer. Front. Oncol. 2019, 9, 1449. [Google Scholar] [CrossRef] [PubMed]
- LeBouder, E.; Rey-Nores, J.E.; Rushmere, N.K.; Grigorov, M.; Lawn, S.D.; Affolter, M.; Griffin, G.E.; Ferrara, P.; Schiffrin, E.J.; Morgan, B.P.; et al. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J. Immunol. 2003, 171, 6680–6689. [Google Scholar] [CrossRef] [PubMed]
- Kuroishi, T.; Tanaka, Y.; Sakai, A.; Sugawara, Y.; Komine, K.; Sugawara, S. Human parotid saliva contains soluble toll-like receptor (TLR) 2 and modulates TLR2-mediated interleukin-8 production by monocytic cells. Mol. Immunol. 2007, 44, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Dulay, A.T.; Buhimschi, C.S.; Zhao, G.; Oliver, E.A.; Mbele, A.; Jing, S.; Buhimschi, I.A. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J. Immunol. 2009, 182, 7244–7253. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Murthy, A.; Khokha, R. Clipping, shedding and RIPping keep immunity on cue. Trends Immunol. 2008, 29, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Raby, A.C.; Le Bouder, E.; Colmont, C.; Davies, J.; Richards, P.; Coles, B.; George, C.H.; Jones, S.A.; Brennan, P.; Topley, N.; et al. Soluble TLR2 reduces inflammation without compromising bacterial clearance by disrupting TLR2 triggering. J. Immunol. 2009, 183, 506–517. [Google Scholar] [CrossRef]
- Pan, D.; Das, A.; Liu, D.; Veazey, R.S.; Pahar, B. Isolation and characterization of intestinal epithelial cells from normal and SIV-infected rhesus macaques. PLoS ONE 2012, 7, e30247. [Google Scholar] [CrossRef]
- Dayer, J.M.; Oliviero, F.; Punzi, L. A Brief History of IL-1 and IL-1 Ra in Rheumatology. Front. Pharmacol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Bailer, R.T.; Holloway, A.; Sun, J.; Margolick, J.B.; Martin, M.; Kostman, J.; Montaner, L.J. IL-13 and IFN-gamma secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. J. Immunol. 1999, 162, 7534–7542. [Google Scholar] [CrossRef]
- Pham, T.N.Q.; Meziane, O.; Miah, M.A.; Volodina, O.; Colas, C.; Beland, K.; Li, Y.; Dallaire, F.; Keler, T.; Guimond, J.V.; et al. Flt3L-Mediated Expansion of Plasmacytoid Dendritic Cells Suppresses HIV Infection in Humanized Mice. Cell Rep. 2019, 29, 2770–2782.e5. [Google Scholar] [CrossRef]
- Tan, A.C.; Mifsud, E.J.; Zeng, W.; Edenborough, K.; McVernon, J.; Brown, L.E.; Jackson, D.C. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol. Pharm. 2012, 9, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Proud, P.C.; Tsitoura, D.; Watson, R.J.; Chua, B.Y.; Aram, M.J.; Bewley, K.R.; Cavell, B.E.; Cobb, R.; Dowall, S.; Fotheringham, S.A.; et al. Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model. EBioMedicine 2021, 63, 103153. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Xu, X.; Wang, J.; Huang, L.; Peng, J.; Yu, T.; Zhou, Y.; Cheng, K.; Liu, S. TLR1/2 Agonist Enhances Reversal of HIV-1 Latency and Promotes NK Cell-Induced Suppression of HIV-1-Infected Autologous CD4(+) T Cells. J. Virol. 2021, 95, e0081621. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boby, N.; Williams, K.M.; Das, A.; Pahar, B. Toll-like Receptor 2 Mediated Immune Regulation in Simian Immunodeficiency Virus-Infected Rhesus Macaques. Vaccines 2023, 11, 1861. https://doi.org/10.3390/vaccines11121861
Boby N, Williams KM, Das A, Pahar B. Toll-like Receptor 2 Mediated Immune Regulation in Simian Immunodeficiency Virus-Infected Rhesus Macaques. Vaccines. 2023; 11(12):1861. https://doi.org/10.3390/vaccines11121861
Chicago/Turabian StyleBoby, Nongthombam, Kelsey M. Williams, Arpita Das, and Bapi Pahar. 2023. "Toll-like Receptor 2 Mediated Immune Regulation in Simian Immunodeficiency Virus-Infected Rhesus Macaques" Vaccines 11, no. 12: 1861. https://doi.org/10.3390/vaccines11121861
APA StyleBoby, N., Williams, K. M., Das, A., & Pahar, B. (2023). Toll-like Receptor 2 Mediated Immune Regulation in Simian Immunodeficiency Virus-Infected Rhesus Macaques. Vaccines, 11(12), 1861. https://doi.org/10.3390/vaccines11121861





