Immunization Effects of a Novel α-Synuclein-Based Peptide Epitope Vaccine in Parkinson’s Disease-Associated Pathology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Peptide Epitope
2.2. Immunizations
2.3. Open Field Test
2.4. Rotarod Performance Test
2.5. Protein Extraction from Mouse Brain
2.6. Western Blot Analysis
2.7. Immunofluorescence Staining
2.8. ELISA
2.9. Statistical Analysis
3. Results
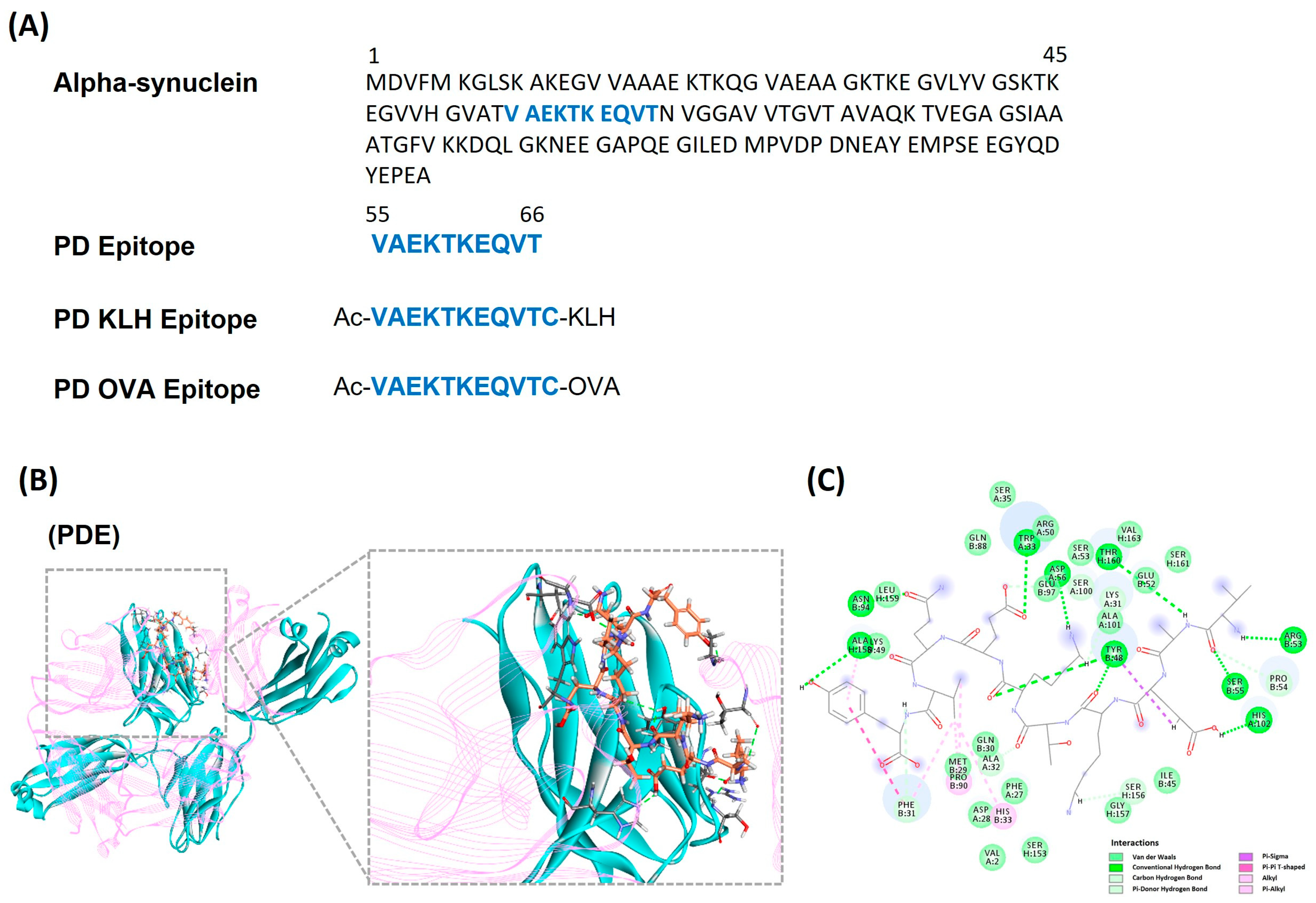
3.1. Identifying and Designing α-Synuclein Based Peptide Epitope Vaccine
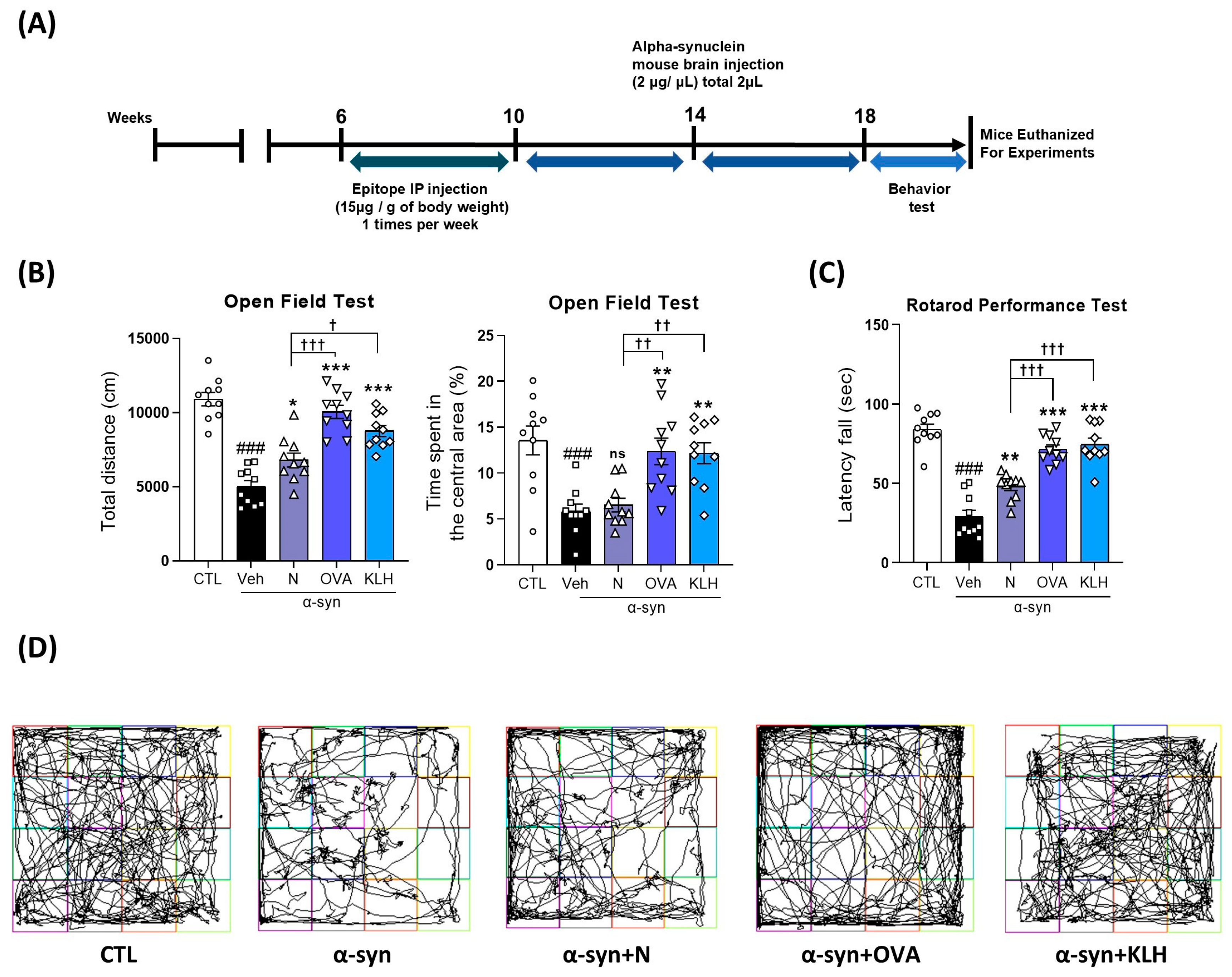
3.2. Immunization Epitope Vaccine Abrogated Motor Dysfunction in α-syn Induced PD Mouse Model
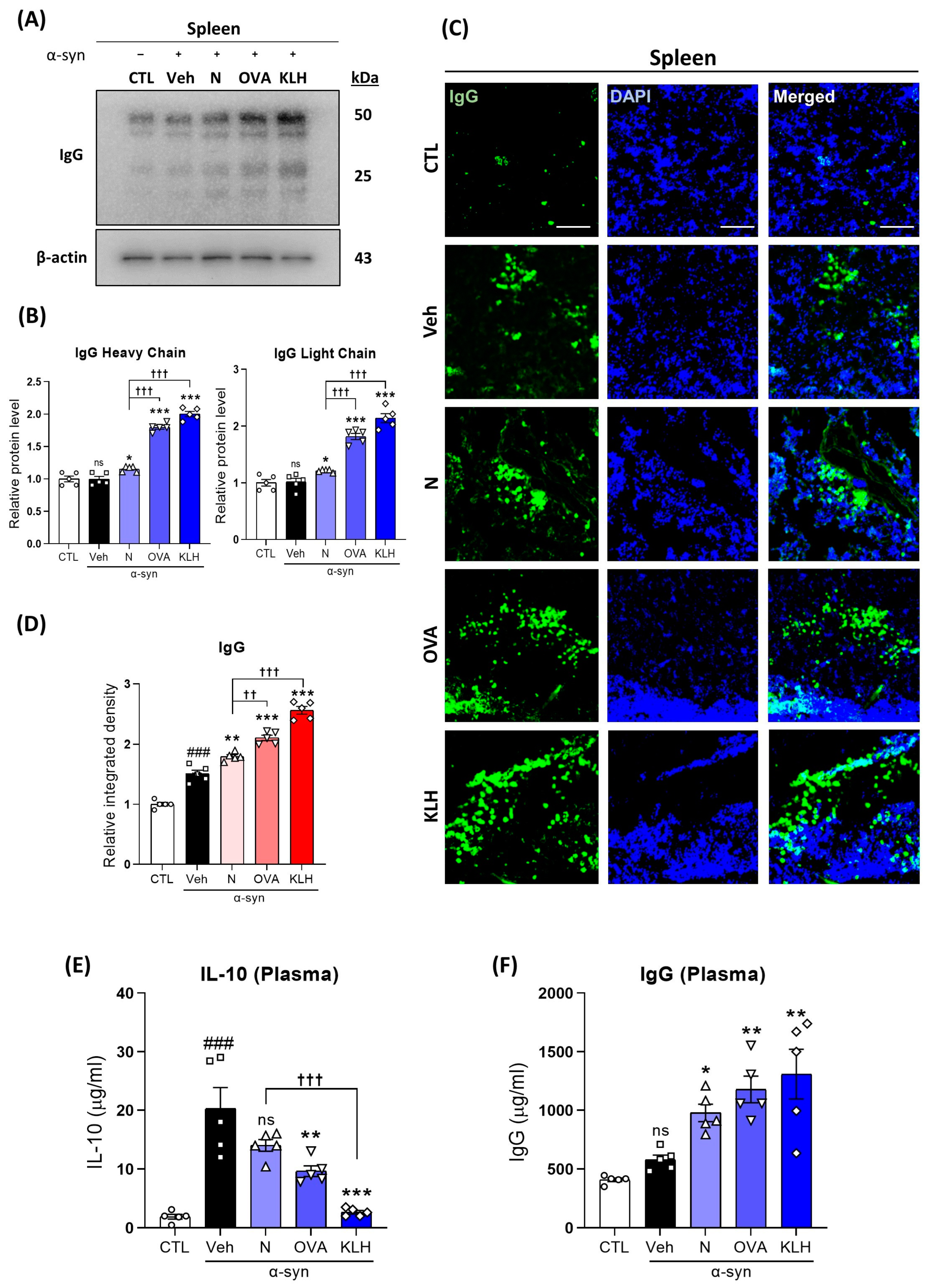
3.3. Changes in IgG in the Spleen and Plasma after the Administration Epitope Vaccines in α-syn-Induced PD Mouse Model
3.4. Immunization with Epitope Vaccine Eliminated α-syn Protein Aggregation and Increased Autophagy in α-syn-Induced PD Mouse Model

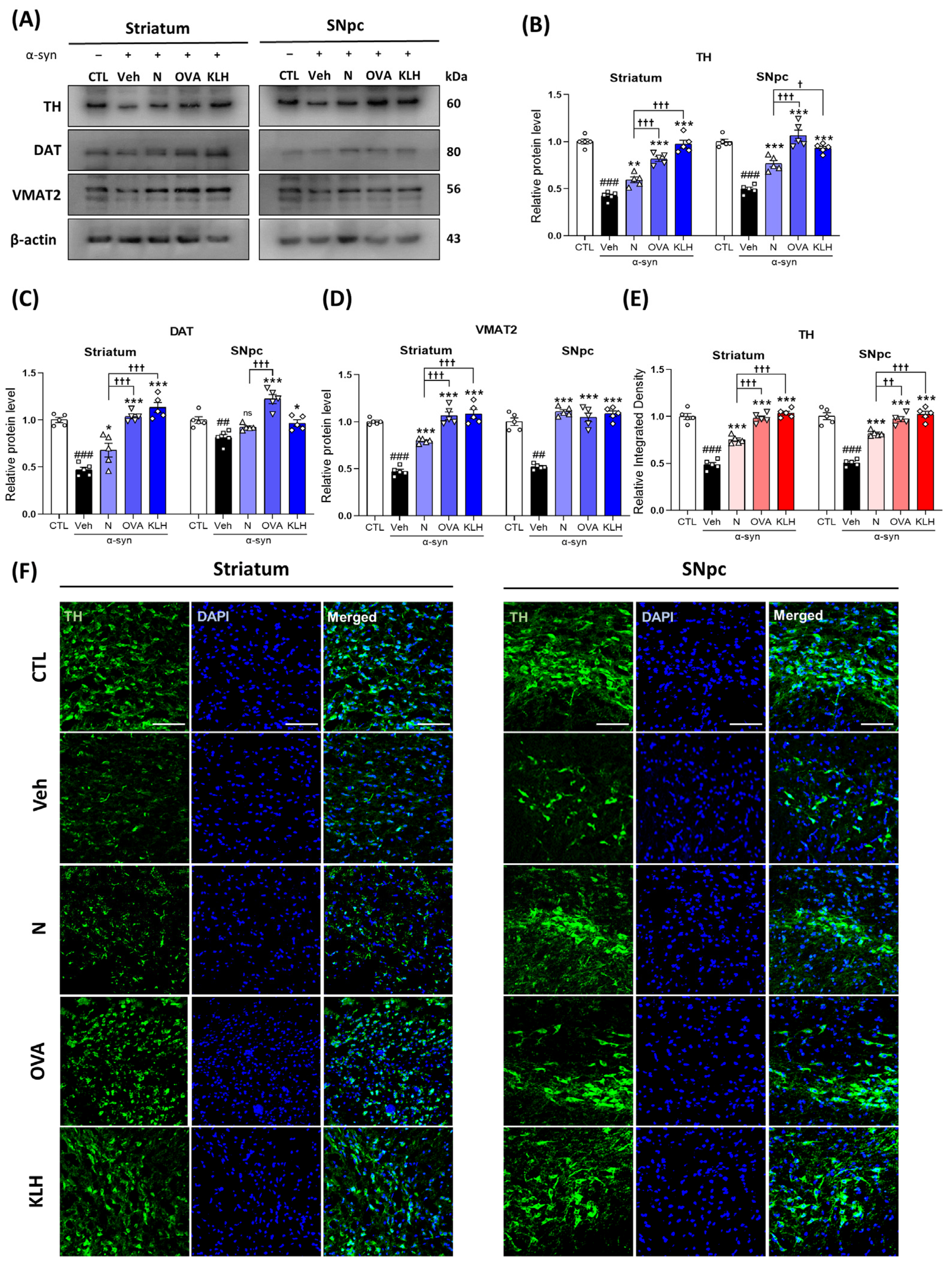
3.5. Immunization with Epitope Vaccine Reversed Dopamine-Related Markers in α-syn-Induced PD Mouse Model
3.6. Epitope Vaccine Immunization Decreases Glial Cell Activation in α-syn-Induced PD Mouse Model
3.7. Epitope Vaccine Immunization Reduced the Expression of Inflammatory Cytokines in α-syn-Induced PD Mouse Model


4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, J.; Dong, W.; Qin, Y.; Xie, J.; Xiao, J.; Xu, J.; Wang, H. Roflupram exerts neuroprotection via activation of CREB/PGC-1alpha signalling in experimental models of Parkinson’s disease. Br. J. Pharmacol. 2020, 177, 2333–2350. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.M.; Heneka, M.T. Role of neuroinflammation in neurodegeneration: New insights. Alzheimers Res. Ther. 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Migdalska-Richards, A.; Schapira, A.H. The relationship between glucocerebrosidase mutations and Parkinson disease. J. Neurochem. 2016, 139 (Suppl. S1), 77–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Thomas, J.L.; Su, Z.L.; Yeh, W.K.; Monzel, A.S.; Bolognin, S.; Schwamborn, J.C.; Yang, C.H.; Lin, H.Y. Epitope imprinting of alpha-synuclein for sensing in Parkinson’s brain organoid culture medium. Biosens. Bioelectron. 2021, 175, 112852. [Google Scholar] [CrossRef] [PubMed]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Perez, R.G.; Hastings, T.G. Could a loss of alpha-synuclein function put dopaminergic neurons at risk? J. Neurochem. 2004, 89, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Tehranian, R.; Dietrich, P.; Stefanis, L.; Perez, R.G. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J. Cell Sci. 2005, 118, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.G.; Waymire, J.C.; Lin, E.; Liu, J.J.; Guo, F.; Zigmond, M.J. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002, 22, 3090–3099. [Google Scholar] [CrossRef]
- Ingelsson, M. Alpha-Synuclein Oligomers-Neurotoxic Molecules in Parkinson’s Disease and Other Lewy Body Disorders. Front. Neurosci. 2016, 10, 408. [Google Scholar] [CrossRef]
- Wersinger, C.; Prou, D.; Vernier, P.; Sidhu, A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003, 17, 2151–2153. [Google Scholar] [CrossRef]
- Oaks, A.W.; Sidhu, A. Synuclein modulation of monoamine transporters. FEBS Lett. 2011, 585, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Bridi, J.C.; Hirth, F. Mechanisms of alpha-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Wood, H. Alpha-Synuclein-activated microglia are implicated in PD pathogenesis. Nat. Rev. Neurol. 2022, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Luo, J.H.; Jin, J.H. Role of microglial activation induced by alpha-synuclein in pathogenesis of Parkinson’s disease. Zhejiang Da Xue Xue Bao Yi Xue Ban 2012, 41, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Cairns, N.J.; Lantos, P.L.; Goedert, M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 1998, 251, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Lee, V.M.; Trojanowski, J.Q. Synucleinopathies: Clinical and pathological implications. Arch. Neurol. 2001, 58, 186–190. [Google Scholar] [CrossRef]
- Sorrentino, Z.A.; Giasson, B.I.; Chakrabarty, P. Alpha-Synuclein and astrocytes: Tracing the pathways from homeostasis to neurodegeneration in Lewy body disease. Acta Neuropathol. 2019, 138, 1–21. [Google Scholar] [CrossRef]
- Schenk, D.B.; Koller, M.; Ness, D.K.; Griffith, S.G.; Grundman, M.; Zago, W.; Soto, J.; Atiee, G.; Ostrowitzki, S.; Kinney, G.G. First-in-human assessment of PRX002, an anti-alpha-synuclein monoclonal antibody, in healthy volunteers. Mov. Disord. 2017, 32, 211–218. [Google Scholar] [CrossRef]
- Volc, D.; Poewe, W.; Kutzelnigg, A.; Luhrs, P.; Thun-Hohenstein, C.; Schneeberger, A.; Galabova, G.; Majbour, N.; Vaikath, N.; El-Agnaf, O.; et al. Safety and immunogenicity of the alpha-synuclein active immunotherapeutic PD01A in patients with Parkinson’s disease: A randomised, single-blinded, phase 1 trial. Lancet Neurol. 2020, 19, 591–600. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Meng, Y.; Yan, X.J.; Liu, S.; Wang, G.Q.; Cao, Y.P. Immunization with Abeta3-10-KLH vaccine improves cognitive function and ameliorates mitochondrial dysfunction and reduces Alzheimer’s disease-like pathology in Tg-APPswe/PSEN1dE9 mice. Brain Res. Bull. 2021, 174, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Mareljic, N.; Michaelsen, M.; Parhizkar, S.; Heindl, S.; Nuscher, B.; Farny, D.; Czuppa, M.; Schludi, C.; Graf, A.; et al. Active poly-GA vaccination prevents microglia activation and motor deficits in a C9orf72 mouse model. EMBO Mol. Med. 2020, 12, e10919. [Google Scholar] [CrossRef] [PubMed]
- Schenk, D.; Barbour, R.; Dunn, W.; Gordon, G.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; Khan, K.; et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999, 400, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Rockenstein, E.; Adame, A.; Alford, M.; Crews, L.; Hashimoto, M.; Seubert, P.; Lee, M.; Goldstein, J.; Chilcote, T.; et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 2005, 46, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Knecht, L.; Folke, J.; Dodel, R.; Ross, J.A.; Albus, A. Alpha-synuclein Immunization Strategies for Synucleinopathies in Clinical Studies: A Biological Perspective. Neurotherapeutics 2022, 19, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Paumier, K.L.; Luk, K.C.; Manfredsson, F.P.; Kanaan, N.M.; Lipton, J.W.; Collier, T.J.; Steece-Collier, K.; Kemp, C.J.; Celano, S.; Schulz, E.; et al. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis. 2015, 82, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choe, K.; Lee, H.J.; Park, T.J.; Kim, M.O. Neuroprotective effects of osmotin in Parkinson’s disease-associated pathology via the AdipoR1/MAPK/AMPK/mTOR signaling pathways. J. Biomed. Sci. 2023, 30, 66. [Google Scholar] [CrossRef]
- Lee, H.J.; Choe, K.; Park, J.S.; Khan, A.; Kim, M.W.; Park, T.J.; Kim, M.O. O-Cyclic Phytosphingosine-1-Phosphate Protects against Motor Dysfunctions and Glial Cell Mediated Neuroinflammation in the Parkinson’s Disease Mouse Models. Antioxidants 2022, 11, 2107. [Google Scholar] [CrossRef]
- Shah, S.; Yoon, G.; Chung, S.; Abid, M.; Kim, T.; Lee, H.; Kim, M. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer’s disease neuropathological deficits. Mol. Psychiatry 2017, 22, 407–416. [Google Scholar] [CrossRef]
- Ali, T.; Rehman, S.U.; Khan, A.; Badshah, H.; Abid, N.B.; Kim, M.W.; Jo, M.H.; Chung, S.S.; Lee, H.G.; Rutten, B.P.F.; et al. Adiponectin-mimetic novel nonapeptide rescues aberrant neuronal metabolic-associated memory deficits in Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 23. [Google Scholar] [CrossRef]
- Ullah, R.; Jo, M.H.; Riaz, M.; Alam, S.I.; Saeed, K.; Ali, W.; Rehman, I.U.; Ikram, M.; Kim, M.O. Glycine, the smallest amino acid, confers neuroprotection against D-galactose-induced neurodegeneration and memory impairment by regulating c-Jun N-terminal kinase in the mouse brain. J. Neuroinflamm. 2020, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Rehman, S.U.; Shah, F.A.; Kim, M.O. Acute dose of melatonin via Nrf2 dependently prevents acute ethanol-induced neurotoxicity in the developing rodent brain. J. Neuroinflamm. 2018, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI 3/Akt/GS k3β pathway in the mouse hippocampus. J. Pineal Res. 2015, 59, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998, 4, 1318–1320. [Google Scholar] [CrossRef]
- Giasson, B.I.; Murray, I.V.; Trojanowski, J.Q.; Lee, V.M. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J. Biol. Chem. 2001, 276, 2380–2386. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Lee, V.M. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 2014, 9, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.K.; Ho, H.A.; Perez-Acuna, D.; Lee, S.J. Modeling alpha-Synuclein Propagation with Preformed Fibril Injections. J. Mov. Disord. 2019, 12, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Benner, E.J.; Mosley, R.L.; Destache, C.J.; Lewis, T.B.; Jackson-Lewis, V.; Gorantla, S.; Nemachek, C.; Green, S.R.; Przedborski, S.; Gendelman, H.E. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 9435–9440. [Google Scholar] [CrossRef]
- Harris, J.R.; Markl, J. Keyhole limpet hemocyanin (KLH): A biomedical review. Micron 1999, 30, 597–623. [Google Scholar] [CrossRef]
- Beck, L.; Spiegelberg, H.L. The polyclonal and antigen-specific IgE and IgG subclass response of mice injected with ovalbumin in alum or complete Freund’s adjuvant. Cell Immunol. 1989, 123, 1–8. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front Immunol 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R. Immunological functions of splenic B-lymphocytes. Crit. Rev. Immunol. 1992, 11, 395–417. [Google Scholar] [PubMed]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Parkinsons Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef] [PubMed]
- Xilouri, M.; Brekk, O.R.; Stefanis, L. Autophagy and Alpha-Synuclein: Relevance to Parkinson’s Disease and Related Synucleopathies. Mov. Disord. 2016, 31, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.A.; Hayat, M.A. Immunotoxicology, Immunopathology, and Immunotherapy; Academic Press, an Imprint of Elsevier: London, UK; San Diego, CA, USA, 2018; pp. 149–169. [Google Scholar]
- Spencer, B.; Potkar, R.; Trejo, M.; Rockenstein, E.; Patrick, C.; Gindi, R.; Adame, A.; Wyss-Coray, T.; Masliah, E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in α-synuclein models of Parkinson’s and Lewy body diseases. J. Neurosci. 2009, 29, 13578–13588. [Google Scholar] [CrossRef] [PubMed]
- Stykel, M.G.; Humphries, K.M.; Kamski-Hennekam, E.; Buchner-Duby, B.; Porte-Trachsel, N.; Ryan, T.; Coackley, C.L.; Bamm, V.V.; Harauz, G.; Ryan, S.D. α-Synuclein mutation impairs processing of endomembrane compartments and promotes exocytosis and seeding of α-synuclein pathology. Cell Rep. 2021, 35, 109099. [Google Scholar] [CrossRef]
- Alter, S.P.; Lenzi, G.M.; Bernstein, A.I.; Miller, G.W. Vesicular integrity in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2013, 13, 362. [Google Scholar] [CrossRef]
- Chen, R.; Furman, C.A.; Gnegy, M.E. Dopamine transporter trafficking: Rapid response on demand. Future Neurol. 2010, 5, 123. [Google Scholar] [CrossRef]
- Beraud, D.; Hathaway, H.A.; Trecki, J.; Chasovskikh, S.; Johnson, D.A.; Johnson, J.A.; Federoff, H.J.; Shimoji, M.; Mhyre, T.R.; Maguire-Zeiss, K.A. Microglial activation and antioxidant responses induced by the Parkinson’s disease protein alpha-synuclein. J. Neuroimmune Pharmacol. 2013, 8, 94–117. [Google Scholar] [CrossRef]
- Reynolds, A.D.; Stone, D.K.; Mosley, R.L.; Gendelman, H.E. Nitrated alpha-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J. Immunol. 2009, 182, 4137–4149. [Google Scholar] [CrossRef]
- Sulzer, D.; Alcalay, R.N.; Garretti, F.; Cote, L.; Kanter, E.; Agin-Liebes, J.; Liong, C.; McMurtrey, C.; Hildebrand, W.H.; Mao, X.; et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 2017, 546, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Shavali, S.; Combs, C.K.; Ebadi, M. Reactive macrophages increase oxidative stress and alpha-synuclein nitration during death of dopaminergic neuronal cells in co-culture: Relevance to Parkinson’s disease. Neurochem. Res. 2006, 31, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, C.; Lee, S.J. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxidative Med. Cell Longev. 2010, 3, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Heng, Y.; Yuan, Y.H.; Chen, N.H. Pathological alpha-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 2017, 265, 30–37. [Google Scholar] [CrossRef]
- Sanchez-Guajardo, V.; Tentillier, N.; Romero-Ramos, M. The relation between alpha-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience 2015, 302, 47–58. [Google Scholar] [CrossRef]
- Prajapati, P.; Sripada, L.; Singh, K.; Bhatelia, K.; Singh, R.; Singh, R. TNF-alpha regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim. Biophys. Acta 2015, 1852, 451–461. [Google Scholar] [CrossRef]
| Antibody | Host | Application | Manufacturer | Catalog # | Concentration |
|---|---|---|---|---|---|
| α-syn | Mouse | WB/IF | Santa Cruz Biotechnology, Dallas, TX, USA | SC58480 | 1:1000/1:100 |
| TH | Mouse/Rabbit | WB/IF | Santa Cruz Biotechnology Cell Signaling, Danvers, MA, USA | SC25269 E2L6M | 1:1000/1:100 |
| DAT | Rat | WB | Santa Cruz Biotechnology | SC32259 | 1:1000 |
| VMAT2 | Mouse | WB | Santa Cruz Biotechnology | SC374079 | 1:1000 |
| IgG | Mouse | WB/IF/ELISA | Santa Cruz Biotechnology MyBioSource, San Diego, CA, USA | SC515946 MBS2708011 | 1:1000/1:100 |
| IL-10 | Mouse | ELISA | MyBioSource | MBS704754 | 1:100 |
| GFAP | Mouse | WB/IF | Santa Cruz Biotechnology | SC33673 | 1:1000/1:100 |
| Iba-1 | Mouse | WB | Santa Cruz Biotechnology | SC398406 | 1:1000 |
| TNF-α | Mouse | WB/IF | Santa Cruz Biotechnology | SC52746 | 1:1000/1:100 |
| IL-1β | Mouse | WB | Santa Cruz Biotechnology | SC32294 | 1:1000 |
| Beclin1 | Mouse | WB | Santa Cruz Biotechnology | SC48341 | 1:1000 |
| LC3B | Rabbit | WB | Abcam, Cambridge, UK | AB48394 | 1:1000 |
| p62 | Mouse | WB | Santa Cruz Biotechnology | SC48402 | 1:1000 |
| PSD-95 | Mouse | WB | Santa Cruz Biotechnology | SC71933 | 1:1000 |
| NeuN | Rabbit | IF | Cell Signaling | D4G4O | 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Ahmad, R.; Choe, K.; Kang, M.H.; Park, T.J.; Kim, M.O. Immunization Effects of a Novel α-Synuclein-Based Peptide Epitope Vaccine in Parkinson’s Disease-Associated Pathology. Vaccines 2023, 11, 1820. https://doi.org/10.3390/vaccines11121820
Park JS, Ahmad R, Choe K, Kang MH, Park TJ, Kim MO. Immunization Effects of a Novel α-Synuclein-Based Peptide Epitope Vaccine in Parkinson’s Disease-Associated Pathology. Vaccines. 2023; 11(12):1820. https://doi.org/10.3390/vaccines11121820
Chicago/Turabian StylePark, Jun Sung, Riaz Ahmad, Kyonghwan Choe, Min Hwa Kang, Tae Ju Park, and Myeong Ok Kim. 2023. "Immunization Effects of a Novel α-Synuclein-Based Peptide Epitope Vaccine in Parkinson’s Disease-Associated Pathology" Vaccines 11, no. 12: 1820. https://doi.org/10.3390/vaccines11121820
APA StylePark, J. S., Ahmad, R., Choe, K., Kang, M. H., Park, T. J., & Kim, M. O. (2023). Immunization Effects of a Novel α-Synuclein-Based Peptide Epitope Vaccine in Parkinson’s Disease-Associated Pathology. Vaccines, 11(12), 1820. https://doi.org/10.3390/vaccines11121820






